Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
Article Publishing History
Received: 12/07/2020
Accepted After Revision: 28/09/2020
Rare actinobacteria have received significant attention which are commonly categorized as strains other than Streptomyces or isolates with less frequency of isolation under normal parameters. These rare actinomycetes may produce important enzymes and some antibiotics. L-glutaminase is an amidohydrolase, produced by a variety of microorganisms including bacteria, yeast and fungi and catalyzed the deamination of L-glutamine to glutamic acid and ammonia. It has extensive applications as an antitumor agent. About 11 actinomycetes isolates were obtained from saline water from the Red Sea Coast on starch nitrate agar containing 10 % NaCl and some antibiotics (25 µg /ml nystatin, 25 µg/ml novobiocin and 25 µg/ml cycloheximide. at 45°C. All the isolates were screened for L- glutaminase production using phenol red as indicator. Out of 11 isolates, 5 showed excellent production and the isolate MM11 was the most active one. According to morphological and physiological characters, it was identified as identified as species of genus Streptomyces. Identification was confirmed using 16SrDNA and the isolate was identified as Streptomyces sp. MM11 and was similar to Streptomyces barkulensis strain RC1831 with 95% similarity level. Thus, it was identified as Streptomyces barkulensis MM11.
Maximal enzyme production was detected in medium containing L-glutamine as carbon and nitrogen sources, respectively at pH 9.0, 40°C and after 7 days. It was clear that addition of yeast extract decreased the enzyme production. The enzyme was collected; partially purified using column chromatography. The molecular weight was determined to be 44 kD. Brine shrimp lethality test was used to predict the cytotoxic effect of the L-glutaminase. The obtained enzyme showed no toxicity and excellent antitumor activities against two cancer cell lines. . In conclusion, using submerged fermentation, L-glutaminase was produced by Streptomyces barkulensis MM 11 using maltose and glutamine as carbon and nitrogen sources and optimizing the growth conditions enhanced the enzyme production which can be used as antitumor agent with no toxicity.
L-glutaminase, antitumor, toxicity, Streptomyces, 16srRNA, screen, isolation, Antibiotic
Aly M. M, Alsiny M. N, AbuRas M. M, Jastaniah S. D. Antitumor L-Glutaminase Production by Rare Actinomycetes Obtained from Marine Water Using Submerged Fermentation. Biosc.Biotech.Res.Comm. 2020;13(3).
Aly M. M, Alsiny M. N, AbuRas M. M, Jastaniah S. D. Antitumor L-Glutaminase Production by Rare Actinomycetes Obtained from Marine Water Using Submerged Fermentation. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2D4wRBe
Copyright © Aly et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Actinomycetes are a heterogeneous group of Gram-positive bacteria with high G+C content in their DNA >55%. They are one of the most diverse groups of filamentous or non-filamentous bacteria, among which some genera produce spores and exhibit powdery growth (Laycock et al., 2013 Takahashi and Nakashima, 2018). The phylum actinobacteria represents one of the largest among the 30 major phyla within domain Bacteria. There are 6 classes, 18 orders, 14 suborders, 63 families and 374 genera were recorded (Subramani and Aalbersberg, 2012). The genus Streptomyces belongs to actinomycetes and contains the largest number, about 600 species (Han et al., 2015). It has an enormous biosynthetic potential that remains unchallenged without a potential competitor among other microbial groups (Solanki et al., 2008).
Rare actinobacteria are commonly categorized as strains other than Streptomyces or actinobacterial strains with less frequency of isolation under normal parameters ((Berdy, 2005, Baltz, 2006). Isolation of actinomycetes for novel compounds from conventional habitats had led to rediscovery of known compounds. However, there is still an urgent need for discovering novel secondary metabolites to combat the problem of arising number of resistant pathogenic bacteria and increasing need of more efficient enzymes in pharmaceutical industries (Rangseekaew and Pathomaree, 2019).
Consequently, the search for novel products has switched to rare genera of actinomycetes from normal habitats or to discovery of strains/species found in unusual or unexplored habitats. Almost every group of organisms isolated from marine environment has unique structures. New and rare actinomycetes have been isolated from the marine environment. Rare actinomycetes may be a source of L-glutaminase (L-glutamine amidohydrolase E.C 3.5.1.2) which is a hydrolytic enzyme that catalyzes the deamination of L-glutamine to glutamic acid and ammonia. L-glutaminase has received significant attention with respect to its extensive applications in pharmaceuticals as an anti-leukemic agent and in food industry as a flavor enhancer (Nakadai and Nasuno, 1989, Nandakumar et al., 2003, Dhevagi et al., 2017 Subramani and Sipkema, 2019).
Another great application of L-glutaminase is in biosensors for monitoring glutamine levels in mammalian (Balagurunathan et al., 2010). L-glutaminases are widely distributed in bacteria, yeast and fungi (Nandakumar et al., 2003). L-glutaminases production have been reported from E. coli), Bacillus subtilis (Dubey et al., 2015), Proteus morganni, Proteus vulgaris, Xanthmonas juglandis, Erwnia carotovora, Erwnia aroideae, Serratia marcescens, Enterobacter coacae, Klebsiella aerogenes and Aerobacter aerogenes (Wade et al., 1971). Also, L-glutaminase synthesis has been reported from Streptomyces rimosus (Keerthi et al., 1999), Streptomyces sp.-SBU1 (Krishnakumar et al., 2011) and Streptomyces avermitilis (Abdallah et al., 2013).
L-glutaminase was isolated from Penicillium crustosum, Emericella nidulans and Mucor circinelloides, grown on the selective medium and the effects of pH, L-glutamine concentrations, temperatures, and incubation periods on glutaminase production was studied. They recorded maximum production at pH 8, temperature 30 ºC, with 0.6% of the L- glutamine. The enzyme was extracted and purified with gel filtration and ion exchange (DEAE Sephadex A50). The molecular weight has 70 kDa and showed cytotoxic activities against two cell lines (LD50: 0.067 – 0.079 mg/ml), (Khalil et al., 2020).
Recently, Masisi et al. (2020) studied the role of glutaminase in cancer, primarily focusing on breast cancer and they address the role played by oncogenes and tumour suppressor genes in regulating glutaminase. They also discussed the current therapeutic approaches to targeting glutaminase. L-glutaminase productions from microbial sources are become urgent need to over produce the enzyme with new and novel character (Prabhu and Chandrasekaran, 1997). The present study reported the production, purification and characterization of extracellular glutaminase enzyme for biotechnological applications from one of the rare actinomycetes.
MATERIAL AND METHODS
Samples collections for rare actinomycete isolation:
Marine water samples were collected from Red Sea Cost in Jeddah city, western region, Saudi Arabia. The collected samples were taken to laboratory in sterile plastic bags and stored at 4◦Cuntil used. From each sample, 0.1 ml was spread on each Petri dish plate containing Starch nitrate medium prepared with 10 % NaCl and containing 25 µg /ml nystatin, 25 µg/ml novobiocin and 25 µg/ml cycloheximide. All plates were incubated at 45°C for 5 days. The colonies which showed powdery growth were selected and transferred to slants of the same medium and preserved at 4°C. For long preservation (more than six months), strains were kept in 20% glycerol and stored at -80°C for further study (Aly et al., 2015).
Screening and selection of L-glutaminase producing isolates:The strains were preliminary tested for L-glutaminase production by streaking on minimal glutamine agar medium (MGA) plates, containing (g/l): KCl, 0.5; MgSO4.7H2O, 0.5; KH2PO4, 1.0; FeSO4.7H2O, 0.1; ZnSO4.7H2O, 1.0; glutamine 5 as the sole carbon and nitrogen source and phenol red 0.012 as a pH indicator. All plates incubated at 45°C for 5 days. Formation of pink zones around the microbial growth indicated the positive reaction (Balagurunathan et al., 2010, Balagurunathan and Subramanian, 1993).
Secondary screening for L-glutaminase production in liquid medium was carried out by inoculating the strains that showed positive result in rapid screening, in medium containing (g/l): L-glutamine, 20; yeast extract, 0.5; K2HPO4, 1.0; KH2PO4, 1.0; MgSO4.7H2O, 0.1; NaCl, 1.0 (Wakayama et al., 2005). After growth, the clear supernatant was used as crude enzyme (Dura et al., 2002). L-glutaminase production was measured according L-glutaminase assay method described by (Imada et al.,1973). One international unit of L-glutaminase was defined as the amount of enzyme that liberates one μMol of ammonia under optimum conditions. The enzyme yield was expressed as Units/ml (U/ml).
Identification of the bacterial isolate: The bacterial isolate that showing the highest L-glutaminase production was identified using morphological, physiological, biochemical and molecular studies. Molecular characterization was determined after extraction of DNA (Kumar et al., 2010). PCR amplification of the 16S rDNA of the Streptomyces sp. was performed using two primers: 9F (5′-GAGTTTGATCCTGGCTCAG- 3′) and 1541R (5′-AAGGAGGTGATCCAACC- 3′) as recommended by Hall et al. (1999).
L-glutaminase assay: Imada et al. (1973) was used for L-glutaminase assay and the mixture was incubated at 37°C for 30 min. The addition of 0.5 ml of 1.5 M trichloroacetic acid was used to stop the reaction. Enzyme activity was determined (U/ml). One unit of L-glutaminase is the amount of the enzyme that produced a μMol of ammonia.
Effect of growth factors on L-glutaminase production: Impact of different parameters, temperature, pH value, and yeast extract and incubation period on L-glutaminase production by Streptomyces barkulensis was investigated (Aly et al., 2017).
Purification of the L-glutaminase: After precipitation with 80% NH4SO4, the enzyme was purified using Sephadex G100 column chromatography and DEAE-Cellulose column chromatography. The active peak was collected, lyophilized and enzyme molecular weight was detected (Aly et al., 2017).
Toxicity and antitumor activity:The brine shrimp lethality test was used to predict the cytotoxic effect of the natural products (Meyer et al., 1982). Varying concentrations of L-glutaminase was added to sea water, containing a counted number of live brine shrimp larvae. Control brine shrimp larvae were incubated in a mixture of sea water. After 24hr., the average number of larvae that survived in each vial was determined. The mean mortality level was plotted against the logarithm of concentrations, the concentration killing fifty percent of the larvae (LC50) was determined (Meyer et al., 1982).
Similarly, the antitumor activity against Ehrlich carcinoma and Lymphoma cell line were determined. The cells were grown in RPMI 1640 medium (Sigma, USA) with 10% fetal calf serum (Gibco, USA) at 37˚C under a humidified atmosphere consisting of 95% air and 5% CO2 for 48 hr. Cells were treated with different doses of the L-glutaminase for 24 hours, centrifuged for 2 min at 1500 g and counted using hemacytometer after staining with trypan blue and removing the supernatant. The percentage of cell viability was assessed to determine the 50 % lethal dose by which 50% of cells are killed (LD50) (Al-Footy et al.,2016).
RESULTS AND DISCUSSION
About 11 actinomycetes isolates were obtained from saline water of the Red Sea Coast in Jeddah city. The isolation medium was starch nitrate agar supplemented with 10% NaCl and 25 µg /ml nystatin, 25 µg/ml novobiocin and 25 µg/ml cycloheximide. The incubation temperature was 45°C. All the isolates were screened for L- glutaminase production using L-glutamine as carbon and nitrogen sources. Phenol red was added to the medium as indicator. Out of 11 isolates, 5 bacterial isolate showed the maximum growth and the diameter of the pink zone was ranged from 22 to 37 mm (Table 1). The most active isolates were grown in liquid medium containing L-glutamine for 5 day. The isolate MM11 was the most active isolate for L-glutaminase production (Figure 1). The selected isolate was grown on different agar media, growth, color and pigment production were recorded (Table 2). Moreover, some physiological characters and resistant to some antibiotics were determined (Table 3). The growth on different carbon and nitrogen sources were reported in Table 4.
Table 1. Source of some bacterial isolates from marine water and their L-Glutaminase production on minimal agar medium (Pink zone diameter/ mm) and in liquid broth medium (u/ml).
| Bacterial isolate | Source | Color of the isolate | Growth on agar medium | L- glutaminase detection | |
| on solid agar (diameter of pink zone, mm) | In liquid broth medium (U/ml) | ||||
| MM1 | Marine water | Blue | +++ | 33±3.31 | 12.10 ±2.19 |
| MM5 | Marine water | Dark gray | +++ | 25±0.94 | 6.68 ±1.39 |
| MM9 | Marine water | Blue | +++ | 24±5.31 | 10.01±1.22 |
| MM11 | Marine water | Yellowish white | +++ | 37±3.39 | 18.14 ±2.04 |
| MM13 | Marine water | Dark gray | +++ | 22±0.39 | 6.13 ±1.34 |
| LSD 6.66 | |||||
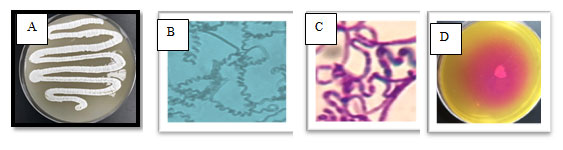
Figure 1: The selected isolate MM 11 on starch nitrate agar containing three antibiotic (A), on slide agar (B), stained with Gram stain (C) and screened for L-glutaminase using phenol red as indicator (D)
Table 2.The selected actinomycetes MM11 on different media after growth for 5 days at 30°C.
| Media used | Growth | Color of aerial mycelium | Color of substrate mycelium | Presence of soluble pigment |
| Starch- nitrate agar | Heavy | Pale yellow | Yellow | No pigment |
| Yeast extract- malt extract agar (ISP-2) | Heavy | White | Yellowish white | No pigment |
| In-organic salts-starch iron agar (ISP-4) | Poor | White | White | No pigment |
| Glycerol asparagine agar (ISP-5) | Heavy | White | White | No pigment |
| Tyrosine agar (ISP-6) | Moderate | White | Yellowish white | No pigment |
| E-Medium (ISP-9) | Poor | White | White | No pigment |
Table 3. Antibiotic susceptibility and physiological and biochemical tests of the selected isolate MM11
| tests | Result | tests | Result |
| Gelatine production | _ | Amikacin | Sensitive |
| Melanin production | _ | Ceftazidime | Resistant |
| Starch hydrolysis | + | Aztreonam | Resistant |
| Catalase | + | Piperacillin | Resistant |
| Oxides | + | Imipenem | Sensitive |
| Indole test | + | Ciprofloxacin | Sensitive |
| Methyl red test | + | Ampicillin | Sensitive |
Table 4.Effect of different carbon and nitrogen sources in ISP-9 medium on growth of the selected isolate MM11
| Carbon source | Utilization | Nitrogen source | Utilization |
| Negative control | ++ | Ammonium sulfate | + |
| Positive control
(Glucose) |
+++ | Ammonium
chloride |
+ |
| Sucrose | ++ | Sodium nitrate | ++ |
| Starch | +++ | Potassium nitrate | + |
| Lactose | +++ | Glycine | ++ |
| Dextrose | – | Peptone | +++ |
| Maltose | + | Yeast extract | +++ |
| Glycerol | + | Vanillin | +++ |
| Xylose | – | Asparagine | +++ |
+++: Good utilization, ++: Moderate utilization, +: Poor utilization, -: No utilization
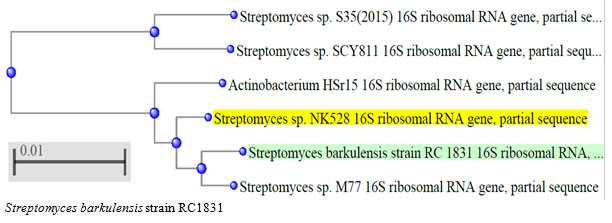
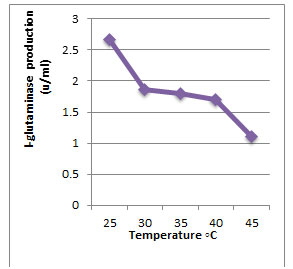
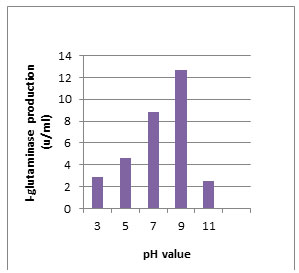
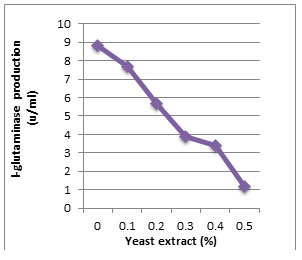
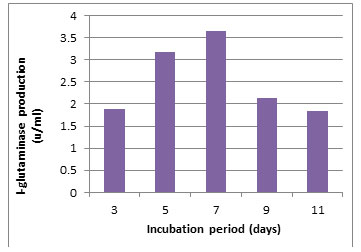
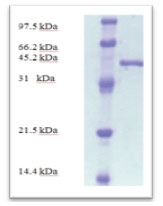
According to morphological and physiological characters, the most active isolate was identified as species belong to genus Streptomyces. Identification was confirmed using 16SrRNA and the isolate was identified as Streptomyces barkulensis (Figure 2). Maximum L-glutaminase production was detected in broth medium contained L- glutamine as carbon and nitrogen sources at 45°C, pH 9.0, no yeast extract addition and after 7 days (Figures 3, 4, 5 and 6). Addition of yeast extracts decreased L-glutaminase production. In broth medium, the selected bacterium was grown in Lab. scale production, the enzyme was collected and purified. After precipitation with 80% NH4SO4 the crude enzyme was partially purified using column chromatography. Molecular weight of the purified L-glutaminase was determined using gel electrophoresis. The molecular weight was of the pyre enzyme was determined to be 44 kDa (Figure 8). Brine shrimp lethality test was used to predict the cytotoxic effect of the L-glutaminase. The obtained enzyme showed no toxicity and excellent antitumor activities against two cancer cell line (Table 5).
Table 5. Toxicity (mg/ml) against Artimia salina and the antitumor activities (LD50, mg/ml) of l-glutaminase
| Antitumor activity (LD50) | Toxicity against Artimia salina (LC50) | Tested
Product |
|
| Erlish cell line | Lymphoma cell line | ||
| ≥77.0* | ≥77.0* | ≥77.0* | Filtrate |
| 7.5 | 5.0 | ≥55.3* | L-glutamimase |
| ≥ 3.6 | 3.6 | ≥ 3.0 | Control (cis platin) |
* : significant results compared to control
Figure 2: The phylogenetic tree of the isolate MM11 and the most related genera
Figure 3: Effect of the temperature on production of L-glutaminase in broth medium by the isolate MM11
Figure 4: Effect of the pH value on production of L-glutaminase in broth medium by the isolate MM11
Figure 5: Effect of the yeast extract addition on production of L-glutaminase in broth medium by the isolate MM11
Figure 6: Effect of the incubation period on the production of L-glutaminase in broth medium by the isolate MM11
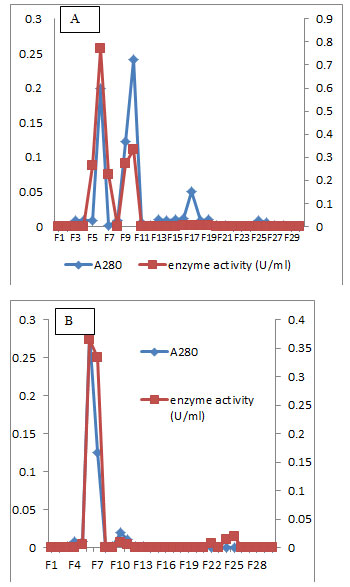
Figure 7: Elution profile of L-glutaminase of the isolate MM11 after sephadex G-100 column chromatography (A) and DEAE-Cellulose column chromatography (B).
The chance of isolating new actinomycete strains with novel character is difficult and need special methods to be isolated. Addition of NaCl to the isolation medium enhanced salt tolerant actinomycetes. Similarly, addition of antibiotics to the growth medium enhanced rare actinomycete isolation. Selective isolation of actinomycetes for L-glutaminase production is of great interest for preparing new antitumor agents. Out of 11 actinomycetes isolates, 5 isolates (45.5%) were highly producer of L-glutaminase and the most active isolate was Streptomyces barkulensis Screening was performed based on the activity of glutaminase (33 u/ml). Similarly, Out of 102 actinomycete isolates, only 6 Streptomyces isolates recorded L-glutaminase activities (Abdallah et al., 2012).
Figure 8: SDS-PAGE analysis of the purified enzymes after DEAE-cellulose column chromatography
Detection of L-glutaminase in this study was recorded by diameter of the pink zone (mm) and the same method was used (Abdallah, et al., 2012). Similarly, L-glutaminase was obtained by Streptomyces avermitilis (Omura et al., 2001) and Streptomyces labedae (Han et al., 2012). Effect of various physicochemical factors on L- glutaminase production by Streptomyces species was detected. It was found that under the best growth conditions, rapid L- glutaminase production was found (Tobin et al., 2001). On the other hand, there is a dire need for the discovery of new drugs to effectively target the life-threatening diseases like cancers. The application of enzymes in diverse biotechnological industries tends to the discovery of novel enzymes.
Members of the class actinobacteria especially Streptomyces spp. have long been recognized as prolific sources of useful bioactive enzyme like L-glutaminase (Nathiya et al., 2011). Therefore, current actinomycetes isolation programs are reoriented toward largely unexplored and extreme environments. The present study has been focused on the isolation and identification of different actinomycetes from extreme habitats in Saudi Arabia. Marine environments were studied in order to unravel the diversity of actinomycetes and determine their potential as a resource for biotechnological applications. The findings obtained from this study revealed that the most active isolate was of genus Streptomyces (Nonomura, 1974).
L-Glutaminase, an amidohydrolase enzyme has been a choice of interest in the treatment of lymphoblastic leukaemia. The isolate was identified as Streptomyces sp. Effect of physicochemical factors namely temperature, pH, yeast extract concentration, and incubation period on the production of L-glutaminase from the Streptomyces was carried out. The enzyme production was found to be optimum in medium containing L-glutamine as carbon and nitrogen source at pH 9, temperature 45◦C The L-glutaminase produced from Streptomyces sp. was purified by ammonium sulphate precipitation, dialysis method and ion exchange chromatography. After the purification of the enzyme by ion exchange chromatography, it has 44 kDa. Similar results were obtained by (Okami (1986), Ōmura et al., 2001).
CONCLUSION
Rare actinomycetes were found in extreme environments and need special method to be isolated. Streptomyces barkulensis isolated from marine water in medium containing three different antibiotics. It was characterized and identified. The previous isolate showed excellent activity of L- glutaminase production. Optimization of growth conditions enhanced the production process. Using Lab. scale production the enzyme was purified and characterized. It showed no toxicity and moderate antitumor activity. Thus, it can be developed for medical uses.
ACKNOWLEDGEMENTS
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G-415-247-37). The authors therefore acknowledge with thanks DSR for technical and financial support.
REFERENCES
Abdallah, N.A., Amer, S.K., Habeeb, M.K. (2012). Screening of LGlutaminase produced by actinomycetes isolated from different soils in Egypt. International Journal of ChemTech Research 4: 1451-1460.
Abdallah, N.A., Amer, S.K., Habeeb, M.K. (2013). Production, purification and characterization of L-glutaminase enzyme from Streptomyces avermitilis. African Journal of Microbiology Research 7: 1184-1190.
Al-Footy, K. O., Alarif ,W. M., Zubair S., Ghandourah M. A., and Aly, M. M. (2016). Antibacterial and cytotoxic properties of isoprenoids from the red sea soft coral, Lobophytum sp. Tropical Journal of Pharmaceutical Research; 15 (7): 1431-1438
Aly, M. M., Tork, S., Al-Garni, S. and Kabli, S. (2015). Production and characterization of phytase enzyme from Streptomyces luteogrises R10isolated from decaying wood sample. IJ of Agriculture and Biology. Vol. 17(3):515–522.
Aly, M., Kadi., R. H., Aldahlawi, A. M., Alkhatib M H, Wali A N (2017). Production Of The Antitumor L-Glutaminase Enzyme From Thermotolerant Streptomyces sp. D214, Under Submerged Fermentation Conditions. Journal of Experimental Biology and Agricultural Sciences, 5(6).878-885.
Balagurunathan R and Subramanian A (1993). Studies On marine Streptomyces nigrifaciens (P-9). I. Taxonomy and standardization of antibiotic production. Ciencias Marinas19: 435-443.
Balagurunathan R, Radhakrishnan M, Somasundaram ST (2010) L-glutaminase producing actinomycetesfrom marine sediments– selective isolation, semi quantitative assay and characterization of potential strain. Australian Journal of Basic and Applied Sciences 4: 698-705.
Baltz, R.H. (2006). Marcel Faber Roundtable: is our antibiotic pipeline unproductive because of starvation, constipation or lack of inspiration?.Ind Microbiol Biotechnol, 33:507-513.
Berdy, J. (2005). Bioactive microbial metabolites. Antibiot, 58:1-26; doi: 10.1038/ja.2005.1.
Dhevagi, P., Brundha, A., Geetha, K., Gobu, R., Manju, K.A.A. and Poorani, E. (2017). A preliminary study on the antimicrobial activity of marine actinomycetes. Environ Biol, 38:483-88.
Dubey R, Paul A, Prity N (2015) Isolation, production and screening of anti-cancer enzyme L-glutaminase from Bacillus subtilis. International Journal of Pharma and Bio Sciences 5: 96- 105.
Dura MA, Flores M, Toldrá F (2002). Purification and characterization of a glutaminase from Debaryomyces spp. International journal of Food Microbiology 76: 117-126.
Hall V, O’Neill GL, Magee JT, Duerden BL (1999) Development of amplified 16S ribosomal DNA restriction analysis for identification of Actinomyces species and comparison with pyrolysis-mass spectrometry and conventional biochemical tests. Journal of Clinical Microbiology 37: 2255-2261.
Han J., Cho M. and Kim S., 2012. Ribosomal and protein coding gene based multigene phylogeny on the family Streptomycetaceae. Sys. Appl. Microbiol., 35, 1-6.
Han, X., Zheng, J., Xin, D., Xin, Y., Wei, X. and Zhang, J. (2015). Streptomyces albiflavescens sp. nov., an actinomycete isolated from soil. Int J Syst Evol Microbiol, 65:1467-1473.
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of microrganisms. Journal of General Microbiology 76: 85-99.
Keerthi TR, Suresh, PV, Sabu A, Rajeevkumar S, Chandrasekaran M (1999) Extracellular production of L-glutaminase by alkalophilic Beauveria bassiana BTMF S10 isolated from marine sediment. World Journal of microbiology and Biotechnology 15: 751-752.
Khalil M S., Moubasher M.H., El-Zawahry M. M, Michel M M. (2020). Evaluation of antitumor activity of fungal L-glutaminase produced by Egyptian isolates. Letters in Applied NanoBioScience, Volume 9, Issue 1, 2020, 924 – 930.
Krishnakumar S, Rajan RA, Ravikumar S (2011) Extracellular production of L-Glutaminase by marine alkalophilic Streptomyces sp.-SBU1 isolated from Cape Comorin coast, Indian Journal of Geo-Marine Sciences 40: 717-721.
Kumar V, Bharti A, Gusain O, Bisht GS (2010) An improved method for isolation of genomic DNA from filamentous actinomycetes. Journal of Engineering and Technology Management 2: 10-13.
Laycock, B., Halley, P., Pratt, S., Werker, A. and Lant, P. (2013). The chemomechanical properties of microbial polyhydroxyalkanoates. Prog Polym Sci, 38:536-583.
Masisi, B K , El Ansari R., Alfarsi L., Rakha E A, Green, A. R. Craze M. L. (2020). The role of glutaminase in cancer. Histopathology, Volume 76, Issue 4, Pages 498-508.
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols, DE, Mclaughlan JL (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45: 31-34.
Nakadai T and Nasuno S (1989) Use of glutaminase for soy sauce made by Koji or a preparation of proteases from Aspergillus oryzae. Journal of Fermentation and Bioengineering 67: 158-162.
Nandakumar R, Yoshimune K, Wakayama M, Moriguchi M (2003). Microbial glutaminase: biochemistry, molecular approaches and applications in the food industry. Journal of Molecular Catalysis B: Enzymatic 23:87-100.
Nathiya K, Nath SS, Angayakanni J, Palaniswamy M (2011). Optimised production of L-glutaminase: A tumor inhibitor from Aspergillus flavus cultures on agroindustrial residues. African Journal of Biotechnology 10: 13887- 13894.
Nonomura H (1974) Key for classification and identification of 458 species of the streptomycetes included in ISP. Journal of Fermentation Technology 52: 78- 92.
Okami Y (1986). Marine microorganisms as a source of bioactive agents. Microbiology Ecology 12: 65-78.
Omura, S., Ikeda, H., Ishikawa, J., Hanamoto, A., Takahashi, C., Shinose M., Takahashi Y., Horikawa H., Nakazawa H., Osonoe T., Kikuchi H., Shiba T., Sakaki Y. and Hattori M. (2001) Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA, , 98, 1221:12220.
Prabhu, G.N. and Chandrasekaran, M. (1997). Impact of process parameters on L-glutaminase production by marine Vibrio costicola in solid state fermentation using polystyrene as an inert support. Process Biochemistry 32: 285-289.
Rangseekaew, P. and Pathom-aree, W. (2019). Cave actinobacteria as producers of bioactive metabolites. Front Microbiol, 10:387; doi:10.3389/fmicb.2019.00387.
Solanki, R., Khanna, M. and Lal, R. (2008). Bioactive compounds from marine actinomycetes. Indian J Microbiol, 48:410-431.
Subramani, R. and Aalbersberg, W. (2012). Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res, 167: 571-580; 10.1016/j.micres.2012.06.005.
Subramani, R. and Sipkema, D. (2019). Marine rare actinomycetes: a promising source of structurally diverse and unique novel natural products. Mar Drugs, 17:249.
Takahashi, Y. and Nakashima, T. (2018). Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiot, 7:45.
Tobin P.R., Olosimbo O.A., Anu K., Maria J.O., Jozsef B. and Bernard M. M., (2001). The effect of inoculum size on the lag phase of Listeria monocytogenes. Int. J. Food Microbiol., 2001, 70,163-173
Wade HE, Robinson HK, Phillips BW (1971) Asparaginase and glutaminase activities of bacteria. Journal of General Microbiology 69:299-231.
Wakayama M, Yamagata T, Kamemura A, Bootim N, Yano S, Tachiki T, Yoshimune K, Moriguchi M (2005). Characterization of salt-tolerant glutaminase from Stenotrophomonas maltophilia NYW-81 and its application in Japanese soy sauce fermentation. Journal of Industrial Microbiology and Biotechnology 32: 383–390.