Centre for Biological Sciences, Department of Biochemistry, K.S. Rangasamy
College of Arts and Science (Autonomous), Tiruchengode, Tamil Nadu, India.
Corresponding author email: sarabioc@gmail.com
Article Publishing History
Received: 29/10/2020
Accepted After Revision: 15/12/2020
Diabetes is a metabolic disorder which manifests itself in an extensive diversity of complications including neurodegeneration. The treatment with antioxidants has been reported to produce beneficial effect in various types of neurodegenerative diseases. In the present study diabetes was induced by the administration of streptozotocin (STZ; 45 mg/kg body weight) and nicotinamide (NAD; 110 mg/kg body weight) to rats by intraperitoneally and hyperglycemia was confirmed by the elevated glucose levels (˃250 mg/dL) in the blood. Then we investigated the effect of the administration of Asiatic acid (AA) is a triterpene and an active constituent found in large quantities in Centella asiatica, on biochemical parameters, acetylcholinesterase (AChE) activities, lipid peroxidation, enzymatic (superoxide dismutase, catalase, glutathione peroxidase) and nonenzymatic (glutathione) antioxidants in brain of normal (non diabetic) and STZ-NAD-induced diabetic rats.
Changes in blood glucose, insulin, insulin resistance, lipid peroxidation, enzymatic and nonenzymatic antioxidants and activities of AChE were observed in normal control and experimental diabetic rats. Administration of AA (40 mg/kg body weight) for 45 days to STZ-NAD-induced diabetic rats showed a significant (P<0.05) reduction in plasma glucose, insulin resistance, and brain lipid peroxidation and AChE activity while brain enzymatic and non-enzymatic antioxidants levels are increased. The therapeutic effect of AA was compared with gliclazide, a well-known antioxidant and antihyperglycemic drug. AA supplementation significantly ameliorated all alterations induced by STZ-NAD in rats. Taken together, our study clearly depicts that AA, as a powerful antioxidant, prevents AChE, oxidative damage in the STZ-NAD-induced diabetic rats representing their possible therapeutics to be investigated in brain disorders associated with diabetes.
Antioxidants, Asiatic Acid, Acetylcholinesterase, Diabetes Mellitus
Swapna K, Uddandrao V. V. S, Vadivukkarasi S, Saravanan G. Asiatic Acid Attenuate Type 2 Diabetes Mellitus Induced Alterations in Acetylcholinesterase and Antioxidant System of Brain in Rats. Biosc.Biotech.Res.Comm. 2020;13(4).
Swapna K, Uddandrao V. V. S, Vadivukkarasi S, Saravanan G. Asiatic Acid Attenuate Type 2 Diabetes Mellitus Induced Alterations in Acetylcholinesterase and Antioxidant System of Brain in Rats. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3kUA2Mv
Copyright © Swapna et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus (DM) is probably highest growing metabolic syndrome in the globe and its increasing incidence puts a large burden on society and the public health sector. DM is spreading like an epidemic disease and India emerges to be the capital. DM is characterized by the accumulation of glucose in the blood which causes hyperglycemia, often leads to various macrovascular and microvascular complications. The neurological consequences of DM in the Central Nervous System (CNS) are now receiving greater attention. Diabetic Neuropathy (DNP) affects nearly 50% of diabetic patients with either type 1 or type 2 DM and involves both the peripheral and central nervous systems. During diabetic state, oxidative stress is caused by hyperglycemia that may be amplified an autocatalytic cycle of metabolic stress, tissue damage, and cell death leading to a simultaneous increase in free radical production and compromised inhibitory and scavenger mechanisms. Many studies proved that high levels of lipidperoxidation and low antioxidant defenses increase the vulnerability of the CNS to oxidative damage which causes alteration brain energy metabolism (Ramkumar et al., 2004; Saravanan and Ponmurugan, 2013; Brahmanaidu et al., 2016; Sathibabu et al., 2019; Uddandrao et al., 2020).
The pathogenesis of DM is managed by insulin and oral administration of hypoglycemic medicines (Saravanan et al., 2009). Increasing of side effects and disease caused by drugs and chemotherapeutics agents provoke awareness toward natural products to minimize hazards like tissue and vascular complications caused by these drugs. Thus, new, relatively non-toxic, therapeutic representatives are required to treat hyperglycemia, which also would correct DNP (Saravanan and Ponmurugan, 2013; Sathibabu Uddandrao et al., 2018). According to several in vitro and in vivo studies, in animals and human, dietary terpenoid compounds alter hyperglycemia, dyslipidemia and insulin resistance (IR), and improve oxidative stress and inflammation markers. In addition, they can inhibit the progression of many complications of DM involving retinopathy, nephropathy, neuropathy and cardiovascular disease (Moneim et al., 2017; Parim et al., 2019).
Asiatic acid (AA) is a triterpene found abundantly in Centella asiatica (L.), a perennial herbaceous creeper of the Apiaceae family, commonly used as the major constituent in a salad, juices and as ingredient in culinary dishes (Rameshreddy et al., 2018; Uddandrao et al., 2019). AA has an extensive array of biological functions including antihyperlipidemic, antihyperglycemic and anti-inflammatory activities (Swapna et al., 2019; Kalidhindi et al., 2020). However, no information pertaining to the anti-neurodegenerative effects of AA in the diabetic brain effects of is available. Thus, the present investigation aimed to assess the beneficial effects of AA against brain neurodegeneration in type 2 diabetic rats.
MATERIAL AND METHODS
Chemicals: AA (2α, 3β, 23-trihydroxyurs-12-en-28-oic acid was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). All the reagents used in the experiments were analytical grade reagents of the highest purity.
Animals: Male Wistar rats were obtained from Department of Biochemistry, Muthyammal College of Arts and Science, Rasipuram, Tamil Nadu, India. The experimental rats were maintained under standard laboratory conditions (temperature: 22°C ± 2°C; humidity: 40%-60%) and were permitted food and water adlibitum. The rats, initially weighing 180–200g were divided into four groups of six each (n=6). All procedures involving laboratory animals were in accordance with the institutional animal ethical committee of Muthyammal College of Arts and Science (Approval No: IAEC/MCAS/05/2017).
Induction of DM: The overnight fasted rats were made diabetic by a single intraperitoneal injection of freshly prepared STZ (45 mg/kg BW) in citrate buffer (0.1 M, pH 4.5), 15 min after the intraperitoneal administration of Nicotinamide (110 mg/kg BW) in 0.9% normal saline. Hyperglycemia was confirmed by the elevated glucose levels (Above 250 mg/dL) in blood, determined at 72 h and then on day 7 after injection.
Experimental design: After the successful induction of experimental DM, the rats were divided into four groups each comprising a minimum of six rats.
Group 1: Normal control
Group 2: Diabetic control
Group 3: Diabetic rats orally treated with AA (40 mg/kg BW) in vehicle solution for 45 days through an intragastric tube (Swapna et al., 2019).
Group 4: Diabetic rats orally treated with Gliclazide (5 mg/kg BW) in vehicle solution for 45 days through an intragastric tube (Pulido et al., 1997).
Body weight and blood glucose level measurements were conducted periodically. At the end of the experiments, all the animals were anaesthetized using mild chloroform then decapitated. Brain structures were removed and separated into cerebral cortex and hippo campus. Blood samples were collected into tubes containing 2% sodium oxalate as an anticoagulant. The samples were centrifuged at 250 ×g for 5 min at 4°C, and then the plasma was immediately removed and stored at −20°C until analyzed. Blood glucose level was determined by using Span Diagnostic kit, Mumbai, India.
Determination of blood glucose, insulin and IR: Blood glucose level was estimated spectrophotometrically using reagent kit purchased from Spinreact Co. (Spain). Insulin levels in serum were estimated using specific ELISA kits (R&D Systems Inc., USA) according to the manufacturer’s instructions. Insulin resistance was calculated by homeostasis model assessment of IR (HOMA-IR) as described by Rameshreddy et al. (2018).
Determination of antioxidants in brain: Brain tissues were weighed and rinsed in ice-cold saline. A 10% tissue homogenate was prepared using 0.025 M Tris-HCl buffer, pH 7.5. After centrifugation at 2000 rpm for 10 min, the clear supernatant was used for further biochemical assays. The supernatants thus obtained were used for the estimation of thiobarbituric acid substances (TBARS) (Fraga et al., 1988), assay of reduced glutathione (GSH) (Ellman, 1959), superoxide dismutase (SOD) (Kakkar et al., 1984), catalase (CAT) (Aebi, 1984), glutathione peroxidase (GPx) by Paglia and valentine (1967) and glutathione-S-transferase (GST) activity was assayed by the method of Habig and Jackoby (1981).
Determination of Cholinergic dysfunction: Cholinergic dysfunction was assessed by measuring acetylcholinesterase (AChE) levels in cerebral cortex and hippo campus according to the method of Ellman et al. (1961).
Statistical analysis: All the results were expressed as the Mean ± S.D. (n=6). All the grouped data were statistically evaluated with graph pad prism software. Hypothesis testing methods included ANOVA followed by bonferroni multiple comparison test. Significance level at P<0.05 was considered to indicate statistical significance.
RESULTS AND DISCUSSION
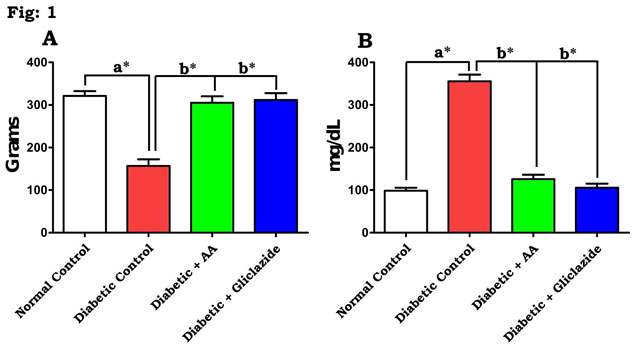
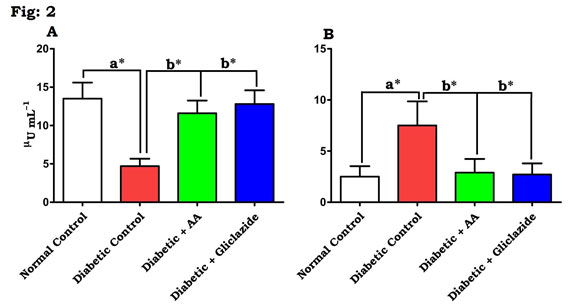
In this study, DM was induced through STZ-NAD in rats. The mechanisms by which STZ-NAD brings about its diabetic state include selective devastation of pancreatic insulin secreting β-cells, which makes the cells less active (Pari and Srinivasan, 2010; Brahmanaidu et al., 2017) and leads to deprived glucose utilization by tissues. In the present study, STZ-NAD treatment to experimental animals confirmed the nature of DM as evidenced by decreasing body weight, insulin, and hyperglycemic condition and increased IR. This is in line with previous reports (Swapna et al., 2019). Figure 1 & 2 depicts the level of body weight changes (Fig. 1A), blood glucose (Fig. 1B), insulin (Fig. 2A) and IR (Fig. 2B) in control and experimental rats.
Figure 1: Effect of AA on (A) body weight changes and (B) blood glucose in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 2: Effect of AA on (A) insulin and (B) IR in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
There was a significant (P<0.05) elevation in blood glucose level, IR and concomitant decreased level of body weight and insulin were noticed in STZ-NAD induced diabetic rats when compared with normal control. Administration of AA or gliclazide caused reduction in blood glucose, IR and increase in body weight and insulin notably (P<0.05) when compared to the diabetic rats. AA produced hypoglycemic effect perhaps by enhancing the peripheral utilization of glucose, correcting the impaired hepatic glycolysis and limiting its gluconeogenic development parallel to insulin. In case of AA, the mechanism of reduction of blood glucose level might be due to increased peripheral uptake of glucose and increased sensitivity of insulin receptors (Saravanan et al., 2009; Uddandrao et al., 2019).
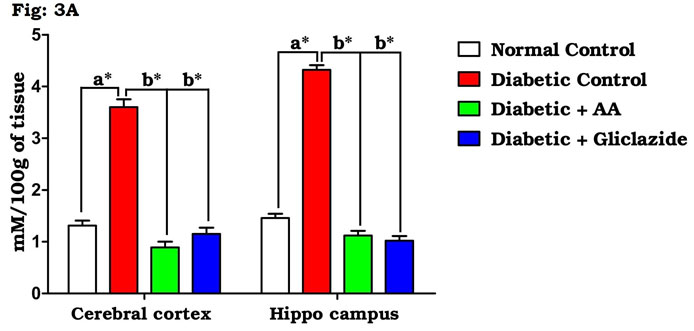
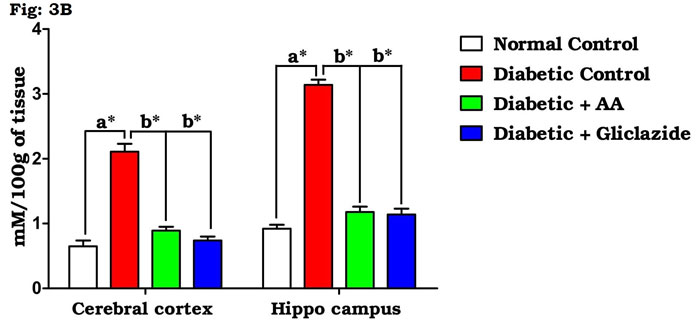
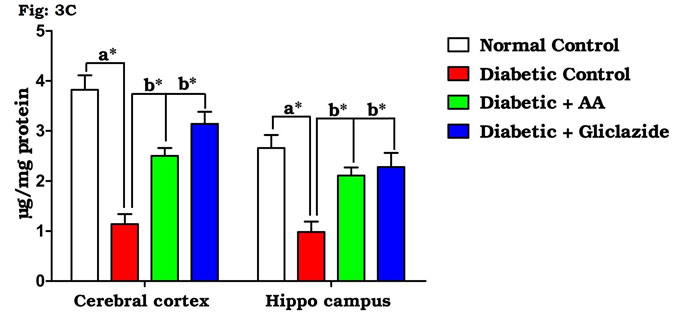
As summarized in figure 3A-C, there was a significant increase in the levels of brain TBARS (Fig. 3A) and hydroperoxides (Fig. 3B) in diabetic rats as compared to control rats. Treatment of diabetic rats with AA and gliclazide markedly ameliorated these altered parameters. On the contrary, brain GSH level (Fig. 3C) was obviously declined in diabetic rats and was significantly increased as a result of administration of AA and gliclazide. It is well known that chronic hyperglycemia causes an imbalance in the oxidative status of the nervous tissue which results damaging of brain through a peroxidative mechanism.
Figure 3A: Effect of AA on brain tissue lipid peroxidation in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 3B: Effect of AA on brain tissue hydroperoxides in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 3C: Effect of AA on brain tissue GSH levels in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
This may be because the brain contains relatively high concentrations of easily peroxidizable fatty acids. Vulnerability of brain induced by oxygen free radicals seems to be more due to the fact that the brain utilizes about one-fifth of the total oxygen demand of the body and it is not particularly enriched (Pari and Latha, 2004; Saravanan and Ponmurugan, 2013). Elevated metabolic rate, high lipid content and relative lack of antioxidant enzymes system might be the factors susceptible to oxidative damage of brain when compared to other organs. Thus, oxidative stress is considered the main player of many neurodegenerative diseases (Samarghandian et al., 2014; Uddandrao Sathibabu et al., 2017).
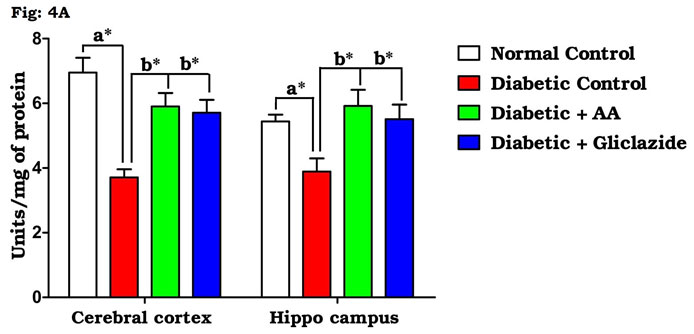
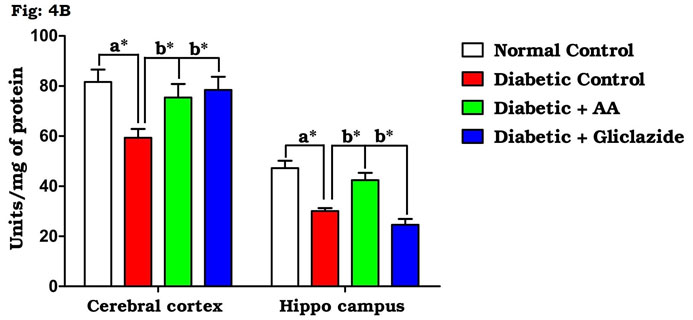
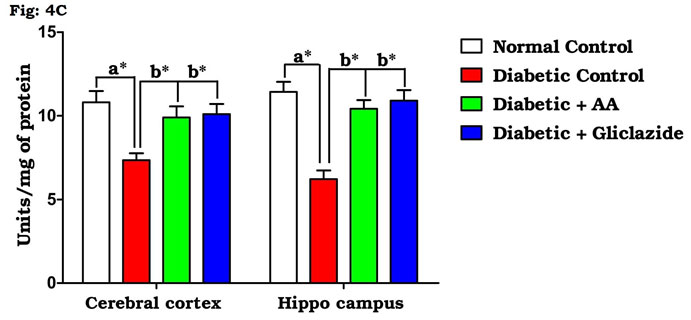
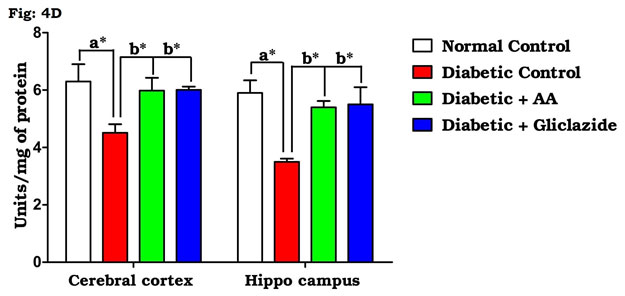
Many studies on both diabetic humans and experimentally induced diabetic rats have revealed that hyperglycemia induces oxidative stress that may disturb brain function (Saravanan and Ponmurugan, 2013). In brain, increased levels of TBARS and hydroperoxides resulted from higher levels of LPO. Increased LPO under diabetic conditions can be due to increased oxidative stress in the cell as a result of depletion of antioxidant scavenger systems (Raza et al., 2015). Figure 4A-D indicates the level of the activities of antioxidant enzymes including SOD (Fig. 4A), CAT (Fig. 4B), GPx (Fig. 4C) and GST (Fig. 4D). There was a significant decrease in the activities of antioxidant enzymes in brain of diabetic rats as compared to control rats.
Figure 4A: Effect of AA on brain tissue SOD activity in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 4B: Effect of AA on brain tissue CAT activity in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 4C: Effect of AA on brain tissue GPx activity in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Figure 4D: Effect of AA on brain tissue GST activity in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
Oral administration of AA and gliclazide potentially improved these altered activities. Based on the above-mentioned data, AA has powerful free radical scavenging and antioxidant activities in the diabetic brain. More specifically, it has been shown that hippocampus of STZ-induced diabetic rats has increased oxidative stress and impaired antioxidant defense systems (Samarghandian et al., 2014). In the present study, a significant increment of TBARS and hydroperoxides and concomitant decrease in the activities of antioxidant enzymes (SOD, CAT, GPx and GST) as well as GSH content were observed in the brain of diabetic rats, which is brought back to normal by AA or gliclazide treatment. This might be due to antioxidant nature of AA that counteract the free radical generation during diabetes (Swapna et al., 2019).
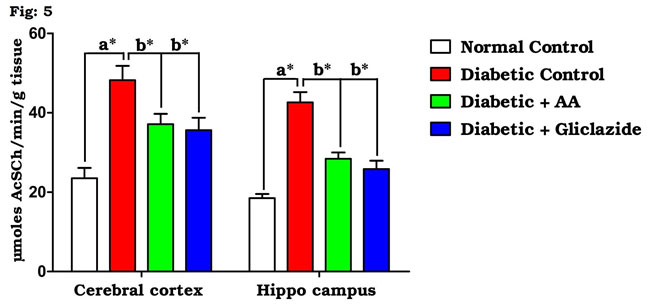
Figure 5: Effect of AA on brain AChE activities in control and experimental animals. Values are expressed as mean±S.D, n=6, aSignificantly different from normal control, bSignificantly different from diabetic control, *P<0.05.
AChE, a significant biological component of the membrane, contributed to its integrity and changed in permeability occurring during synaptic transmission and conduction. Many studies have demonstrated that learning and memory can be modified by drugs affecting the central cholinergic system. Cholinergic transmission is terminated mainly by acetylcholine hydrolysis via the enzyme AchE. One of the hallmarks of this mechanism is impaired cholinergic neurotransmission (Cunnane et al., 2011; Balbaa et al., 2017). The activities of tissue AChE in normal and experimental rats are shown in figure 5. The brain of diabetic rats showed significant increase (P<0.05) in tissue AChE activity. Significant prevention in the activities of tissue AChE was observed in diabetic rats after supplementing AA or gliclazide. Beheshti et al (2016) reported that the metabolism of glucose and impaired insulin signaling in brain is associated with acetylcholine synthesis and the reduced glucose levels in the brain may have a negative effect on cognitive function.
During the diabetic state, the alterations in the lipid membrane could be a key factor in the modification of the conformational state of the AChE molecule and would explain the changes in the activity of this enzyme. It is due to the increased level of free radical formation which promoted increased LPO, having as major consequence oxidative deterioration of the cellular membranes. Thus, free radical scavengers and antioxidants have been shown to prevent neurodegeneration. Reduced GSH has been demonstrated to be an important marker for oxidative stress in the cortex and hippocampus (Pocernich et al., 2012; Salama et al., 2016; Mehta and Banerjee, 2017). In the present study, decreased GSH level in cortico-hippocampal lysates of STZ-NAD animals was observed which ultimately leads to increase in AChE activities in the different brain regions. AA treated experimental animals showed a decrease in AchE and increase in GSH levels, which was comparable to control animals. Earlier, Tuzcu and Baydas (2006) and Kuhad et al., (2008) reported that the antioxidant therapy may decrease the AChE activity induced by the diabetic state. In line with this, AA, an antioxidant may be responsible for preventing cholinergic dysfunction in diabetic rats (Rameshreddy et al., 2018; Swapna et al., 2019).
CONCLUSION
In conclusion, administration of AA rise antioxidant enzymes to a relevant extent in STZ-NAD induced diabetic animals and to retard brain AChE associated with hyperglycaemic development experimentally. From this study, altering the antioxidant enzymes will be a promising new pharmacological approach to treat brain disorders associated with DM. Further investigation into the neuroprotective potential and mechanisms of AA is required to determine, whether it can be an effective cure for cognitive impairment.
ACKNOWLEDGEMENTS
The authors K. Swapna and V. V. Sathibabu Uddandrao are equally contributed to this study. The authors thank Muthyammal College of Arts and Science, Rasipuram, Tamilnadu, India for providing facilities to do animal studies and also express heartfelt thanks to the management of K. S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, Tamilnadu for their support and encouragement.
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
Aebi, H. (1984) Catalase in vitro, Methods in Enzymology, 105, 121-126.
Balbaa, M., Shaymaa, A., and Sofia Khalil, A. (2017) Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs, PLOS ONE, 16, 15-28.
Beheshti, F., Khazaei, M., and Hosseini, M. (2016) Neuropharmacological effects of Nigella sativa. Avicenna Journal of Phytomedicine, 6(1), 104-116.
Brahmanaidu, P., Sathibabu Uddandrao, V.V., Pothani, S., Naik, R.R., Begum, M.S., Varatharaju, C., and Saravanan, G. (2016) Effects of S-Allylcysteine on Biomarkers of the Polyol Pathway in Rats with Type 2 Diabetes. Canadian Journal of Diabetes, 40, 442-448.
Brahmanaidu, P., Sathibabu Uddandrao, V.V., Sasikumar, V., Naik, R.R., Pothani, S., and Saravanan, G. (2017) Reversal of endothelial dysfunction in aorta of streptozotocin–nicotinamide-induced type-2 diabetic rats by S-allylcysteine. Molecular and Cellular Biochemistry, 43, 225-232.
Cunnane, S., Nugent, S., Roy, M., Courchesne-Loyer, A., Croteau, E., and Tremblay, S., (2011) Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition, 27, 3-20.
Ellman, G.L. (1959) Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70-77.
Ellman, G.L., Courtney, D.K., Andres, V., and Featherstone, R.M. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88-95.
Fraga, C.G., Leibouitz, B.E., and Toppel, A.L. (1988) Lipid peroxidation measured as TBARS in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radical Biology and Medicine, 4, 155-61.
Habig, W.H., and Jakoby, W.B. (1981) Assays for differentiation of glutathione S-transferases. Methods in Enzymology, 77, 398-405.
Kakkar, B., Das, P., and Viswanathan N (1984). A modified spectrophotometric assay of SOD. Indian Journal of Biochemistry and Biophysics, 21, 130-132.
Kalidhindi, S., Uddandrao, V.V.S., Sasikumar, V., Raveendran, N., and Ganapathy, S. (2020) Mitigating Perspectives of Asiatic Acid in the Renal Derangements of Streptozotocin-Nicotinamide Induced Diabetic Rats. Cardiovascular & Hematological Agents in Medicinal Chemistry, 18 (1), 37-44.
Kuhad, A., Sethi, R., Kanwaljit, K., and Chopra, I. (2008) Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sciences, 83, 128-134.
Mehta, B.K., and Banerjee, S. (2017) Characterization of Cognitive Impairment in Type 2 Diabetic Rats. Indian Journal of Pharmaceutical Sciences, 79 (5), 785-793.
Moneim, A.A., Ahmed, I., and Sanaa, M. (2017) Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metabolic Brain Disease, 32 (4), 1279-1286.
Paglia, D., and Valentine, W. (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70, 158-169.
Pari, L., and Latha, M. (2004) Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipid peroxidation in STZ diabetic male Wistar rats. BMC Complementary and Alternative Medicine, 4, 16-24.
Pari, L., and Srinivasan, S. (2010) Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomedicine & Pharmacotherapy, 64, 477-481.
Parim, B., Sathibabu Uddandrao, V.V., and Saravanan, G. (2019) Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Failure Reviews, 24 (2), 279-299.
Pocernich, C.B., and Butterfield, D.A. (2012) Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochimica et Biophysica Acta, 1822, 625-30.
Pulido, N., Suarez, A., and Casanova, B. (1997) Gliclazide treatment of streptozotocin diabetic rats restores GLUT4 protein content and basal glucose uptake in skeletal muscle. Metabolism, 46, 10-13.
Rameshreddy, P., Sathibabu Uddandrao, V.V., Brahmanaidu, P., Vadivukkarasi, S., Ramavat, R., Suresh, P., Swapna, K., Kalaivani, A., Parvathi, P., Tamilmani, P., and Saravanan, G. (2018) Obesity-alleviating potential of asiatic acid and its effects on ACC1, UCP2, and CPT1 mRNA expression in high fat diet induced obese Sprague-Dawley rats. Molecular and Cellular Biochemistry, 442 (1-2), 143-154.
Ramkumar, K.M., Latha, M., and Venkateswaran, S. (2004) Modulatory Effect of Gymnema montanum Leaf Extract on Brain Antioxidant Status and Lipid Peroxidation in Diabetic Rats. Journal of Medicinal Food, 7 (3), 366-371.
Raza, H., John, A., and Howarth, F.C. (2015) Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cellular Physiology and Biochemistry, 35 (3), 1241-1251.
Salama, S.M., Gwaram, N.S., AlRashdi, A.S., Khalifa, S.A.M., Abdulla, M.A., Ali, H.M., and El-Seedi, A. (2016) Zinc morpholine complex prevents HCl/ethanol-induced gastric ulcers in a rat model. Scientific Reports, 6, 29646-60.
Samarghandian, S., Azimi-Nezhad, M., and Samini, F. (2014) Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed Research International, 920857, 1-12.
Saravanan, G., and Ponmurugan, P. (2013) Attenuation of streptozotocin-induced alterations in acetylcholinesterase and antioxidant system by S-allylcysteine in rats. Food Bioscience, 4, 31-37.
Saravanan, G., Ponmurugan, P., Senthil Kumar, G.P., and Rajarajan, T. (2009) Antidiabetic properties of S-allyl cysteine, a garlic component on streptozotocin-induced diabetes in rats. Journal of Applied Biomedicine, 7, 151-9.
Sathibabu Uddandrao, V.V., Brahmanaidu, P., and Saravanan, G. (2019) Restorative Potentiality of S-allylcysteine Against Diabetic Nephropathy Through Attenuation of Oxidative Stress and Inflammation in Streptozotocin-Nicotinamide-Induced Diabetic Rats. European Journal of Nutrition, 58 (6), 2425-2437.
Sathibabu Uddandrao, V.V., Brahmanaidu, P., Nivedha, P.R., Vadivukkarasi, S., and Saravanan, G. (2018) Beneficial Role of Some Natural Products to Attenuate the Diabetic Cardiomyopathy through Nrf2 Pathway in Cell Culture and Animal Models. Cardiovascular Toxicology, 18 (3), 199-205.
Swapna, K., Sathibabu Uddandrao, V.V., and Saravanan, G. (2019) Effects of asiatic acid, an active constituent in Centella asiatica (L.): restorative perspectives of streptozotocin-nicotinamide induced changes on lipid profile and lipid metabolic enzymes in diabetic rats. Comparative Clinical Pathology, 28, 1321-1329.
Tuzcu, M., and Baydas, G. (2006) Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. European Journal of Pharmacology, 537, 106-110.
Uddandrao Sathibabu, Brahmanaidu, and Saravanan. (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovascular & Hematological Agents in Medicinal Chemistry, 15, 71-77.
Uddandrao, V.V.S., Parim, B., Ramavat, R., Pothani, S., Vadivukkarasi, S., and Ganapathy, S. (2020) Effect of S-allylcysteine against diabetic nephropathy via inhibition of MEK1/2-ERK1/2-RSK2 signalling pathway in streptozotocin-nicotinamide-induced diabetic rats. Archives of Physiology and Biochemistry, doi: 10.1080/13813455.2020.1811731.
Uddandrao, V.V.S., Rameshreddy, P., Brahmanaidu, P, Ponnusamy, P., and Balakrishnan, S. (2019) Antiobesity efficacy of asiatic acid: down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Archives of Physiology and Biochemistry, 10.1080/13813455.2018.1555668.