Department of Biotechnology Sri Sathya Sai College for Women, Bhopal, India.
Corresponding author Email: orjitnandi@gmail.com
Article Publishing History
Received: 15/04/2019
Accepted After Revision: 25/06/2019
Symbiotic nitrogen fixation is a key to the nitrogen nutrition to the legumes. The most important agents for the symbiotic nitrogen fixation are the bacteria of the genus Rhizobium, which invade the root hairs of leguminous plant and develop nodules on the roots, where nitrogen fixation occurs.Rhizobium promotes growth of plants by fixing nitrogen from the atmosphere and is also a biocontrol agent which inhibits growth of pathogens. The biocontrol effect is due to the secretion of secondary metabolites.The present study describes the physiological, biochemical characterization and antagonistic activity of Rhizobium species were isolated from root nodules of leguminous plant. The Rhizhobium spp. were rod shaped, gram negative and mucous producing. Antifungal activity of Rhizobium spp. isolates were tested against three fungi which are potential phytopathogens on legumes. Inhibition zones were observed, hence Rhizobium spp. can be used as biocontrol agent.
Antagonistic activity, antifungal activity, Biocontrol effect, inhibition zone, Rhizobium spp.
Nandi R. G, Bara J. K, Shrivastava P. Antimicrobial Activity of Rhizobium Japonicum and Bradyrhizobium Japonicum on Different Plant Pathogenic Fungal Strains. Biosc.Biotech.Res.Comm. 2019;12(2).
Nandi R. G, Bara J. K, Shrivastava P. Antimicrobial Activity of Rhizobium Japonicum and Bradyrhizobium Japonicum on Different Plant Pathogenic Fungal Strains. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2IoUTFE
Copyright © Nandi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Chemicals used to control plant diseases contaminate the soil environment , degrade its fertility and also defile underground water, causing health risk. Thus, biocontrol agents emerge as an alternate to those antifungal chemicals, these are inexpensive, eco-friendly and have no harmful effects on human , animals and plants (Deshwal et al., 2003). Legumes establish a symbiotic interaction with soil bacteria termed as Rhizobia. These bacteria in association with legumes can fix atmospheric N and through this feature. Hence, they are introduced into agricultural systems to improve soil fertility, plant growth and limit the use of chemical fertilizers (Ouma et al., 2016 Srinivasan, 2017 Yassine, 2018).
Rhizobia not only fix nitrogen from atmosphere and supply it to plants but also promote the growth of plants. Rhizobia synthesize phyto-hormones and solubilization of minerals act as a biocontrol agent and can inhibit the growth of pathogens. Due to the secretion of secondary metabolites such as antibiotics and HCN by rhizobia, they have the capacity to restrict the growth of fungal pathogen. In iron stress conditions in rhizobia, siderophore production provides an added advantage, resulting in the exclusion of pathogen due to iron starvation. Rhizobacteria possessing all these features are also referred as plant growth promoting rhizobacteria (PGPR).Not only this but these bacteria also protect plants against flooding, drought, salt, flower wilting, metals, organic contaminants, and both bacterial and fungal pathogens, (Glick, 2014, Subramanium et al., 2015, Srinivasan, 2017 and Yassine, 2018).
Legumes fix atmospheric nitrogen only in association with a bacterial symbiont of the genus Rhizobium. Rhizobia have been arbitrarily divided into two classes: fast growing and slow growing. Normally, commercial cultivars of the soybean Glycine max are nodulated only by slow-growing Rhizobium japonicum (Keyser et al., 1982). The isolate appeared to be effectively nodulate only the wild soybean Glycine soja and an unbred soybean cultivar from China (G. max cv. Peking) Rhizobium japonicum 191 is a member of a salt-tolerant group of R. japonicum recently described by (Keyser et al., 1982).
Bradyrhizobium japonicum is a Gram negative bacterium belonging to rhizobia group associated with roots of soybean and have the capacity to fix N2 in the presence of nitrogenase enzyme (Baoling et al., 2007) .This bacterium and nitrogenase enzyme both are very sensitive for the environmental conditions. The commercially introduced strains must compete with highly adapted indigenous rhizobia for legume nodulation under specific physiological, biological and environmental soil conditions. Soil acidity limits symbiotic nitrogen fixation by limiting Rhizobium survival in soils, as well as reducing nodulation (Ibekwe et al, 1995). Chakraborty and Purkayastha, (1984) reported that some strains of Bradyrhizobium japonicum produce rhizobitoxine which can protect soybean crops from the infection of Macrophomina phaseolina, which is a charcoal rot fungus of leguminous crops. Rhizobium has shown to reduce root-rot of soybeans caused by Phytophthora megasperma. Rhizobial mechanism such as improvement in intake of plant nutrients by altering root morphology, production of siderophore (Antoun et al., 1998; Arora et al., 2001; Chabot et al., 1996; Srinivasan, 2017) to meet the iron requirement of the plant under iron stressed conditions and lowering of ethylene through ACC deaminase enzyme are some example with direct positive effects on non leguminious plant growth. B. japonicum strain inhibit the growth of seven pathogenic microorganisms causing disease in soybean was studied by (Balasundaram and Sarbhoy 1998) The fast growing rhizobial strains were found to completely inhibit the growth of white sclerotia of S. rolfsii. Different strains of Rhizobium and Bradyrhizobium have been reported to inhibit the growth of M. phaseolina (Deshwal et al., 2003; Arora et al., 1998). The rhizobia having antagonistic property showed more competency in root hair infection in host plants as compared to non- biocontrol rhizobia. Rhizobia also appear to influence the plant defense mechanism by stimulating the production of phytoalexins by plants. Antibiotics produced by rhizobia have been found to play an important role in disease control. HCN, a secondary metabolite produced by several microorganisms, has deleterious effect on the growth of some microbes (Knowles, 1996).
Studies conducted on numerous plant microbe interaction have shown that such antagonistic rhizobacteria could function by competition and antibiosis i.e. by producing antimicrobial compounds like bacteriocin (Rodelas et al., 1998; Joseph et al., 1983) but also indirectly induces systemic resistance against plant diseases. The enzyme system of bacteria during nodulation in the roots supplies constant source of reduced nitrogen to the host plant and the plant in turn provides nutrients and energy for the activities of the bacteria (Singh et al., 2008). It has also been evaluated that Rhizobium increases plant growth by various ways such as production of plant growth hormones, vitamins, siderophores, by solubilisation of insoluble phosphates, induction of systemic disease resistance and enhancement in stress resistance (Hussain et al., 2009; Yassine, 2018).
Some Rhizobium spp. have shown antimicrobial activities towards Pseudomonas spp. (Kacem et al., 2009) Aspergillus niger (Yuttavanichakul et al., 2012) .In the present study Rhizobium was isolated from the root nodules of soybean and its antagonistic activity was studied against pathogenic fungi such as Aspergillus niger and Fusarium oxysporum.
Material and Methods
Isolation of nitrogen fixing bacteria from soybean root nodules
Soybean plants were uprooted carefully from the soil so that intact roots can be obtained without destroying the nodules. Healthy soybean nodules were detached from the root and further isolation of root nodulating rhizobia was carried out (Vincent, 1970).
The separated root nodules were washed in tap water to remove the adhering soil particles from nodule surface. Fresh root nodules from soybean were collected and surface sterilized with 70% ethanol and 0.1% mercuric chloride and washed thrice with sterile distilled water. Root nodules were crushed in saline solution. Test isolate was isolated by spreading 0.1ml crushed root nodule suspension on YEM (Yeast extract mannitol, pH.7.0) agar plate and incubated at 36°C.Colonies of test isolate were observed in 2-3 days (Singh et al., 2008) were further used for morphological and biochemical characterization. To check the antibacterial activity, rhizospheric bacteria were isolated by serial dilution of soil. All the experiments were carried out in triplicates.Bradyrhizobium japonicum (RJ(s)TAL102) was collected from M.P. State Agro Industries, Bhopal.
Morphological characterization
Morphological characters such as shape, colour, size elevation, margin, opacity and gram staining were performed for identification of the bacteria (Gachande and Khansole, 2011) and for further biochemical test.
Biochemical characterization
All the tests were carried out with triplicates. For characterization of bacteria, the acid production test (Jordan, 1984), oxidase test (Kovaks, 1956), catalase test (Graham and Parker, 1964), methylene blue test, starch hydrolysis test (Oliveria et al., 2007), glucose peptone agar test (Kleczkowska et al., 1968), gelatin hydrolysis test (Sadowsky et al., 1983), growth in NaCl (Sadowsky et al., 1983), citrate utilization test gram staining and motility were performed.
Temperature tolerance
Effect of different physical parameters on the growth of test isolate was studied by keeping plates at different Differences in the range of growth temperatures were investigated by incubating bacterial cultures in YEM agar at 32°,34°,36°,38° and 40°C.
Antifungal activity
The antifungal effect of test isolate were evaluated against pathogenic fungi (Aspergillus niger, Alternaria alternate and Fusarium oxysporum) by filter paper method. Fluconazole antifungal was used as a positive control and saline as negative control. Bacterial suspension was made and filter paper was dipped into it and placed on the surface of assay plates labelled as Control. The plates were incubated for 24 – 48 hour at 28°C to observe antifungal activity and the zone of inhibition were recorded (Arfaoui et al.,2006).
Results and Discussion
The colonies isolated from roots of soybean were entire, opaque with regular margin, milky white, translucent, circular in shape, shiny, raised (convex), sticky consistency, musky colour of the colony and 2-4mm in diameter. The isolated bacterium was aerobic, non spore forming, pink coloured gram negative rods and motile. Rhizobium showed positive results for acid production test, catalase test, oxidase test ,starch hydrolysis test, glucose peptone agar test and NaCl test. Also the negative result were seen for gelatin hydrolysis and methylene blue test.The optimum temperature was 28°C.There is a gradual decrease in a colony count was observed after 40°C and growth was totally absent at temperature 44°C in broth.
Both the isolates of Rhizobium inhibited the growth of Aspergillus niger , Alternaria alternata and Fusarium oxysporum which is pathogenic fungi and affects on the yield of crop plants. The zone of inhibition (in mm) was recorded and measured.
The test isolates were identified as Rhizobium japonicum and Bradyrhizobium japonicum by morphological and biochemical characterization. These characteristics were found to be similar with the standard characteristics of the Rhizobium and thus this indicates that the isolated microorganisms are Rhizobium japonicum and Bradyrhizobium japonicum.
Table 1: Biochemical characteristics of the isolate
| TEST | REMARK | |
| Rhizobium japonicum | Bradyrhizobium japonicum | |
| Acid production test | +ve | +ve |
| Catalase test | +ve | +ve |
| Oxidase test | +ve | +ve |
| Methylene blue test | -ve | +ve |
| Starch hydrolysis test | +ve | +ve |
| Glucose peptone agar test | +ve | +ve |
| Gelatin hydrolysis test | -ve | +ve |
| NaCl test | +ve | +ve |
| Gram staining | Gram negative rod shaped | Gram negative rod shaped |
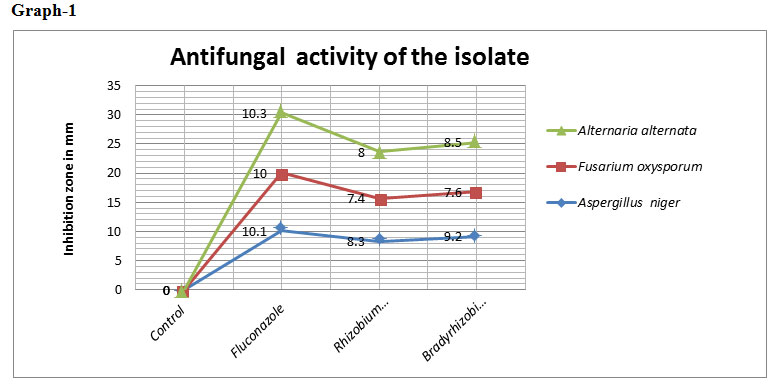
Table 2: Antifungal activity of the isolate
| S.no. | Treatments | Aspergillus niger | Fusarium oxysporum | Alternaria alternata |
| 1. | Rhizobium japonicum | 8.3 | 7.4 | 8.0 |
| 2. | Bradyrhizobium japonicum | 9.2 | 7.6 | 8.5 |
| 3. | Fluconazole | 10.1 | 10.0 | 10.3 |
| 4. | Control | 0 | 0 | 0 |
*Note: Inhibition zone measured in mm
 |
Graph 1 |
The zone of inhibition (in mm) recorded was 8.3 for Aspergillus niger ,7.4 for Fusarium oxysporum and 8 for Alternaria alternata from Rhizobium japonicum and for Bradyrhizobium japonicum the zone of inhibition was recorded 9.2 for Aspergillus niger , 7.6 for Fusarium oxysporum and for 8.5 Alternaria alternata.. Rhizobia have been reported to inhibit significantly the growth of Fusarium spp. and Aspergillus spp. (Srinivasan, 2107). Antifungal activity of Bradyrhizobium japonicum against Fusarium oxysporum has been reported by Mariastuti et al., (2018) that inhibition of Fusarium oxysporum by Bradyrhizobium japonicum ranged from 19% to 54.9%. Thus, indicating Rhizobium japonicum and Bradyrhizobium japonicum as a biocontrol agent.
Conclusion
The aim of the study was screening of Rhizobium spp. (Rhizobium japonicum and Bradyrhizobium japonicum) and determine its antifungal activity against Aspergillus niger , Alternaria alternata and Fusarium oxysporum causing various root rot diseases.In our investigation the antifungal activity of Rhizobium spp. were found to inhibit fungal growth showing inhibition zone, suggesting production of certain antifungal metabolites. Rhizobium spp. could be effectively used as a biocontrol agent in the form of bio-inoculant against fungal pathogen but enhancement in its antifungal properties would prove to be more efficacious. Therefore efforts are required to understand biocontrol mechanism of rhizobia against fungi. Genetic engineering approach can also be used to introduce the genes coding for the synthesis of antifungal and antimicrobial metabolites into rhizobial strains selected for use in biocontrol.
References
Antoun H., Beauchamp C.J., Goussard N., Chabot R. and Lalande R. (1998), Potential of Rhizobium and Bradyrhizobium species as growth promoting rhizobacteria on non – legumes: effect of radishes ( Raphanus sativus). Plant soil 204: 57-67.
Arfaoui A., Sifi B., Boudabous A., El-Hadrami I. and Cherif M. (2006). Identification of Rhizobium isolates possessing antagonistic activity against Fusarium oxysporumSPP. Ciceris, The causal agent of Fusarium wilt of Chickpea. J. Pl. Pathol. 88(1): 67-75.
Arora N. K., Kang S. C. and Maheshwari D. K., (2001) Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci, 81:673-677.
Balasundaram V.R. and Sarbhoy A.K., (1988) Inhibition of plant pathogenic fungi by Rhizobium japonicum. Indian Phytopathol, 128.
Baoling H., Chengquon L., Bo W., and Liqin F. (2007) A rhizobia strain isolated from root nodule of gymnosperm Podocarpusm acrophyllus.Chin. Ser C-Life Sci.;50; 1-6.
Chabot R., Antoun H.,and Cescas, M.P. (1996) A growth promotion of maize and lettuce by phosphate – solubilizing Rhizobium leguminosarum biovar phaseoli .Plant and soil, 184 :311- 321.
Chakraborty U. and Purkayastha R.P., (1984), Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina, Can J Microbiol, 30:285.
Deshwal V. K., Dubey R. C. and Maheshwari D.K, (2003) Isolation of plant growth-promoting strains of Bradyrhizobium (Arachis) with biocontrol potential against Macrophomina phaseolina causing charcoal rot of peanut, Curr Sci, 84:443.
Deshwal V.K., Pandey P., Kang S.C. and Maheshwari D.K.,(2003), Rhizobia as a biological control against soil borne plant pathogenic. Indian Journal of Experimental Biology 41:1160-1164.
Gachande B.D. and Khansole G.S. (2011). Morphological, cultural and biochemical characteristics of Rhizobium japonicum and Bradyrhizobium japonicum of Soybean. Biosci. Discov. 2(1): 1-4.
Glick B.R. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res.;169: 30–39.
Graham P.H. and Parker C.A. (1964). Diagnostic features in the characterization of root nodule bacteria of legumes. Pl. Soil. 20: 383-396.
Hussain M.B., Mehboob I., Zahir Z.A., Naveed M. and Asghar H.N. 2009. Potential of Rhizobium for improving growth and yield of rice (Oryza sativa L.). Soil Environ. 28(1): 49-55.
Ibekwe A.M., Angle J.S., Chaney R.L.,Van Berkum P. (1995) Sewage sludge and heavy metal effects on nodulation and nitrogen fixation of legumes. J Environ. Quality, vol. 24, , no. 6, p. 1199-1204.
Jordan D.C. (1984). Family ll Rhizobiaceae in Bergeys manual of systematic bacteriology, Vol. l (eds. By N. R. Krieg and J.G. Holt Williams and Wilkins Co. Baltimore M.D.), pp.232-242
Joseph M. V., Desai J. D. and Desai A. J. (1983) Production of Antimicrobial and Bacteriocin-Like Substances by Rhizobium trifolii. Appl .Environ. Microbiol .45(2): 532–535.
Kacem M., Kazouz F.,Merabet C., Rezki M., de Lajudie P. And Bekki A. (2009) Antimicrobial activities of Rhizobium strains against Pseudomonas savastanoi the agent responsible for the olive knot disease in Algeria Grasasaceites, 60(2):139-146
Keyser H. H., Bohlool B. B., Hu T. S., and Weber D. F.(1982). Fast-growing rhizobia isolated from root nodules of soybean. Science 215:1631-1632.
Kleczkowska J., Nutman P.S., Skinner F.A. and Vincent J.M. (1968). The identification and classification of Rhizobium. Identification Methods of Microbiologists, Part B, (eds. Fibbs B. M. &Shapton D. A., London), pp.51-65
Knowles C.J., (1996) Microorganism and cyanide, Bacteriol Rev, 40: 652.
Kovaks N. (1956). Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 178: 703
Mariastuti H.D., Listiyowati S., Wahyudi A. T., 2018, Antifungal activity of soybean rhizosphere actinomycetes producing bioactive compounds against Fusarium oxysporum. 19 (6): 2127-2133.
Oliveira A.N., Oliveira L.A., Andrade J.S. and Junior A.F.C. (2007). Rhizobia amylase production using various starchy substances as carbon substrates. Braz. J. Microbiol. 38(2): 208-216.
Ouma EW, Asango AM, Maingi J, Njeru EM. 2016. Elucidating the potential of native rhizobial isolates to improve biological nitrogen fixation and growth of common bean and soybean in smallholder arming systems of Kenya. International Journal of Agronomy: 1-7.
Rodelas B., González,López J. , Salmerón V., Martı́nez-Toledo M.V., Pozo C. (1998) Symbiotic effectiveness and bacteriocin production by Rhizobium leguminosarum viceae isolated from agricultural soils in Spain .Applied Soil Ecology Vol 8; 51-60.
Sadowsky M.J., Keyser H.H. and Bohlool B.B. (1983). Biochemical characterization of fast and slow growing rhizobia that nodulate soybean. Int. J. Syst. Bacteriol. 33: 716-722.
Singh B., Kaur R. and Singh K. 2008. Characterization of Rhizobium strain isolated from the roots of Trigonella foenumgraecum (fenugreek). Afri. J. Biotechnol. 7(20): 3671-3676.
Srinivasan T. Studies on Antifungal Activity of Siderophores Produced by Rhizobium spp Isolated from Groundnut (Arachis hypogaea). Journal of Agricultural Science and Food Research, 8:4.
Subramaniam G., Arumugam S., Rajendran V., Rajeev K.V., Gowda L. L., and Lakshmanan K., 2015. Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech; 5(4): 355–377.
Vincent J.M. (1970). A manual for the practical study of root nodule bacteria. IBP Handbook No. 15. Blackwell publication, oxford, U.K.
Yassine M., Imen H., Issam B.S., Sonia M., Mouldi S. and Omrane B. (2018) .Potential of Rhizobia in Improving Nitrogen Fixation and Yields of Legumes. Intech Open Science Open Mind.
Yuttavanichakul W., Lawonga P., Wongkaew S., Teaumroong N., Boonkerd N. and Tittabutr P. (2012) Improvement of peanut rhizobial inoculant by incorporation of plant growth promoting rhizobacteria (PGPR) as biol control against the seed borne fungus ,Aspergillus niger. Biologica Control 63:87-97.


