1Post Graduate and Research Department of Biotechnology, Mohamed Sathak
College of Arts and Science, Sholinganallur, Chennai, India.
2Post Graduate and Research Department of Biochemistry, Mohamed Sathak
College of Arts and Science, Sholinganallur, Chennai, India.
Corresponding author email: syedmicro555@gmail.com
Article Publishing History
Received: 11/06/2021
Accepted After Revision: 18/09/2021
The marine source variety allows the selection of polysaccharides isolated from seaweeds with specific characteristics that are completely absent in polysaccharides from terrestrial plants. Algal polysaccharides and their structural diversity constitute a source of several biological capacities that may represent an interesting tool for novel therapeutic benefits and industrial applications, including nutraceuticals, pharmaceuticals, and functional foods. Currently, sulfated polysaccharides are found principally as recipients in feed, food and pharmaceutical formulations, but the discovery of surprising biological capacities makes these polymers a very exciting research field. For a vision towards the future, the use of algal polysaccharides in medicine is expected to considerably progress.
Padina is a widely available brown alga in the marine coastal region and gained great attention of researchers all over the world. Padina can be used as food, fodder, plant growth promoter and bio-fertilizer. The brown alga is well known and is utilized for its various pharmacological properties like antimicrobial, insecticidal, antioxidants, antibiotics, anti-inflammatory, hypo-allergenic, hepatoprotective and antidiabetic activities. However, the current work aimed to evaluate in vitro antioxidant activity of L-Fucose- potent polysaccharide isolated from Padina gymnospora. L-Fucose was extracted with ethanol and acetone from brown algae Padina gymnospora, followed by isolation and purification process.
The polysaccharide composition was assessed using high-performance liquid chromatography. Free radical scavenging activity of purified L-Fucose was evaluated using different in vitro systems such as DPPH radical scavenging assay, Hydrogen peroxide, Nitric oxide, Ferric reducing antioxidant power, Deoxyribose Radical scavenging assay, ABTS Radical cation scavenging assay, Superoxide radical scavenging assay, Superoxide Dismutase scavenging assay. Based on the results obtained, we conclude that L-Fucose isolated from Padina gymnospora have potential radical scavenging activity.
Antioxidant Activity, Dpph, L-Fucose, Lipid Peroxidase (Lpo), Radical Scavenging Activity
Swaminathan R, Ali M.S, Anuradha V, Abinaya R, Ananthalakshmi J.S, Yogananth N. Antioxidant Potential of Fucose Isolated from the Marine Macroalgae Padina gymnospora. Biosc.Biotech.Res.Comm. 2021;14(3).
Swaminathan R, Ali M.S, Anuradha V, Abinaya R, Ananthalakshmi J.S, Yogananth N. Antioxidant Potential of Fucose Isolated from the Marine Macroalgae Padina gymnospora. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3lIbCJy“>https://bit.ly/3lIbCJy</a>
Copyright © Swaminathan et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Brown seaweed (Phaeophyceae) is the largest and most complex type of algae, having brown, olive or yellowish–brown in color. There are about 1800 species of brown seaweed, broadly distributed from tropical to polar zones of ocean in the world (Harris et al. 2011). Marine plants have long been recognized as producers of biologically active substances. Potential activities of some marine plants like mangroves, seaweeds, sea grasses and lichens have been reported from India. Marine secondary metabolites are secretory products of marine microbial species, sponges, seaweeds, and another marine biota. Due to increasing demand shown as an output of the research towards search of therapeutic molecules from natural sources, greater interest is growing on marine organisms especially seaweeds (Maheswaran et al. 2013; Kumar et al. 2021).
Seaweeds are extraordinary sustainable resources found within the marine ecosystem which have been explored as a source of food and feed and about 50% of the global photosynthesis is being contributed through marine algae (Neelam 2005). Seaweeds have been used in traditional medicine for many centuries, and are of potential interest for the pharmaceutical and food industries (Guaratini et al. 2012). Seaweed is a preferable bioactive compound as it comprises stable antioxidants as compared to terrestrial plants and helps in preventing oxidative stress and other mammalian diseases (Kumar et al. 2021).
Seaweeds or marine macroalgae are primitive non-flowering plants with absence of true root, stem and leaves in their structural system. There are a lot of reports proving on antibacterial activity of solvent extracts from different marine algae. Several bioactive compounds from the seaweeds have shown pharmacological properties, primarily for treating deadly diseases like tumor, Acquired Immuno Deficiency Syndrome (AIDS), rheumatic arthiritis etc (Guaratini et al. 2012; Kumar et al. 2021).
Brown algae contains a broad spectrum of acid polysaccharides which constitutes alginic acids, comprising of uronic acid; the homo fucans, consisting of sulfated fucan and the heterofucans, that contain portions of mixed neutral sugars and uronic acids in addition to sulfated fucose. In all these polysaccharides, difference in branched structures, a varied distribution of sulfate and occasionally acetyl groups may be observed (Castro et al. 2016). Free radicals are highly reactive molecules with an unpaired electron that are produced by radiations or as by-products of metabolic products of metabolic processes.
The free radicals in excited state initiate chain reaction within biological system which lead to disintegration of cell membrane and cell compound, including lipids, protein and nucleic acids. Antioxidants are defensive compounds released by the cell during such oxidative stress to scavenge free radicals such as peroxide, hydro peroxide or lipid peroxyl and decreases the levels of oxidative stress and prevent the development of complication associated with oxidative stress related disease. Thus, naturally occurring antioxidants are non-toxic without any deleterious side effects when compared to the chemically synthesised (Wu et al. 2008; Castro et al. 2016; (Kumar et al. 2021).
Sulfated polysaccharides (SP) of marine resources are the anionic polymers that occurs abundantly in most of the macroalgal community. Fucoidan represents the SPs exclusively of marine brown algae whereas agar and carrageenan are present mainly in those SPs that occur in the red algae. Green algae have a heterogeneous class of SPs with different sugar residues such as glucuronoxylorhamnans, glucuronoxylor rhamnogalactans, and xyloarabinogalactans (Na et al. 2010; Costa et al. 2010).
Fucoidan refers to structural polysaccharide composed mainly on sulfated L-fucose, and consists of less than 10% of othercontributing monosaccharides. The term sulfated fucan can be used to define heterofucans containing sulfated fucose and neutral sugars. However, fucans and fucoidans are often interchangeably used to describe the SPs of seaweeds. Fucoidans are extracted from brown seaweeds such as Ecklonia cava, Saccharina longicruris, Fucus vesiculosus, Ascophyllum nodusum, and Undaria pinnatifida (Ramli et al. 2020; Kumar et al. 2021).
Fucoidan is a sulphated polysaccharide containing important biological activities due to having a different amount of sulphate group in its chemical structure. Fucoidans are a series of sulphated polysaccharides that occurs widely in the cell walls of brown macroalgae. Fucoidans are reported to exhibit diverse physiological and biologically therapeutic properties. In addition, the pharmacological potential of fucoidans tends to increase with their degree of sulfation and they can be easily extracted from the source using either by percolation in hot water or an acid solution. These polymers generally occur in the intercellular tissues or mucilaginous matrix of brown algae. However, the structure of algal fucans varies among species and sometimes also among different parts of the seaweed of same species (Rocha et al. 2005; Kumar et al. 2021).
Thus, each purified sulfated L-fucose is a unique novel compound and thus can be explored as a potential lead compound or a prodrug. Many research has proved the anti-inflammatory activity of a fucoidan from the alga Fucus vesiculosus, called sulphated fucan. During inflammatory response, fucoidans are potent inhibitor of migration of leucocytesto the site of inflammation, which is contributed by its interaction with P and L-selectin (Zang et al. 2001; Klintman et al. 2002; Cardoso et al. 2010). Sulfated fucans from the Fucales and Laminariales orders has been reported to prevent recruitment of leucocytes in an inflammation model studied in rats (Cardoso et al. 2010; Paiva et al. 2011; Kumar et al. 2021). However, the present study was an attempt to determine the antioxidant potential of the L-Fucose purified from Padina gymnospora.
MATERIAL AND METHODS
Padina gymnospora was collected from coastal water bodies in Rameshwaram. These algae were washed using tap water, dried under sunlight and then dried in an oven at 60 °C. Finally, they were crushed into a fine powder and stored in a 4 °C refrigerator for further analysis. The method for extraction of fucoidan from brown macroalgae as described in the past studies, were followed with some modification (Yang et al. 2008; Rodriguez-Jasso et al. 2011; Foley et al. 2011).
Ten grams of algal powder was mixed with 100 mL of 85% ethanol and incubated in shaker for around 12 h at room temperature to remove lipids and pigments. The solutions were then subjected to centrifugation for 10mins at 3273g to remove the supernatant. The remaining sediment was repeatedly washed with acetone to remove any contaminants and left to dry at room temperature overnight. Five grams of the sediment of was extracted with 200 mL of deionized water for 1 h upon hot plate at 65 °C and stirred occasionally. The mixture was again subjected to centrifugation at 3273 × g for 10 min.
1% CaCl2 was added to the supernatant to precipitate the alginate and the solution was subsequently added to 95% ethanol to obtain a final ethanol concentration of 30% (v/v). Finally, the fucoidan was recovered after centrifuging at 3273 × g for 10 min. The fucoidan extract obtained was lyophilised and stored at 4°C. The commercial Fucoidan from Padina gymnospora (Sigma, USA) were used as a reference to check the purity of the experimentally recovered fucoidan. 200 mg of extracted fucoidan was separately dissolved in 20 ml of distilled water and heated at reflux with 0.75 ml of 3.0M HCl for 3 h. After cooling, the mixture was centrifuged at 3000 rpm and 1.0 M NaOH was added to neutralise the supernatant solution and poured over 100 ml of ethanol. Then the precipitate was redissolved in distilled water and freeze dried.
The polysaccharide content was assessed using an HPLC system comprising a pump, injection valve with a 20-μL sample loop, PL Hi-Plex H column and refractive index detector. 10 mg of the sample was treated with 2 mL of 2 M trifluoroacetic acid at 121°Cfor 1 h. After trifluoroacetic acid hydrolysis, the reaction medium was dried with a vacuum concentrator, and distilled water was added to redissolve the sample. The resultant mixture was neutralized to approximately pH 7 by using 1N NaOH. One milligram per millilitre of polysaccharide sample was injected into the HPLC system. The column was kept in a 65°C column oven (COLBOX), and distilled water was used as the mobile phase at a flow rate of 0.6 mL/min.
The data were analyzed using the software Chromera Perkin Elmer system. The antioxidant activity such as DPPH radical scavenging assay, Hydrogen peroxide scavenging activity, Nitric oxide scavenging activity, Ferric reducing antioxidant Power (FRAP), Deoxyribose Radical Scavenging Activity, ABTS [2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonicacid)] Radical Cation Scavenging Assay, Superoxide radical scavenging activity (SO), Superoxide Dismutase Scavenging Assay (SOD) (Suganya et al. 2017).
The statistical analyses for all the experiments were done using Excel 2013 through statistical formula. Experimental data were expressed as mean ± SD and IC 50 values were calculated. The experiment was performed in triplicates for all the test samples (Suganya et al. 2017).
RESULTS AND DISCUSSION
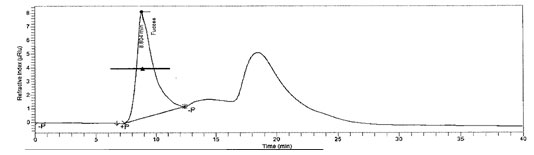
Chemical composition of L-Fucose result: Collected algae was first cleaned with filtered seawater to remove contamination and then dipped in tap water to remove salt. The fucose content of sample was found to be12% and HPLC chromatogram of L-Fucose standard and L-Fucose extracted from was depicted in Figure 1 and figure 2 respectively. In recent years, a broad series of polysaccharides from edible seaweeds have emerged as an important class of bioactive natural products, possessing many important properties of pharmacological relevance.
Fucose, sulfate, and L-fucose can be used to represent the quality of the fucoidan. Cho et al. (2010) reported that the bioactivity of fucoidan was positively correlated with sulfate content (Yangthong et al. 2009; Cho et al. 2010). Fucoidan is a sulphated polysaccharide containing important biological activities due to having a different amount of sulphate group in its chemical structure. It has anticoagulant, immunomodulation, anticancer, antiviral, anticomplement, antithrombotic, and antiproliferative activity (Hentai et al. 2019).
Figure 1: Standard L-Fucose
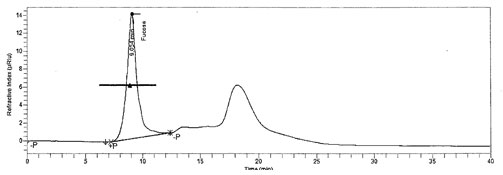
Figure 2: L-Fucose in Padina gymnospora
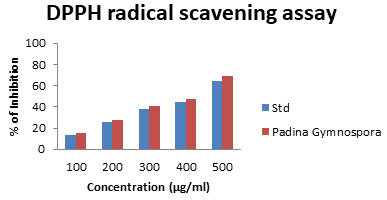
DPPH radical scavenging assay: DPPH is extensively utilized stable free radical mediator used to evaluate the radical scavenging efficacy of plant extracts. In the presence of hydrogen donating antioxidant, stable DPPH radical is converted into a non-radical component (DPPH-H), due to this reaction that the colour of the DPPH solution is converted from purple to yellow. In this assay, all the tested polysaccharide samples showed high DPPH radical scavenging capacities.
The present study indicates DPPH scavenging activity for L-Fucose in Padina gymnospora as 15.70 ± 1.012 to69.31±2.08 (Fig. 3), which is high level that of L-Fucose in the present study. DPPH is a compound that possesses a nitrogen free radial and is readily destroyed by a free radical scavenger. This assay was carried out to assess the anti-oxidative potential of compounds functioning as proton radical scavengers or hydrogen donors in an in vitro system (Singh et al. 2004). Moreover, Kumar et al. (2021) reported that, DPPH is a stable free radical appears in purple color in methanol/ethanol turns colorless by reduction in the presence of hydrogen donating antioxidants (Kumar et al. 2021).
Figure 3: DPPH radical scavenging assay
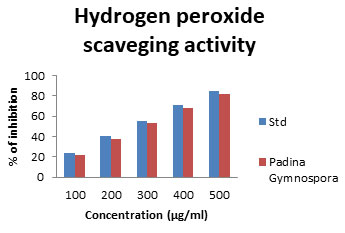
Hydrogen peroxide scavenging activity: Hydrogen peroxide itself being very unreactive, becomes toxic to cells as a consequence of increase in concentration of hydroxyl radicals in the cells (Halliwell 1991). Hence, compounds with good hydrogen peroxide scavenging ability are considered physiologically important. The commercial fucoidan sample exhibited the most potent hydrogen peroxide scavenging activity in contrast the other polysaccharide samples showed 22.26-81.71% inhibition Fig 4. The measurement of H2O2 scavenging activity is one amongst the potent methods for determining the ability of antioxidants to decrease the level of pro-oxidants such as H2O2 (Czochra et al. 2002). However, it can cross biomembranes and can slowly oxidize a number of reactive compounds leading to cell death (Kumar et al. 2021).
Figure 4: Hydrogen Peroxide scavenging assay
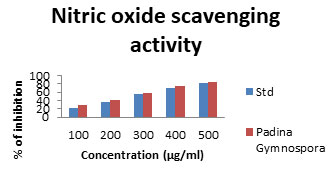
Nitric oxide scavenging assay: Nitric oxide radical is an important signalling molecule in human body; however, the accumulation of this radical creates adverse side effects. Therefore, compounds with high nitric oxide radical scavenging activities are important, but fewer compounds have been documented for their NO scavenging ability (Kim et al. 2007; Kumar et al. 2021). The nitric oxide scavenging assay was performed with L-Fucose samples along with the standard. Based on percentage of inhibition and different concentration ranges 100 – 500 μg/ml the result was given in Figure5.The inhibition was founded in L-Fucose with percentage of 28.71% to 84.69%. The low inhibition was recorded in standard (23% to 82.45%). All the test samples possess higher percentage of inhibition when compared with standard ascorbic acid which produced (Kumar et al. 2021).
Figure 5: Nitric Oxide scavenging assay
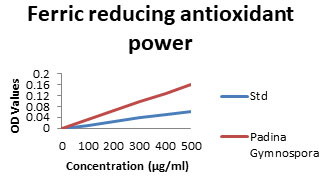
Ferric reducing antioxidant Power (FRAP): In ferric reducing Antioxidant power (FRAP), the antioxidant activity was determined based on the ability of the components in the samples to reduce Ferric (III) to Ferrous in a redox linked colorimetric reaction that involves single electron transfer. The L-Fucose which is usually present in brown seaweeds is potent antioxidant. In the present study results showed increased level (26.48-88.67%) which is shown in Fig 6. Oxygen derived free radicals or reactive oxygen species (ROS) formed in the within biological system during energy producing metabolic process, plays an important role in pathophysiology of a number of diseases (Cuzzocrea et al. 2001; Li et al. 2006; Bhuyar et al. 2021).
Figure 6: FRAP scavenging assay
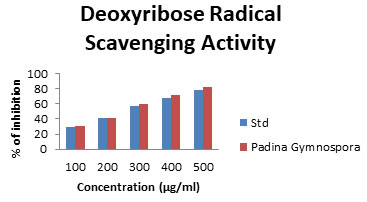
Deoxyribose Radical Scavenging Activity: Oxidative stress induced by the free radicals has been gained a vital importance as it forms the root cause of about 200 human diseases. Free radicals are highly reactive molecules with unpaired electrons and are generated during various cellular processes. They represent an essential part of metabolism and aerobic life. Many of the reactive oxygen species (ROS) and reactive nitrogen species (RNS) are grouped under free radicals. The Deoxyribose radical assay was expressed as percentage activity of ascorbic acid control at 100 to 500 µg/mL and the data are shown in Fig.7. The polysaccharides are arranged from the highest activity, which ranged from 30.78 to 81.94% (Hybertson et al. 2011; Finosh et al.2013; Bhuyar et al. 2021).
Figure 7: Deoxyribose scavenging assay
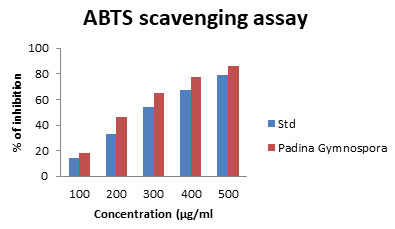
ABTS Scavenging Activity: The ABTS radical reactions involves the transfer of electrons and the process take place faster rate when compared with that of DPPH radicals. In the present study the ABTS radical scavenging activity from Padina gymnospora (18.56 to 86.19) was given in fig 8. There are numerous reports in the literature on the antioxidant capacity of algae. Bhuyar et al. (2021) reported that, antioxidant potential in which the Padina gymnospora showed 15.56 to be the best antioxidants and scavenging among all the polysaccharides studied. The hydroalcoholic and aqueous extracts of many seaweeds have been studied and reported for antioxidant activity by their inhibitory activity on lipoxygenase activity, DPPH radical and deoxyribose assays (Jimenez-Excriget al. 2001; Lekameera et al. 2007; Lekameera et al. 2008; Bhuyar et al. 2021).
Recently, several marine alginate derivatives like those of sulphated fucoidans from Laminaria japonica the brown seaweed, agar-like sulfated galactans from Nori, the red seaweed and sulphated polysaccharides from Fucus vesiculosus, have been reported to possess antioxidant activity. This property is contributed by the presence of reductones that are reported to be terminators of free radical chain reaction (Duh 1998; Xue et al. 2001; Ruperez et al. 2002; Duan et al. 2006; Bhuyar et al. 2021).
Figure 8: ABTS scavenging assay
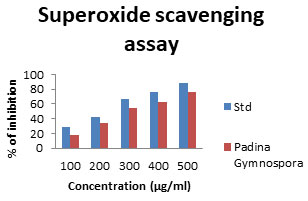
Superoxide radical scavenging activity (SO): Superoxide radicals are a highly toxic species generated by many biological and photochemical reactions. Although, the superoxide radical was a weak oxidant in most organisms, it could produce hydrogen peroxide and hydroxyl radicals through dismutation and other reactions and is the major source of free radicals formed in vivo. Moreover, superoxide radical and its derivatives are cell-damaging through causing damage to the DNA and membrane of the cell (MacDonald et al. 2003; Yuan et al. 2005). The SO antioxidant assay was found to be 18.33±75.92 %at 100μg/ml which gradually increases with increase in concentration (Fig. 9). The reducing properties are generally associated with the presence of reductions. The relation between polysaccharide structure and function was also analysed (Yuan et al. 2005; Bhuyar et al. 2021).
Figure 9: Superoxide scavenging assay
Superoxide Dismutase radical scavenging activity (SOD): The radical-scavenging activity of L-Fucose extracted from Padina gymnospora varied in a range from 18.50 to 84.64 % on SOD radicals at a concentration of 100 to 500 mg/ml. Antioxidants can grant protection from oxidative damages and prevent the onset of many chronic diseases. They are naturally present in our body (endogenous) and the additional supplementation can be done through the diet (exogenous). Natural antioxidants like ascorbic acid (vitamin C), α-tocopherol, and carotenoids are readily absorbed through diet (Padayattyet al. 2003; Bhaskar 2013). Butyl hydroxyanisole (BHA) and butyl hydroxytoluene (BHT) are commercial synthetic antioxidants which are proven to cause major side effects leading to cancer. Hence natural antioxidants always gained importance as safe, cost effective food supplements (Bhuyar et al. 2021).
CONCLUSION
he finding of the present study states that, the total sulfated polysaccharide in Padina gymnospora showed a potential free radical scavenging ability responsible for the antioxidant activity. The results showed an equally good radical scavenging activity when compared to the standard. Several Preparative approach and analytical techniques in isolation and purification of the compound may be thus helpful in obtaining sufficient quality of the bioactive principle and determination of its radical inhibiting capacity along with its median inhibitory concentration will be helpful to assess the drug potentiality of compounds from the natural source. Further studies are required to assess antioxidant based therapeutic potential of Padina gymnospora.
Financial support and sponsorship: There was no outside financial support and sponsorship.
Conflicts of interests: Authors declare no conflicts of interests to disclose.
REFERENCES
Arunkumar K, Raja R, Kumar V B S, et al. (2021). Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. Journal of Food measurement and Characterization, Vol 15 Pages 567-576.
Bhaskar A N (2011) Phytochemical screening and in vitro antioxidant activities of the ethanolic extract of Hibiscus rosasinensis L. V.G3. Annals of Biological Research, Vol 2 No 5 Pages 653-661.
Bhuyar P, Sundararaju S, Rahim M H A, et al. (2021). Antioxidative study of polysaccharides extracted from red, green and brown marine macroalgae/seaweed. Applied Science, Vol 3 Pages 485-496.
Cho M, Lee L and You S G (2010) Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules, Vol 16 Pages 291-297.
Costa L S, Fidelis G P, Cordeiro S L, et al. (2010) Biological activities of sulphated polysaccharides from tropical seaweeds. Biomedical Pharmacotherapy, Vol 64 Pages 21‑28.
Czochra M P and Widensk A (2002) Spectrophotometric determination of hydrogen peroxide scavenging activity. Journal of Anlitica Chem Acta, Vol 452 Pages 177-184.
Duan X J, Zhang W W and Li X M (2006) Evaluation of antioxidant property of extract and fractions obtained from a red alga Polysiphoni aurceolata. Food Chemistry, Vol 95 Pages 37-43.
Finosh G T and Jayabalan M (2013) Reactive oxygen species—Control and management using amphiphilic biosynthetic hydrogels for cardiac applications. Advance Journal of Bioscience and Biotechnology, Vol 4 No 12 Pages 1134-1146.
Foley S A, Mulloy B and Tuohy A (2011) An unfractionated fucoidan from Ascophyllum nodosum: extraction, characterization, and apoptotic effects in vitro. Journal of Natural Products, Vol 74 Pages 1851-1861.
Gulcin I (2006) Antioxidant and antiradical activities of L-Carnitine. Life Sciences, Vol 78 Pages 803-811.
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry and role in human disease American. Journal of Medical, Vol 91 Pages 14-22.
Harris J B and Hans P (2011) A decade of change in the seaweed hydrocolloids industry. Journal of Applied Phycology, Vol 23 No 3 Pages 321–335.
Hentati F, Barkallah M, Ben A, et al. (2019). Quality characteristics and functional and antioxidant capacities of algae-fortified fish burgers prepared from common barbel (Barbus barbus). Biomedical Research, Vol 12 Pages 14-23.
Hybertson B M, Gao B, Bose S K et al. (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Molecular Aspects Medicine, Vol 32 No 4- 6 Pages 234-246.
Jimenez-Excrig A, Jimenez-Jimenez I and Pulido R (2001) Antioxidant activity of fresh and processed edible seaweeds. Journal of Science of Food and Agriculture, Vol 81 No 5 Pages 530-534.
Kahl R and Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z FürLebensm-Unters –Forsch, Vol 196 Pages 329–338.
Kim S H, Choi D S, Yasanthaathukorala, et al. (2007) Antioxidant activity of sulphated polysaccharides isolated from Sargassum fulvellem. Journal of food science and nutrition, Vol 12 Pages 65-73.
Lekameera R, Vijayabaskar P and Murugan A (2007) Potential antioxidant activity of the brown alga Lobophora varigata Seaweed. Research Utilization, Vol 29 No 1&2 Pages 55-61.
Lekameera R, Vijayabaskar P and Somasundaram S T (2008) Evaluating antioxidant property of brown Alga Colpomenia sinuosa (DERB. ET SOL). African Journal of Food Science, Vol2 Pages 126-130.
Li Y F, Guo C J, Yang J J, et al. (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chemistry, Vol 96 Pages 254-260.
MacDonald J, Galley H F and Webster N R (2003) Oxidative stress and gene expression in sepsis. British Journal of Anaesthesia, Vol 90 Pages 221–232.
Maheswaran M L, Padmavathy S and Gunlan B (2013) Screening and Characterization of Marine Seaweeds and its Antimicrobial Potential against Fish Pathogens International. Journal of Fisheries and Aquatic Studies, Vol 1 No1 Pages 1-13.
Monsuang Y, Nongporn H T, and Wutiporn P (2009) Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Foods for Human Nutrition, Vol 64, Pages 218-223.
Na Y S, Kim W J, Kim S M, et al. (2010) Purification, characterization and immune stimulating activity of water‑soluble polysaccharide isolated from Capsosiphon fulvescens. International Journal of Immunopharmacology, Vol 10 Pages 364‑370.
Padayatty S J, Katz A and Wang Y (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of American College Nutrition, Vol 22 Pages 18–35.
Ramli A N M, Badrulzaman S Z S, Hamid H A, et al. (2021) Antibacterial and antioxidative activity of the essential oil and seed extracts of Artocarpus heterophyllus for effective shelf-life enhancement of stored meat. J Food Process Preserv, 45:e14993
Rodriguez-Jasso R M, Mussatto S I, Pastrana L, et al. (2011) Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydrate Polymers, Vol 86 Pages 1137-1144.
Ruperez P, Ahrazem Q and Leal J A (2002) Potential antioxidant activity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. Journal of Agriculture. Food Chemistry, Vol 50 No 4 Pages 840-845.
Saikat S, Chakraborty R and Sridhar C (2010) Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. International Journal of Pharmaceutical Sciences Review and Research, Vol 3 No 1 Pages 91-100.
Sharique A A, S Salim, Sahani T, et al. (2012c) Serotinergic receptors as novel target for optimizing skin pigmentary responses in Indian bullfrog Hoplobatrachus tigerinus. British Journal of Pharmacology, Vol 165 No 5 Pages 1515-1525.
Singh N and Rajini P S (2004) Free radical scavenging activity of an aqueous extract of potato peel. Food Chemistry, Vol 85 Pages 611-616
Suganya V, Anuradha V, Syed Ali M, et al. (2017) In vitro antioxidant activity of microencapsulated and non-encapsulated astaxanthin. Asian Journal of Science and Technology, Vol 8 No 11 Pages 6391-6404.
Will Castro L S E P, Gomes Castro A J, da S Nascimento Santos M (2016) Effect of galactofucan sulfate of a brown seaweed on induced hepatotoxicity in rats, sodium pentobarbital-induced sleep, and anti-inflammatory activity. Journal of Applied Phycology, Vol 28 Pages 2005–2017.
Xue C H, Fang Y and Lin A (2001) Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. Journal of Applied Phycology, Vol 13 No 1 Pages 67-70.
Yang C, Chung D, Shin I S, et al. (2008) Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Journal of Biological Macromolecules, Vol 43 Pages 433-437.
Yogesh K, Tarafdar A, Badgujar P C (2021) Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. Journal of Food quality. Vol 5753391 Pages 17.
Yuan H, Zhang W, Li X (2005) Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their over sulfated, acetylated, and phosphorylated derivates. Carbohydrates Research, Vol 340 Pages 685–690.
Zayed A, El-Aasr, Ibrahim ARS and Ulber R. (2020) Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Marine Drugs, Vol 18 No 571; Pages 1-31.


