1*, 3Department of Microbiology, Tiruppur Kumaran College for Women, Tirupur, Tamilnadu, India and
2Department of Microbiology,Sri Ramakrishna College of Arts and Science for Women, Coimbatore, Tamilnadu, India.
Corresponding author Email: pavaimicro@gmail.com
Article Publishing History
Received: 19/04/2019
Accepted After Revision: 30/06/2019
Cassia fistula, commonly known as golden shower, purging cassia or Indian laburnum is a flowering plant in the family Fabaceae. The species is native to the Indian subcontinent and adjacent regions of Southeast Asia. Sputum were collected aseptically from patients and cultured on the appropriate bacteriological media. Bacterial isolates were identified by biochemical tests and antimicrobial susceptibility performed by standard methods. Out of 25 specimens, 12 species of various bacteria isolated. The prevalence of bacteria spp. isolated were as follows Staphylococcus sp, Klebsiella sp, Pseudomonas sp , Escherichia coli ,Proteus sp. The susceptibility patterns varied from one bacterial isolates to the other depending on the drug. The susceptibility test against microbial isolates of 15 commercially available antibiotics were used. The Aqueous, Acetone leaf extracts of Cassia fistula was subjected for screening of in vitro antibacterial activity against selected major human pathogenic bacterial strains like Staphylococcus sp, Klebsiella sp, Pseudomonas sp, Escherichia coli ,Proteus sp by agar well diffusion method. Ciprofloxacin antibiotic was used as positive control. The Acetone extraction shows more effective in 19 mm in 100μl concentration against Escherichia coli. Phytochemical analysis of Aqueous, Acetone solvent extracts of Cassia fistula were carried out and results confirmed that alkaloid, flavonoid, phenol, tannin, anthoquinone, saponin, carbohydrate, steroid, terpenoids, triterpenoids, phytosterols are present. Quantitative phyotochemical analysis of Cassia fistula leaves revealed the presence of Alkaloids (13%), Flavanoid(17%), Moisture (15%) , Ash (5%) , protein and carbohydrate as 11500mg/ml and 1160mg/ml respectively. Antioxidant activity of Acetone, aqueous extracts were studied, such as the ferric reducing antioxidant power (FRAP), Hydrogen peroxide Scavenging activity, Fe2+ chelating ability was performed. The chemical constituents of organic plant extracts were purified by column chromatography and were separated by thin layer chromatography (TLC).
cassia fistula, Antibiotic susceptibility testing, Agar well diffusion method, Phytochemical analysis, Antioxidant activity
Pavai I. S, Selvi B. T, Anisha R. Antimicrobial and Antioxidant Activity of the Golden Shower, Cassia fistula. Biosc.Biotech.Res.Comm. 2019;12(2).
Pavai I. S, Selvi B. T, Anisha R. Antimicrobial and Antioxidant Activity of the Golden Shower, Cassia fistula. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2Nd5TvG
Copyright © Pavai et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
A global problem accounting for over 50 million deaths of each year and occurs in both community and health care settings (Zafar et al., 2008).Respiratory infections are the commonest community-acquired infections of humans (Bannister et al., 2006).Respiratory tract infection (RTI) is considered as one of the major public health problems and a leading cause of morbidity and mortality in many developing countries (Sharma et al., 2005, Jacobs et al., 2009 and Bipin Prajapati et al., 2011). An antibacterial is an agent that inhibits bacterial growth or kills bacteria. The term is often used synonymously with the term antibiotics; today, however, with increased knowledge of the causative agents of various infectious diseases, an antibiotic has come to denote a broader range of antimicrobial compounds, including anti-fungal and other compounds. With advances in medicinal chemistry, most of today’s anti-bacteria chemically are semisynthetic modifications of various natural compounds. These include, for example, the beta-lactam antibacterials, which include the penicillins (produced by fungi in the genus Penicillium), the cephalosporins, and the carbapenems. Compounds that are still isolated from living organisms are the aminoglycosides, whereas other antibacterials—for example, the sulfonamides, the quinolones, and the oxazolidinones—are produced solely by chemical synthesis. In accordance with this, many antibacterial compounds are classified on the basis of chemical/biosynthetic origin into natural, semisynthetic, and synthetic, (Bindhu and Sabeena, 2019) .
Many factors play in the emergence of resistance, (WHO, 2012) from poor utilization of antimicrobial agents, to the transmission of resistant bacteria from patient to patient and from healthcare workers to patients and vise versa, to a lack of guidelines for a appropriate and judicious use of antimicrobial agents, to lack of easy-to-use auditing tools for restriction (Aly and Balkhy, 2012).In recent years, the infectious diseases remain the leading cause of death worldwide infections due to antibiotic resistant ability of some microorganisms. However, synthetic antimicrobial agents provide broad spectrum characteristics, but often associated with the adverse effects on the host, including immune suppression, hypersensitivity and severe allergic responses. This situation reinforced the scientist communities looking for eco-friendly alternatives so that novel bioactive therapeutic agents can be made. Herbal medicine has a long history in the treatment of several kinds of disease (Holm et al., 1998). Their use for the treatment of disease has been practiced by man for many years and is still being widely practiced even today (Kokwaro, 1993).
Medicinal plants beer potent antimicrobial potential, many of them uses in traditional system of medicine, which are readily available in rural areas as relatively cheaper than modern medicine. Some investigators reported the medicinal values of this plant such as hypoglycemic properties of leaf, antibacterial and antifertility effects of seed extract. The present work was objected to carryout scientific study on antibacterial activity of the leaf extract of Cassia fistula against nine bacterial species in comparison with the contemporary commonly used antibacterial drug Cephradine. Medicinal plants always played an important role in the health development of mankind. In developing countries, 80% of populations are totally dependent on plants for their primary health care. The global emergence of multidrug resistant bacterial strains is increasing, limiting the effectiveness of current drugs and treatment failure of infections. A noval approach to the prevention of antibiotic resistance of pathogenic species is the use of new compounds from plant origin (Bindhu and Sabeena, 2019).
Materials and Methods
Cassia fistula (leaves) were collected in Tirupur district, Tamil Nadu, India. The freshly collected leaves were washed with water and immediately sprayed with ethanol and dried under shade at room temperature .The dried leaves were powdered in a blender. The powdered plant leaf material was stored in sterile containers for further use. For aqueous extraction 10 gram of dried powder of Cassia fistula was suspended in 100ml cold distilled water and hot water and mixture was soaked for 24 hours. The suspended solid was filtered through what man No.1 filter paper and kept in water bath at 80˚C for 2 hours. The dried crude extracts were stored at 4˚C for further use, ( Negi & Dave 2010, Kulkarni et al., 2015). For solvent extraction 5 gram of dried powder of Cassia fistula suspended in 100ml of solvent Acetone and the mixture was soaked for 24hours.The suspended solid was filtered through What man No.1 filter paper and kept in water bath at 80˚C for 2hours. The dried crude extracts were stored at 4˚C for further use. The 35 Pathogenic sputum samples were collected from various Hospitals in Tirupur. Sputum samples were maintained at 4°C.The collected sputum samples were inoculated on the selective media such as EMB, MacConkey, MSA and Cetrimide agar plates were incubated for 24 hours at 37°C. The growth of bacterial colonies was observed and results were recorded. The collected pathogens were identified on the basis of Gram’s reaction and biochemical characteristics (Macfaddin, 1980) and results were identified with the help of Bergey’s manual of systemic Bacteriology.
Kirby-Bauer disc diffusion method was adopted for susceptibility testing. Penicillin, Azhihaemycin, Cefoxitin, Chloramphenicol, Clindamycin, Erythromycin, Oxacillin, Teicoplarin, Ciprofloxacin, Ofloxatin,Linezolid, Tetracycline, Vancomycin, Rifampicin, Ceftazimide, Ceftriaxone, Cerpodoxime, Cefazolin, cefoperazone, Cephalexin, Cefpriome, Piperacillin, Gentamycin, Cotrimoxazole, Amikacin, Polymixin was used. The antibiotic disc was placed on the surface of the Mueller hinton agar medium by using a sterile forceps. The plates were incubated at 37°C for 24 hrs. The zones of inhibition were measured and tabulated (Bauer et al., 1966).The plant extract was tested for antibacterial activity by standard agar well-diffusion method. The sputum isolates were swabbed uniformly using sterile cotton swab. The wells of 6mm diameter were made on nutrient agar using gel puncture and then 100µl of plant extracts were loaded into the well and allowed to diffuse at room temperature for 2hrs. The plates were incubated at 37ºC for 24hrs. Diameter of the inhibition zones were measured and tabulated (Perez, 1990).
For Qualitative Phytochemical Analysis the plant extracts and acetone, aqueous solutions were assessed for the existence of the phytochemical analysis by using the following standard methods. Wagner’s test was performed to determine alkaloids. 0.5g of extracts was diluted with 10ml of acid alcohol and warmed then filtered. 2ml of dilute ammonia and 5ml of chloroform was added, shaken gently to extract the alkaloidal base. The chloroform layer was extracted with 10ml of Acetic acid and 1ml of Wagner’s reagent was added to the resultant extract. Formation of cream or reddish brown precipitate indicated the presence of alkaloids, (Harborne, 1973). For Flavonoids determination 1 ml of extract, 5 drops of 5% sodium hydroxide was added. An increase in the intensity of yellow colored solution is seen which becomes colorless on the addition of few drops of 2M Hydrochloric acid. To determine the Phenols by using Ferric Chloride Test, few drops of 10% aqueous ferric chloride solution was added to 2 ml of the diluted leaf extract and thoroughly mixed. The presence of phenols indicated the formation of blue or green or violet colour. Ferric Chloride test was used to find tannins, 2ml of extract, few drops of 5% ferric chloride solution were added. The blue color indicated the presence of hydrolysable tannis, the green color indicated the presence of condensed tannins. For saponins test, 5 ml of extract was taken and shaken well for five minutes., after which table foam was formed.
The presence of carbohydrates in solvent extracts was determined by different methods such as, Fehling’s test, Benedict’s test, Molisch’s test and iodine test. For Benedict’s test the 5 ml of Benedict’s qualitative reagent added 0.5ml of the leaf extract and mixed well. Boiled it for 5 minutes in a boiling water bath. Appearance of yellow, green, red or reddish brown colour precipitate showed that presence of reducing sugar. For Glycosides Test 2ml of the diluted leaf extract added 1 ml of aqueous sodium hydroxide solution. Formation of yellow colour indicated the presence of glycosides. 2 ml of the leaf extract (prepared in chloroform) added 2 ml of concentrated sulphuric acid drop by drop along the sides of the test tube. Red colour formed in the chloroform layer, indicated the presence of steroids.5 ml of each plant extract was mixed with 2ml of chloroform and 3ml of concentrated sulpuric acid (98%).Formation of a reddish brown coloured ring between the interfaces indicated the presence of terpenoids.
Few drops of Ninydrin reagent and 1ml of extract were added. Appearance of blue colour indicates the presence of protein. Approximately 2mg of dry extract was shaken with 1ml of chloroform and few drops of concentrated sulfuric acid were added along the sides of the test tube. Formation of red brown color at the interface indicates the presence of triterpenoids.2 ml of aqueous extract was added to 2 ml of 2N HCl & NH3. The appearance of pink red turns into blue violet indicates presence of Anthocyanin. The extract was (2mg) dissolved in 2ml of acetic anhydride and heated to boiling, cooled and 1 ml of concentrated sulfuric acid was added along the sides of the test tube. A brown ring was formed at the junction and the upper layer turned to dark green color indicates the presence of phytosterols. Hydroxyanthraquinone test was performed to determine the anthraquinone glycosides. To 1ml of extract, few drops of 10% potassium hydroxide solution were added, the appearance of red color confirmed the test.
For quantitative estimation – Alkaloids 5 g of the sample was weighted into a 250ml beaker and 200ml of 10% acetic acid in ethanol was added and covered and allowed to stand for 4 hrs. This was filtered and the extract was concentration a water bath to one quarter of the original volume concentrated ammonium hydroxide was added drop wise to the extract until the preparation was complete. The whole solution was allowed to settle and the precipitated was collected and washed with dilute ammonium hydroxide and then filtered. The residue is the alkaloids which was dried and weighed (Harborne, 1973). For flavonoid analysis 10 g of the plant sample was extracted repeatedly with 100ml of 80% aqueous methanol at room temperature. The whole solution was filtered through Whatman filter paper No.42 (125mm). The filtrate was later transferred into a crucible and evaporated into dryness over a water bath and weighed to a constant weight (Bohrm and Abyazan, 1994).The total ash content of a substance is the percentage of inorganic residue remaining after the organic matter has been ignited.2gm of the pulverized samples was placed in a crucible and ignited in a muffle furnace at 555ºc for 6 hours. It was then cooled in desiccators and weighted at room temperature to get the weight of the ash. For the estimation of moisture 2g of sample was placed in a dried pre-weighed petri dish and placed in an oven to dry at 105ᴼC for two hours. The dried samples were weighed and the experiments were repeated until constant weight was obtained. (A. O. A. C 1990).The protein estimation was done by Lowry et al method as per standard protocol (Lowry et al., 1951).
For the estimation of carbohydrates 1.0 g of sample was taken into boiling tubes and hydrolyzed by keeping them in boiling water bath for 30minutes to 1 hr with 5ml of 2.5 N HCL. It was cooled to room temperature and neutralized with solid sodium carbonate until the effervescence ceases and made up the volume to 100ml and centrifuged.0.5ml and 1ml of the supernatant were collected and used for further analysis. The volume was make up to 1.0ml in all the tubes including the sample tubes by the addition of distilled water.4ml of 0.2% Anthrone reagent was added to each test tube followed by heated in a boiling water bath for 10 minutes. Test tubes were cooled at room temperature. Dark green color was appeared on heating the samples. The optical density (OD) value of the colored solution was then measured through 630nm wavelength in a colorimeter against blank. The amount of carbohydrate present in the sample was calculated, using the method of Hedge and Hofreiter, (1962).
Antioxidant activity of the extract was done in reducing assay, chelating assay and scavenging of hydrogen peroxide assay. About 1ml of extract/fractions (50-300μg/ml) was prepared in distilled water and mixed with 2.5ml of phosphate buffer (0.2M,pH6.6) and2.5ml of 1% potassium ferricyanide. The mixture was incubated at 50oC for 20min. 2.5ml of 10% trichloroacetic acid was then added to the mixture and centrifuged at 3000rpm for 10 min.1ml of aliquot of supernatant was mixed with 2.5ml of distilled water and 0.5ml FeCl3 (0.1%) and absorbance was measured at 700nm. Increase in absorbance was interpreted as increased reducing activity.1ml of extract with different concentration was mixed with 3.5ml methanol and then the mixture was mixed with ferrous chloride (2mM, 0.1ml) and ferrozine (1mM, 0.2ml) for 10 min at room temperature. The absorbance was measured at 562 nm against a blank in which the extract was not added. The % age inhibition was calculated as: % Inhibition = BO-B1/BO*100.
Where, BO is the absorbance of control, B1 is the absorbance of reaction mixture (Harpreet Walia et al., 2011). To the Different concentration of the plant extract ranging from (20-100 μg/ml) and (200-1000μg/ml) the hydrogen peroxide prepared with phosphate buffer (PH 7.4) was added in the volume of 0.6ml. Then the mixture was kept at room temperature for 10 minutes and the absorbance was measured at 230 nm using UV-visible spectrophotometer. % of inhibition = Control OD-sample OD / Control OD*100 (Gill et al., 2010). For column chromatography air dried powder Cassia fistula was soaked in methanol for overnight. 0.5 g of crude extract was used for column (6mm×2mm) chromatography. Silica gel (mesh 60-120) was used as column packing material. The column was eluted up to 5 fractions. Collected fractions were applied on TLC using Acetone; Methanol; Acetic acid (75:08:50µl). The spots were detected and the presence of certain compounds such as organic compound, carbohydrate, carboxylic acid and phenol was identified due to colour change by spraying reagents.
Results and Discussion
A total of 35 sputum samples were collected from various hospitals in and around Tirupur. The 10 samples showed no growth and 25 sample shows of respiratory tract infections. From this 25 samples 12 isolates were selected and identified as Staphylococcus sp, E.coli, Pseudomonas sp, Klebsiells sp, and Proteus sp, based on morphological and biochemical characterization. It was shown in Table no. 1. From this 12 bacterial isolates, 8 isolates were gram negative and remaining 4 were gram positive. Based on the gram’s reaction commercial antibiotics were chosen. For gram negative isolates were subjected to the Kirby-bauer disc diffusion method was adopted for susceptibility and zone of inhibition were measured and shown in Table no:2 In this study, five Staphylococcus sp., one Proteus sp., two Klebsiella sp., two Escherichia coli, three Pseudomonas sp., strains were isolated and identified from different types of sputum samples by Biochemical characterization. In the present study, the antibacterial activity of commercial antibiotics against four Staphylococcus sp., was done by disc diffusion method. The Cefotaxime shows Maximum zone of inhibition of range 25mm and Rifampicin shows that Minimum zone of inhibition of range 7mm. Manikandan and Asmath (2013) had reported the susceptibility profile of Staphylococcus aureus with the maximum inhibition of 97% for Amikacin and minimum inhibition of 21.3% for Ampicillin.
Table 1: Morpholological and Biochemical characterization of sputum isolates
| S.no | Bio-chemical test | Iso
1 |
Iso
2 |
Iso
3 |
Iso
4 |
Iso
5 |
Iso
6 |
Iso
7 |
Iso
8 |
Iso
9 |
Iso
10 |
Iso
11 |
Iso
12 |
| 1 | Gram staining | +ve cocci | +ve cocci | +ve cocci | +ve cocci | -ve
Rod |
-ve
rod |
-ve
rod |
-ve
rod |
-ve
Rod |
-ve
rod |
-ve
rod |
-ve
rod |
| 2 | Mannitol salt agar | G | G | G | G | – | – | – | – | – | – | – | – |
| 3 | Macconkey agar | P | P | P | P | NLF | – | – | MLF | MLF | – | – | – |
| 4 | Eosin methylene blue agar | – | – | – | – | – | MS | MS | – | – | – | – | – |
| 5 | Cetrimide agar | – | – | – | – | – | – | – | – | – | BG | BG | BG |
| 6 | Indole | – | – | – | – | – | + | + | – | – | – | – | – |
| 7 | Methyl red | + | + | + | + | + | + | + | – | – | – | – | – |
| 8 | Voges proskauer | + | + | + | + | – | – | – | + | + | – | – | – |
| 9 | Citrate | – | – | – | – | + | – | – | + | + | + | + | + |
| 10 | Catalase | + | + | + | + | + | + | + | + | + | + | + | + |
| 11 | Oxidase | – | – | – | – | – | – | – | – | – | + | + | + |
| 12 | Result | Staph 1 | Staph 2 | Staph 3 | Staph 4 | Pro 1 | E 1 | E2 | K1 | K2 | Ps1 | Ps2 | Ps3 |
Note:‘+’ indicates positive , ‘-‘ indicates negative , NLF-Non lactose fermenting, MLF-Mucoid lactose fermenting, G-Golden color colonies, P-Pink color colonies, BG-Blue green color colonies, Staph-Staphylococcus sp, Pro-Proteus sp, E-E.coli, K-Klebsiella sp, Ps-Pseudomonas sp
In the present study, the antibacterial activity of commercial antibiotics was done by disc diffusion method. The antibiotics Piperacillin, Ciprofloxacin shows Maximum zone of inhibition of range 28mm and Polymixinb shows that Minimum zone of inhibition of range 13mm against Proteus sp. The polymixin shows Maximum zone of inhibition of range 14mm and ciprofloxacin
shows that Minimum zone of inhibition of range 13mm against Escherichia coli. The polymixin shows Minimum zone of inhibition of range 13mm against Klebsiella sp., other antibiotics shows Resistant. The cefoperazone shows Maximum zone of inhibition of range 14mm and Cefotaxime and amikacin shows that Minimum zone of inhibition of range 12mm against Pseudomonas sp.,
Manikandan and Asmath (2013) had reported the susceptibility profile of E. coli with the maximum inhibition of 86% for Amikacin and minimum inhibition of 14% for Sparfloxacin. The susceptibility profile of Pseudomonas aeruginosa with the maximum inhibition of 87% for Amikacin and minimum inhibition of 01% for Ampicillin. Solvent extraction of Cassia fistula leaf powder was extracted using cold water, hot water and acetone. An attempt has been made to assess the antibacterial properties of Cassia fistula for 12 sputum isolates were shown in Table no: 3 &Figure no: 1
Phytochemical analysis of three solvent extracts of Cassia fistula were carried out and results confirmed that alkaloid, flavonoid, saponin, carbohydrate, steroid, terpenoids, triterpenoids, anthraquinones, phytosterols, phenol are present. Kulkarni et al., (2015) reported the phytochemicals analysis of three solvents (Aqueous, Methanol, Petroleum ether) showed the presence of 11 important phytoconstituents viz., alkaloid, flavonoid, saponin, carbohydrate, anthraquinones, phenol compounds, glycosides, fats, gums and mucilages, proteins and amino acid.Antioxidant increasing absorbance indicates an increase in reductive ability. The Cassia fistula leaf extract of acetone, aqeous extract was used for reducing power assay in different concentration of 20,40,60,80,100 µl in absorbance of 700nm and the result was showed in Table no: 5
The compounds were separated by column chromatography. The fractions of F1,F2,F3,F4 were collected and thin layer chromatography was done . In this study the Acetone extract of C. fistula leaves was subjected to purification by column chromatography with the combinations of solvents i.e. Acetone + Hexane (10+90%), Acetone + Ethyl acetate (60+40%), Acetone + Methanol (55:45%), Acetone + Hexane (50:50%), Acetone + Hexane (50:50%) (0.146, 0.33, 0.568, and 0.7355 respectively) and the column was eluted in different fractions.
In this study TLC of selected fractions reported the presence of certain compounds by spraying different reagents such as p-Anisaldehyde gives a range of colors indicates Carbohydrate , Bromocresol Green stain yellow-green on a blue background indicates the presence of Carboxylic acid , Iodine which reveals the appearance of dark spot indicates organic compounds.TLC results of different organic solvents (Methanol, Chloroform, Toluene, Petroleum ether, Ethyl acetate) when used singly the elution resulted in No separation, spots are not clear, Blur spot, Blur spot and No separation respectively were reported by Bhawna Sunil Negi & Bharti P.Dave (2010).
Tabkle 2: Antibiotic sensitivity of isolates for commercial antibiotics
| S.No | Isolates | Resistance | Sensitivity |
| For gram negative isolates | |||
| 1 | Pro 1 | 9 | 6 |
| 2 | E1 | 13 | 2 |
| 3 | E2 | 12 | 3 |
| 4 | K1 | 14 | 1 |
| 5 | K2 | 14 | 1 |
| 6 | Ps1 | 13 | 2 |
| 7 | Ps2 | 13 | 2 |
| 8 | Ps3 | 13 | 2 |
| For gram positive isolates | |||
| 9 | Staph 1 | 13 | 2 |
| 10 | Staph 2 | 13 | 2 |
| 11 | Staph 3 | 13 | 2 |
| 12 | Staph 4 | 13 | 2 |
Note: Staph-Staphylococcus sp., Pro-Proteus sp., E-E.coli., K-Klebsiella sp., Ps-Pseudomonas sp.,
Table 3: Antibacterial activity of C.fistula extracts for Sputum isolates
| S.No | Isolates | Extracts | Zone of inhibition in mm | |||
| 25% | 50% | 75% | 100% | |||
| 1 |
Pro 1 |
C | 0 | 0 | 15 | 16 |
| H | 0 | 12 | 14 | 14 | ||
| A | 13 | 15 | 15 | 18 | ||
| 2 |
E1 |
C | 0 | 13 | 14 | 14 |
| H | 0 | 13 | 13 | 15 | ||
| A | 15 | 17 | 17 | 19 | ||
| 3 |
E2 |
C | 0 | 0 | 13 | 13 |
| H | 0 | 12 | 13 | 15 | ||
| A | 12 | 13 | 15 | 16 | ||
| 4 |
K1 |
C | 0 | 0 | 13 | 13 |
| H | 0 | 11 | 12 | 15 | ||
| A | 14 | 15 | 17 | 17 | ||
| 5 |
K2 |
C | 0 | 12 | 13 | 15 |
| H | 0 | 12 | 13 | 13 | ||
| A | 13 | 14 | 17 | 18 | ||
| 6 |
Ps1 |
C | 0 | 12 | 12 | 16 |
| H | 0 | 13 | 13 | 15 | ||
| A | 12 | 15 | 16 | 18 | ||
| 7 |
Ps2 |
C | 0 | 0 | 13 | 16 |
| H | 0 | 0 | 13 | 14 | ||
| A | 12 | 13 | 15 | 17 | ||
| 8 |
Ps3 |
C | 13 | 15 | 16 | 17 |
| H | 0 | 0 | 14 | 16 | ||
| A | 14 | 15 | 18 | 18 | ||
| 9 |
Staph 1 |
C | 12 | 12 | 15 | 17 |
| H | 12 | 13 | 15 | 15 | ||
| A | 12 | 14 | 15 | 17 | ||
| 10 |
Staph 2 |
C | 11 | 13 | 15 | 15 |
| H | 0 | 10 | 14 | 16 | ||
| A | 13 | 15 | 17 | 18 | ||
| 11 |
Staph 3 |
C | 0 | 0 | 11 | 13 |
| H | 10 | 10 | 12 | 14 | ||
| A | 12 | 13 | 16 | 17 | ||
| 12 |
Staph 4 |
C | 0 | 0 | 13 | 16 |
| H | 0 | 0 | 10 | 13 | ||
| A | 13 | 14 | 16 | 16 | ||
Note: Staph-Staphylococcus sp,Pro-Proteus sp, E-E.coli,K-Klebsiella sp, Ps-Pseudomonas sp,
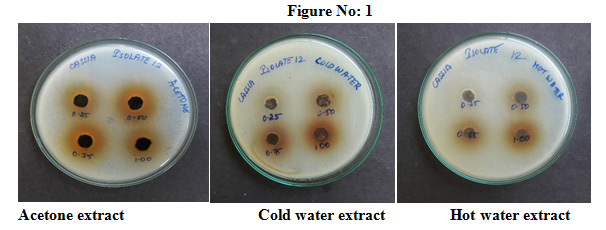 |
Figure 1 |
Table 4: Phytochemical analysis of Cassia fistula for bio active compounds
| S.No. | Parameters | Different Extraction | ||
| cold water | Hot water | Acetone | ||
| 1. | Alkaloid | + | + | – |
| 2. | Flavonoid | + | + | + |
| 3. | Phenol | – | – | + |
| 4. | Tannin | – | – | – |
| 5. | Saponin | + | + | – |
| 6. | Carbohydrate | + | + | + |
| 7. | Glycoside | – | – | – |
| 8. | Steroid | + | + | + |
| 9. | Terpenoids | + | + | + |
| 10. | Proteins | – | – | – |
| 11. | Triterpenoids | + | + | + |
| 12. | Anthocyanin | – | – | – |
| 13. | Anthoquinone | + | + | – |
| 14. | Phytosterols | + | + | + |
‘+’ indicates Presence, ‘–‘ indicates Absence
Table 5: Antioxidant activity of Cassia fistula leaf extracts
| S.No
|
Concentration | Extracts | Reducing assay
(Absorbance at 700 nm ) |
Scavenging assay (% of inhibition) | Chelating assay (% of inhibition) |
| 1 | 20µl | Acetone | 0.95 | 11 | 15 |
| Cold water | 1.04 | 17 | 15 | ||
| Hot water | 0.82 | 10 | 20 | ||
| 2 | 40 µl | Acetone | 1.07 | 28 | 28 |
| Cold water | 1.06 | 22 | 25 | ||
| Hot water | 0.88 | 22 | 38 | ||
| 3 | 60 µl | Acetone | 1.08 | 43 | 42 |
| Cold water | 1.12 | 31 | 36 | ||
| Hot water | 1.02 | 39 | 51 | ||
| 4 | 80 µl | Acetone | 1.36 | 56 | 56 |
| Cold water | 1.30 | 48 | 48 | ||
| Hot water | 1.12 | 48 | 65 | ||
| 5 | 100 µl | Acetone | 1.44 | 64 | 69 |
| Cold water | 1.38 | 55 | 61 | ||
| Hot water | 1.20 | 62 | 77 |
Conclusion
From this present study we conclude that Cassia fistula leaf extracts have significant antimicrobial and antioxidant properties. Sputum isolates had a high level of resistance to examine antibiotics. Acetone extract of Cassia fistula leaves showed 18-19mm zone of inhibition, surprisingly cold water extract also showed 14-17mm zone of inhibition and hot water shown 14-16mm of inhibition. Health sector should educate the public to use natural remedies instead of antibiotics.
References
Anderson, H., Esmail, A., Hollowell, J., Littlejohns, P., and Strachen D. (1993)
Epidemiologically based needs assessment: lower respiratory disease. DHA Project Research Prograrnme; pp 6-12.
O.A.C., (1990) Official Methods of Analysis. Association of Official Analytical Chemists,
The Association: Arlington, VA, Vol. II, 15th ed. Sec.985.29.
Bannister, B. A.; Gillespie S. H. and Jones, J. (2006) Infection: Microbiology and Management. 3rd Black well Publishing.
Bauer, A. W.; Kirby, W. M. M.; Sherris, J. C. and Turck, M. (1966) Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol., Vol 45: pp.493-496.
Bhawna Sunil Negi and Bharti P. Dave (2010) , Evaluation Of In Vitro Antimicrobial Activity
From The Leaves Extract of Cassia fistula Journal of Pure and Applied Microbiology; vol 4(2), pp.557-564.
Bindhu, R.K and K Sabeena (2019).Invitro antibacterial activity of Cassia fistula
Linn methanolic leaf extracts, International journal of basic and clinical
pharmacology,Vol.8/2,PP.270-274.DOI:http://dx.doi.org/10.18203/2319-ijbcp20190146.
Bipin Prajapati., Nitiben Talsania., Sonaliya, K. N. (2011). A study on prevalence of acute respiratory Tract infections (ARI) in under five children in Urban and rural communities of Ahmedabad District, Gujarat, Nat. J. Com. Med., Vol.2(2): pp.255-259.
Gupta RK, 2010. Medicinal and Aromatic Plants, CBS publishers and distributors, 1st edition,2010.
Harborne, JB (1973) Phytochemical methods, London, Chapman & Hall Ltd. pp 49-188.
Harpreet Walia, Subodh Kumar and Saroj Arora, 2011. Journal of Basic and Clinical Pharmacy 2(2) ,pp.115-124.
Hedge J.E, Hofreiter B.T. In Carbohydrate chemistry 17, Academic press, Newyork, 1962.
Iwu M.W., Dunean A.R., Okunji C.O., (1999) New Antimicrobials of Plant Origin, In: Janick J. Perspectives on New Crops and NEW Uses, Alexandria, V.A.: ASHS Press, pp.457-462.
Jacobs, E., Dalhoff, A., and Korfmann, G. (2009) Susceptibility patterns of bacterial isolates from hospitalised patients with respiratory tract infections. Int. J. Antimicrob. Agents, 33: 52–57.
Kokwaro; J.O., (1993) Medicinal plants of east Africa ed.2, Kenya literature bureau, Nairobi.
Kulkarni A, Govindappa M, Channabasava, Chandrappa CP, Ramachandra YL, Koka PS, (2015)
Phytochemical analysis of Cassia fistula and it’s in vitro antimicrobial, antioxidant and anti-inflammatory activities. Adv Med Plant Res, 3(1): 8-17
Lowry O.H, Rosebrough N.J, Farr A.L, Randall R.J. (1951) Protein measurement with the folin phenol reagent, J. Biological chemistry, 193: 265-275.
MacFaddin, J. F. (1980): Biochemical tests for identification of medical bacteria, 2nd ed. Williams and Wilkens, Baltimore, Md.
Mahmoud, A. and Balkhy, H.H. (2012) The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob. Resist. Infect Contl. 1, 26-31 doi: 10.1186/2047-2994-1-26
Manikandan, C. and Asmath, A, (2013) Antibiotic susceptibility of bacterial strains isolated from patients with respiratory tract infections. Int. J. Pure Appl Zool., 1(1):61-69.
Perez C, Paul M and Bazerque P (1990) Antibiotic assay by agar well diffusion Acta Biologiae et Medicine Experimentalis Vol.15: pp 113-115.
Prescott, L. M., Harley, J. R., and Klein, D. A. (2005) Microbiology (6th Ed.) McGraw-Hill. Higher Education, Boston Burride, pp 673-936.
Sarvajeet Gill, Veer Singh Gill and Narendra Tuteja.(2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48: 909-930.
Sharma, R., Sharma, C. L., and Kapoor, B. (2005) Antibacterial resistance: current problems and possible solutions. Indian Journal of Medical Sciences 59, 120-129.
Thirumal, M, Surya S, Kishore G.,(2012) Cassia fistula Linn-Pharmacognostical, Phytochemical and Pharmacological Properties. Rev. Pharm. Sci. 1: 66-78
Veloo, AC., Seme, K., Raangs, E., Rurenga, P., Singadji, Z., Wekema-Mulder, G., and van Winkelhoff, AJ. (2012) Antibiotic susceptibility profiles of oral pathogens.Int J Antimicrob Agents. Nov; 40(5):450-4.
Zafar, A., Hussain, Z., Lomama, E., Sibiie, S., Irfan, S., Khan, E. (2008) Antibiotic susceptibility of pathogens isolated from patients with community-acquired respiratory tract infections in Pakistan- the active study. J. Ayub Med. Coll. Abbottabad., 20(1): 7-9


