School of Biological Engineering & Life Sciences, Shobhit Institute of
Engineering & Technology, Modipuram, Meerut, Uttar Pradesh, India
Corresponding author email: amarprakashgarg@yahoo.com
Article Publishing History
Received: 16/01/2022
Accepted After Revision: 25/03/2022
Vinegar-treated eatables are widely used to improve digestion and are also known for their antimicrobial activity. The evaluated antimicrobial activity of apple cider vinegar (ACV) treated and untreated eatables-ginger (Zingiber officinale), garlic (Allium sativum), onion (Allium cepa), raw papaya (Carica papaya), white radish (Raphanus sativus) and green chilli (Capsicum annum) were analysed against selected common food borne pathogens named Escherichia coli (ATCC8739), Bacillus subtilis, Staphylococcus aureus (ATCC 6539), Shigella flexneri (ATCC 12022), Salmonella typhi (ATCC 14028), Cronobacter sakazakii (ATCC 29544), Vibrio parahaemolyticus (ATCC 17802) and V. cholera (ATCC 3906) using agar well diffusion technique. Different methods for extraction of phytochemicals have been compared. The eatables were soaked in water for 24 hours, then followed by centrifugation which yielded highest number of phytochemicals. All untreated eatables showed high to moderate antimicrobial activities against all test pathogens, while ACV-treated showed higher antimicrobial activities.
Antimicrobial activity, Apple Cider vinegar, Eatables, Extraction Methods, Phytochemicals.
Singh J, Garg A. P. Antimicrobial Activity of Apple Cider Vinegar Treated Selected Vegetables Against Common Food Borne Bacterial Pathogens. Biosc.Biotech.Res.Comm. 2022;15(2).
Singh J, Garg A. P. Antimicrobial Activity of Apple Cider Vinegar Treated Selected Vegetables
Against Common Food Borne Bacterial Pathogens. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3yZfj43“>https://bit.ly/3yZfj43</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Eatables like ginger (Zingiber officinale), garlic (Allium sativum), onion (Allium cepa), white radish (Raphanus sativus), raw- papaya (Carica papaya), and green chili (Capsium annum) are commonly used in different household preparations with and without vinegars, and also in various food products within the industry. Apple cider vinegar (ACV) is known to possess a wide range of biological activities that include antimicrobial, antioxidant, anti-diabetic, anti-inflammatory, anti-hypertensive, immune-stimulatory, anticancer and others (Kalaba et al. 2019; Benedek et al. 2022). Ginger (Zingiber officinale) which belongs to the family-Zingiberaceae, is cultivated for its medicinal and spices/condiment purposes. This spice is also useful in the treatment of common cold, headache, muscular and rheumatic disorders. Ginger has a distinct spicy flavour and a pleasant aroma. The specific fragrance is attributed to its essential oil which is approximately up to 3% (Hara et al. 1998; Unuofin et al. 2021).
Gingerol is the main bioactive compound in ginger. This spice also contains zingerone, shogaol, gingerol, paradol, β-phellandrene, curcumene, cineol, geranyl acetate, terpineol, terpene, borneol, geranyl, limonene, zingiberol, linalool, α- zingiberene, β-sesquiphellandrene, β-bisabolene, zingiberenoland α-farnesene (Manuhara et al. 2018; Yan et al. 2021; Benedek et al. 2022). Ginger has high amounts of antioxidants including phenolic compounds, alanine, and vitamin C. Due to this, ginger is often used by boiling in water to be consumed as a beverage. These antioxidant compounds have an important role in maintaining human health. Besides this, the antioxidants are also widely used as food additives to prevent food damage and add value to the food (Tsai et al. 2005; Habinshuti et al. 2018; Priya et al. 2019; Yan et al. 2021). White Radish (Raphanus sativus) belongs to the family- Brassicaceae. It helps in weight loss and increases the metabolism for improved bodily processes (Gamba et al. 2021).
Similarly, white radish helps in treatment of colon, kidney, intestinal, stomach and oral cancers, it also improves the immunity, removes mucous from throat and fight cold and cough. Onion (Allium cepa) belongs to the family- Liliaceae. Onion has recorded 6000 years BC old roots and is widely used for its several minerals and vitamins. Onion is also used as a medicine. Available research ahs shown that it is better to use raw onion because after boiling it loses its medicinal properties (Elisabetsky 1991; Sami et al. 2021; Benedek et al. 2022).
Papaya (Carica papaya) belongs to the family Caricaceae. It is a rich source of antioxidant and vitamin (A, B, C and E) and some minerals including magnesium and potassium. Papaya has medicinal properties that makes it effective against dyspepsia, hyperacidity, dysentery and constipation. It is also useful in digestion of proteins because it has high proteolytic enzymes (Dawson and Emma 1997; Parin 2020). Chilli (Capsicum annum) belongs to “Solanaceae” family and is rich in vitamins, especially vitamin C and is known for its characteristic non-pungent and pungent taste (Brito-Argaezet al. 2009; Batiha et al. 2020).
Various enteric pathogens are known to adversely affect human health and cause several intestinal diseases. However, these are constituent part of the normal gut microflora of small intestine and act as opportunistic pathogens that are responsible for wide range of infections. E. coli is a Gram’s-ve, facultative anaerobe, rod-shaped, coliform bacterium, commonly found in lower intestine of healthy people and animals. Few strains of E. coli (O157:H7) produces a powerful toxin that damages the lining of the small intestine, which can cause bloody diarrhoea (Mead et al. 1999; Braz et al. 2020; Haindongo et al. 2022).
Salmonella typhi is also a Gram-ve, rod-shaped, flagellated bacterium which causes systemic infection and typhoid fever in humans. It is transmitted through food and water. Shigella is another Gram’s -ve, facultative anaerobe, non-spore forming, non-motile, rod shaped and a leading bacterial pathogen cause of diarrhoea. The most common symptoms are fever, nausea, vomiting, stomach cramps and flatulence. It is also commonly known to cause large and painful bowel movements. Many commercial antibiotics like Azithromycin (Zithromax), Penicillin, Amoxicillin, Trimethoprim, Ciprofloxacin etc. are being used to fight against such enteric pathogens, but use of such antibiotics is not a good choice due to their high cost, multi-drug resistance and side effects e.g., amikacin and gentamycin used against E. coli leads to hearing loss, vertigo and kidney damage (Lewis et al. 2012; Haindongo et al. 2022).
The aim of this study was to determine the antimicrobial activity of untreated and apple cider vinegar treated ginger (Zingiber officinale), garlic (Allium sativum), onion (Allium cepa), white radish (Raphanus sativus), raw-papaya (Carica papaya) and green chilli (Capsicum annum) extracts on different pathogenic bacteria to find out their beneficial uses and to carry out their phytochemical screening of the extracts so as to evaluate the impact of vinegar treatment on their antimicrobial activities.
MATERIAL AND METHODS
All eatables were collected from the local market of Meerut (Uttar Pradesh) India and washed well to clean the soil particles, peeled off and were further washed thoroughly again with clean water. After washing, these were dried under sunlight for 2-3 days and 5 g of each material was soaked in 25 mL of distilled water and 25 mL of apple cider vinegar (ACV) separately for one week. After soaking, these were crushed. Phytochemical analysis and antimicrobial activities were evaluated.
For the physical evaluation of eatables, Evaporable Moisture Content and Total Ash Content were calculated. Evaporable Moisture Content was determined by subtracting the fresh weight from sun dried mass. For Total Ash Content, weighed number of dried eatables was heated at 550°C temperature for 6 h in muffle furnace and the ash content was determined using following formulae:
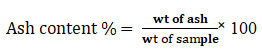
Different methods were used for extraction of phytochemicals from ginger and finally the best was followed for rest eatables. For Soxhlet Method, 5 g dry powder of ginger was dissolved/suspended in 500 mL of distilled water and kept at 70°Cfor 6 to 7 h to evaporate the water, the pellet was dissolved in sterile distilled water to make final volume of 25 mL.
For Heating Method with Filtration, 5g powder of ginger was dissolved/suspended in 25mL of distilled water and heated till boiled, mixed properly and filtered through normal filter paper, final volume was adjusted to 25mL. For Heat without Filtration: 5g powder of ginger was dissolved/suspended in 25 mL of distilled water and heated till boiled, kept for 2 h and just decanted, final volume of extract was adjusted to 25 mL. During Simple Soaking followed by Centrifugation, 5g ginger powder was soaked in 15 mL of distilled water over night and was simply centrifuged at 2000rpm for 15 min.
The extraction procedure was repeated 3 times, each time with 3 mL water and finally all extracts were mixed and final volume of 25 mL was made. Besides this, the antimicrobial activity of different eatables with or without apple cider vinegar (ACV) was evaluated using agar well diffusion method on Müller Hinton agar. The plates were incubated in an upright position at 37±1℃ for 24 to 48 h. and the diameter of zone of inhibition (in mm) was measured against test human pathogens. Lastly, Qualitative standard chemical tests were carried out for phytochemical screening of untreated and ACV- treated eatables using AOAS protocol as described (Trease and Evans 1978; Rawat and Garg 2021).
RESULTS AND DISCUSSION
Moisture evaporated by sundry method revealed that sun dry weight of garlic was highest followed by onion, green chili and ginger (Fig 1) while the ash content of ginger was highest followed by garlic, white radish and onion. It shows that ginger, garlic and white radish contain high amounts of inorganic minerals. Chili had lowest ash content (1.2% only) which suggests that it has lesser mineral contents but its other contents contributes to medicinal and digestive value (Batiha et al. 2020). White radish had highest evaporable moisture content showing its greater and easy availability during digestion (Gamba et al. 2021). On comparison of phytochemical composition of ginger extracted by Soxhlet method, heating with and without filtration, and simple soaking followed by centrifugation, it was found that latter method yielded highest amount of all phytochemicals followed by heating without filtration and Soxhlet method showed poor results (Table1) (Gamba et al. 2021).
It may be due to the loss of vital phytochemicals by evaporation during Soxhlet procedure. Overnight soaking followed by centrifugation avoided the heat treatment which allowed greater release/leaching of phytochemicals. It, was therefore, suggested that overnight soaking followed by centrifugation should be used for phytochemical analysis of biomaterials. Each phytochemical was analyzed using two different tests to ensure their presence or absence in untreated and ACV- treated eatables and the results (Table 2, Fig. 2) revealed that ACV- treated ginger showed tannins; onion and raw papaya possessed terpenoids and green chili gave positive test for saponins while these phytochemicals were absent in the untreated vegetables.
It was also found that no phytochemical was lost due to apple cider vinegar treatment and ACV treatment improved the nutritive value of the vegetables (Benedek et al. 2022).
To assess the antimicrobial activity of untreated and ACV- treated eatables, agar well diffusion assay was used against Escherichia coli, Pseudomonas aeruginosa, Staphyloccus aureus, Shigella flexneri, Salmonella typhi, Cronobacter sakazakii, Vibrio parahaemolyticus, Bacillus subtilis and Vibrio cholera (Table 3, Fig. 3, 4) and comparison of their antimicrobial activity showed that ACV-treated ginger should not be used if suffering from typhoid fever as its treatment reduced antimicrobial activity of ginger against Salmonella typhi (Table 3). ACV-treated green chili either reduced or showed little effects on antimicrobial activity of almost all vegetables. It suggests that green chilies should not be eaten after treatment with apple cider vinegar. ACV-treated white radish and raw papaya generally enhanced antimicrobial activity against all test species except Pseudomonas aeruginosa where it showed little or no effects. ACV-treated onion showed reduced antimicrobial activity against E. coli which suggests that apple cider vinegar-treated onion should not be eaten by a person suffering from colitis.
The presence of medicinally active constitutes like flavonoids, alkaloids, saponin, tannins, anthraquinones, terpenoids and glycosides in untreated and ACV-treated eatables determines their nutritive value and antimicrobial activity (Manuhara et al. 2018; Benedek et al. 2022).
The present study shows that the extracts of eatables possess antimicrobial compounds and are beneficial for health. Vinegars are commonly used as food condiment and preservatives. Apple cider vinegar is also used in the Ayurvedic pharmaceutical industry because of its medicinal properties. Ginger is well known for its antimicrobial activity and is widely used in Ayurveda for various treatments and also in food industry for flavor and aroma (Rajsekhar 2012; Kalhoro et al. 2022). Antibacterial activity of some leafy vegetables has been reported in previous studies against S. aureus, S. pyogenes, B. subtilis, E. coli and P. aeuginosa (Bhat and Al-Daihan 2014; Mahendranathan and Abhayarathne 2021).
Fruits and vegetable are also known for their antimicrobial activity and papaya, potato, cucumber, beet root and ginger have been found to inhibit E. coli, S. aureus, Lactobacillus, and Proteus vulgaris (Narinder et al. 2017; Kumar et al. 2021). Antimicrobial activity of fermented vegetable byproducts of some vegetable has been evaluated in a previous study which showed that fermented extracts of tomato, melon and carrot possessed higher antimicrobial activity than commercial preservatives against Salmonella spp., Lysteria monocytogenes and B. cereus (Ricci et al. 2021).
Hossaini et al. (2020) have evaluated antimicrobial effects of medically relevant green leafy vegetables and have found that ethanolic and methanolic extracts of Azadiracta indica, Coccinia grandis, Ipomoea aquatica and Paederia foetida leaves extracts possess antimicrobial activity against Staphylococcus spp., Klebsiella spp., and Pseudomonas spp., while crude and hot water extract showed almost no effect on bacterial growth (Hossaini et al. 2020; Ricci et al. 2021). Antimicrobial activity of vinegar is attributed to the phenolics, organic acids, microbial metabolites of the fermenter organism as well as its high acid component and has been found effective against E. coli, P. aeruginosa, S. aureus using agar well diffusion technique (Yagnik et al. 2018; Ousaaid et al. 2021; Benedek et al. 2022).
Antimicrobial activity of ginger extract against P. aeruginosa, S. aureus, Proteus mirabilis, E. coli, B. subtilis and S. typhi has been demonstrated in previous studies (Akintobi et al. 2013; Unuofin et al. 2021). In view of the increasing demand of natural products with health promoting attributes, the antimicrobial activity of grape vinegars against S. aureus, E. coli and Candida albicans is reported in the previous study (Antoniewicz et al. 2021). Our results reveal that apple cidar vinegar -treated white radish and raw papaya should be used while ACV-treated ginger should be avoided when suffering from typhoid fever and similarly ACV-treated onion should not be consumed in colitis. Green chili should not be treated with ACV at all and should be used raw (Benedek et al. 2022).
Figure 1: Percent moisture, sun dry and ash contents of eatables used.
Figure 2: Qualitative phytochemical tests for analysis of various groups from eatables

Table 1. Comparison of different extraction methods for estimation of various phytochemical groups using ginger as a model.
| Extraction methods | Phytochemical tests | ||||||
| Flavonoid | Alkaloid | Tannins | Saponins | Anthraquinone | Terpenoid | Glycoside | |
| Soxhlet method | + | + | _ | ++ | _ | + | ++ |
| Heat filtration method | ++ | + | _ | + | _ | ++ | +++ |
| Heat without filtration method | +++ | ++ | _ | +++ | _ | ++ | + |
| Maceration method | ++ | + | _ | ++ | _ | + | ++ |
| Centrifuge method | ++++ | +++++ | _ | +++++ | _ | ++++ | +++++ |
Table 2. Phytochemical analysis of various biomolecules from apple cider vinegar (ACV) treated
and untreated eatable in water extract (WE) using centrifugation method.
|
Phytochemicals |
Eatable’s extract | |||||||||||
| Ginger | Garlic | Onion | Raw papaya | Green chilli | White radish | |||||||
| WE | ACV | WE | ACV | WE | ACV | WE | ACV | WE | ACV | WE | ACV | |
| Alkaloids | + | + | + | + | + | + | + | + | + | + | + | + |
| Saponins | + | + | + | + | + | + | + | + | – | + | + | + |
| Tannins | – | + | + | + | + | + | + | + | + | + | + | + |
| Flavonoids | + | + | + | + | + | + | + | + | + | + | + | + |
| Anthraquinones | – | – | + | + | + | + | – | – | + | + | + | + |
| Terpenoids | + | + | + | + | – | + | – | + | + | + | + | + |
| Glycosides | + | + | + | + | + | + | + | + | + | + | + | + |
Table 3. Zone of inhibition (mm in diam.) exhibited by water extracts (WE) of and apple cider vinegar (ACV) treated eatable’s extracts.
| Test pathogen
|
Garlic | Ginger | Onion | White radish | Green chilli | Raw papaya | |||||||
| WE | ACE | WE | ACE | WE | ACE | WE | ACE | WE | ACE | WE | ACE | ||
| Escherichia coli | 9.5 | 10 | 7.5 | 9 | 7.7 | 8 | 10 | 13.5 | 9 | 10.5 | 9 | 10.5 | |
| Pseudomonas aeruginosa | 7.0 | 7.5 | 7.75 | 10 | 9.5 | 11.25 | 8.25 | 7 | 6.0 | 7.25 | 9 | 10.25 | |
| Staphylococcus aureus | 6.5 | 7 | 8.25 | 10.75 | 7.72 | 12.25 | 9.0 | 11.25 | 8 | 8.5 | 7 | 7 | |
| Shigella flexneri | 9.0 | 11.25 | 9.25 | 12 | 10.25 | 12 | 7.0 | 12.25 | 7.94 | 9.25 | 8.5 | 11.75 | |
| Salmonella typhi | 9.5 | 12 | 7.57 | 9.75 | 7.0 | 11.25 | 8 | 8.25 | 7.68 | 9.75 | 8.5 | 8 | |
| Cronobacter sakazakii | 10.25 | 10.75 | 7.5 | 7.75 | 8.75 | 7.75 | 10.65 | 12.25 | 7 | 7.75 | 9 | 9.75 | |
| Vibrio parahaemolyticus | 8.5 | 11.75 | 9 | 10.75 | 7.5 | 12 | 8.8 | 8.5 | 8.23 | 8.25 | 9 | 12 | |
| Bacillus subtilis | 8.5 | 9 | 9 | 9 | 8.25 | 8.25 | 7.89 | 9.5 | 7.5 | 9.25 | 9 | 9.25 | |
| Vibrio cholera | 7 | 8.75 | 6.5 | 9.5 | 6 | 10.5 | 8.25 | 7.75 | 6 | 8.25 | 8 | 9.25 | |
Figure 3: Zone of inhibition of water extract of different eatables: ginger (Gi), garlic (Ga), raw papaya (Rp) and onion (O) against Vibrio parahaemolyticus plate A; Shigella flexneri plate B; Pseudomonas aeruginosa plate C and Bacillus subtilis plate D.
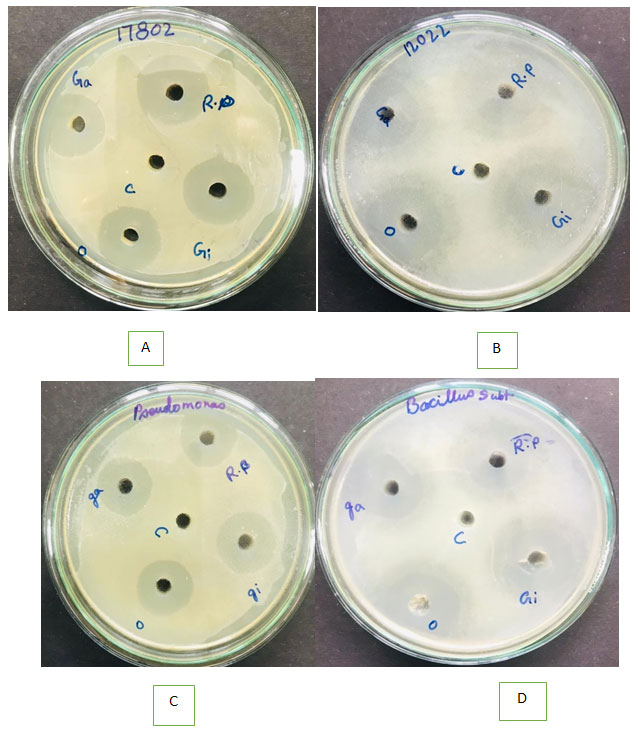
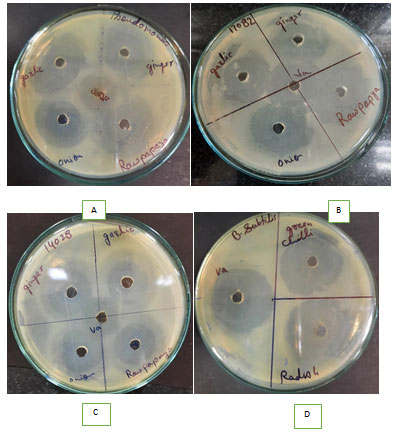
Figure 4: Zone of inhibition of Apple cider vinegar extract of different eatables: ginger, garlic, raw papaya and onion against Pseudomonas aeruginosa plate A, Vibrio parahaemolyticus plate B, Salmonella typhi plate C and Bacillus subtilis plate D and Va denotes apple cider vinegar used as control.
CONCLUSION
The findings of the present study have concluded that overnight soaking followed by centrifugation yields better quality of phytochemicals. Ginger, garlic, onion, raw papaya, green chili and white radish possess high antimicrobial activities against Escherichia coli, Pseudomonas aeruginosa, Staphyloccus aureus, Shigella flexneri, Salmonella typhi, Cronobacter sakazakii, Vibrio parahaemolyticus, Bacillus subtilis and Vibrio cholera and their treatments with apple cidar vinegar improves their antimicrobial activity. ACV- treated white radish and raw papaya should be used while ACV-treated ginger should be avoided in typhoid fever. ACV-treated onion should not be consumed in colitis. Green chili should not be treated with ACV and should be used raw.
ACKNOWLEDGEMENTS
The study was financially supported by the Shobhit Institute of Engineering & Technology (Deemed to-be- University), Modipuram, Meerut (UP) India. Also, the research grade pure vinegar was provided by the firm Vigour of Village Natural Vinegar, Village Food Products, Saradhana, Meerut, Uttar Pradesh.
Conflict of Interests: Authors declare no conflict of interests to disclose.
REFERENCES
Akintobi, OA, Onoh, CC , Ogele, JO et al. (2013). Antimicrobial activity of Zingiber officinale (Ginger) extract against some selected pathogenic bacteria. Nature and Science Vol 1 No1 pages7-15.
Antoniewicz, J, Jakubczyk, K, Kwiatkowski, P et al. (2021). Analysis of antioxidant capacity and antimicrobial properties of selected Polish grape vinegars obtained by spontaneous fermentation. Molecules Vol 26 Pages 4727, Doi: https://doi.org/10.3390/molecules26164727
Batiha, GES, Alqahtani, A, Ojo, OA, et al. (2020). Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. International Journal of Molecular Sciences Vol 21(15) Pages 5179. doi:10.3390/ijms21155179
Benedek, CS, Szakolczi1, O, Makai, G et al. (2022). Evaluation of physicochemical, sensory, and antimicrobial properties of small-scale produced fruit vinegars. Acta Alimentaria Vol 51 No.1 Pages 1–10 DOI: 10.1556/066.2021.00077
Bhat, RS and Al-Daihan, S (2014). Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pacific Tropical Journal of Biomedicine Vol 4 No.3 Pages 189-193, Doi: 10.1016/S2221-1691(14)60230-6
Braz, VS, Melchior, K and Moreira, CG, (2020). Escherichia coli as a multifaceted pathogenic and versatile bacterium. Frontiers in Cellular and Infection Microbiology Pages 793 https://doi.org/10.3389/fcimb.2020.548492
Brito-Argaez L, Moguel -Salazar F, Zamudio F et al. (2009). Characterization of Capsicum Chinese Seed Peptide Fraction with Broad Antibacterial Activity. Asian Journal of Biochemistry Vol 4 Pages 77-87.
Dawson, E (1997). The Medicinal Properties of the Papaya, Carica papaya L. (online). at: http://www.siu.edu/-ebl//
Elisabetsky, E, (1991). Sociopolitical, economical and ethical issues in medicinal plant research. Journal of Ethnopharmacology, Vol 32(1-3), Pages 235-239. Doi: https://doi:org/10.1016/0378-8741(91)90124-v
Gamba, M, Asllanaj, E, Raguindin, PF, et al. (2021). Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends in Food Science & Technology, Vol 113, Pages 205-218. https://doi.org/10.1016/j.tifs.2021.04.045
Guna, PJ, Geetha, L, Sharma, K, et al. (2019). A Comparative Study of Antioxidants and Antimicrobial activity in Vegetables, Leafy Vegetables and Spices, International Journal of Scientific Research in Biological Sciences Vol 6 No1 Pages 80-85.
Janvier, H, Sabine, IA, Colores, U et al. (2018). Antimicrobial and Antifungal Activity of Three Selected Homegrown Vegetables Consumed in Rwanda. European J Med Plants. DOI: 10.9734/EJMP/2018/40226
Haindongo, EH, Funtua, B, Singu, B, et al. (2022). Antimicrobial resistance among bacteria isolated from urinary tract infections in females in Namibia, 2016–2017. Antimicrobial Resistance & Infection Control, 11(1), pp.1-8. doi.org/10.1186/s13756-022-01066-2
Hossaini, F, Munshi, SK and Chakraborty, M (2020). Antimicrobial effects of different extracts of medicinally used green leafy vegetables collected from local market of Dhaka, Bangladesh. Food Research Vol 4 No 3 Pages 860-865. Doi: https://doi.org/10.26656/fr.2017.4(3).017
Kalaba, V, Marjanovic, BZ and Kalaba, D (2019). Antibacterial activity of apple cider vinegar. AGROFOR International Journal Vol 4 No 1 Pages 24-31. Original scientific paper: 10.7251/AGRENGI 1901024K; UDC 613.24:661.741.1:634.11
Kalhoro, MT, Zhang, H and Kalhoro, GM (2022). Fungicidal properties of ginger (Zingiber ofcinale) essential oils against Phytophthora colocasiae. Scientifc Reports Vol 12 Pages 2191-2200. https://doi.org/10.1038/s41598-022-06321-5
Kumar, A and Sharma, VD (1982). Inhibitory effect of garlic (Alium sativum Linn.) on enterotoxigenic Escherichia coli. Indian Journal of Medical Research Vol 76 Pages 66-70 PMID:6764456
Kumar R, Chhatwal S, Arora S, et al. (2013). Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase–lowering effects of garlic in patients with type 2 diabetes mellitus with obesity Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy Vol. 6 Pages 49–56. Doi: https://doi:10.2147/DMSO.S38888.Epub 2013 Jan 19
Kumar, H, Bhardwaj, K, Cruz-Martins, N et al. (2021). Applications of fruit polyphenols and their functionalized nanoparticles against foodborne bacteria: A mini review. Molecules Vol 26 Pages 3447. https://doi.org/10.3390/molecules26113447
Lewis, K (2012). Antibiotics: Recover the lost art of drug discovery. Nature. Vol 4 Pages 439-440. Doi: htpps://doi:10.1038/485439a
Mahendranathan, C and Abhayarathne, A (2021). Antibacterial activities of some medicinal plants and green leafy vegetable: A Review. International Journal of Engineering Applied Sciences and Technology Vol 6 No 4 Pages 78-84. http://www.ijeast.com
Manuhara, GJ, Mentari, GP, Khasanah, LU et al. (2018). Aqueous Extract Composition of Spent Ginger (Zingiber officinale var. Amarum) from Essential Oil Distillation International Conference on Advanced Materials for Better Future 2017 IOP Publishing IOP Conf. Series: Materials Science and Engineering 333 pages 1-5 012069. doi:10.1088/1757-899X/333/1/012069
Mead, PS, Slutsker, L, Dietz, V, et al. (1999). Food-Related Illness and Death in the United States. Emerging Infectious Diseases Vol 5 (5), Pages 607-25. Doi: https: doi:10.3201/eid0505.990502
Omoya FO and Akharaiyi, FC (2012). Mixture of Honey and Ginger Extract for Antimicterial. Assessment on Some Clinical Isolates. International Research Journal of Pharmaceuticals Vol 2 No 5 Pages 127-132: corpus ID:31374206
Ousaaid, D, Hamada, I, Hassan, L et al. (2021). An investigation of Moroccan vinegars: Their physicochemical properties and antioxidant and antibacterial activities. Journal of Food Quality Article ID 6618444, http://doi.org/10.1155/2021/6618444.
Narender, BR, Rajakumari, M, Sukanya B et al. (2017). Antimicrobial activity of peels of different fruits and vegetables. Journal of Pharma Research Vol 7 No 1 Pages 1-7Doi: https://doi.org/10.5281/zenodo.1133694
Rajsekhar, S, Kuldeep, B, Chandakar, A et al. (2012). Spices as antimicrobial agents: A Review. International Journal of Pharmacy Vol 3 No 2 Pages4-9.
Rawat, B and Garg, AP (2021). Characterization of phytochemicals isolated from Cucurbita pepo seeds using UV-VIS and FTIR spectroscopy. Plant Archives Vol 21 No1 Pages 892-899.
Ricci, A, Bertani, G, Maoloni, A et al. (2021). Antimicrobial activity of fermented vegetable by product extracts for food applications. Foods Vol 10 Pages 1092. Doi: https:// doi.org/10.3390/foods10051092
Sami, R, Elhakem, A and Alharbi, M (2021). Nutritional values of onion bulbs with some essential structural parameters for packaging process. Applied Science Vol 11 Pages 2317-2328 https://doi.org/10.3390/app11052317
Sidat PSA, Varachia AIB and Vanshiya SKB (2020). Carica papaya leaves: One of dynamic plant parts having multiple therapeutic activities. Himalayan Journal of Health Sciences Vol 5 No 3 Pages 37-48. DOI 10.22270/hjhs.v5i3.67
Trease, GE and Evans, WC (1978). Pramacognosy. Bailliere Tindall Ltd. London, Vol 11, Pages 78.
Unuofin, JO, Masuku, NPP and Oluwatomiwa K (2021). Ginger from farmyard to town: nutritional and pharmacological applications. Frontiers in Pharmacology Vol 12 Article 779352, https://doi.org/10.3389/fphar.2021.779352
Yagnik, D, Serafn,V and Shah, AJ (2018). Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression Scientific Reports Vol 8 Pages 1732-1744 | DOI:10.1038/s41598-017-18618-x
Yan, H, Zou, D, Zhou G et al. (2021). Metabolomics of ginger based on ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry technology. Food Quality and Safety Vol 5 Pages 1–9 doi:10.1093/fqsafe/fyaa036


