Laboratory of Microbiology and Experimental Medicine, Department of Zoology, University of Gour Banga, Malda-732103, India
Corresponding author email: samtropmed@gmail.com
Article Publishing History
Received: 13/10/2019
Accepted After Revision: 30/11/2019
Fighting bacterial antibiotic resistance is a great challenge, and the researchers are in search of alternative therapies, the effective antibacterial biotherapeutics, in particular. This research aims to explore the antibacterial potentiality of pomegranate (Punica granatum) fruit peel extracts against gram-negative pathogenic bacteria having high MAR (multiple antibiotic resistance) indices. A total of 17 gram-negative pathogenic bacteria: Escherichia coli (n=5), Proteus spp. (n=4), Klebsiella pneumoniae (n=2), Pseudomonas aeruginosa (n=3), Acinetobacter baumannii (n=3), were subjected to susceptibility testing by disc diffusion method using 15 antibiotics, and the MAR indices were calculated. The antibacterial activities of APE (pomegranate fruit peel aqueous extract) and PEE (pomegranate fruit peel ethanolic extract), for the test bacteria, were determined by disc diffusion, while agar dilution technique was followed to determine the MIC (minimum inhibitory concentration) values of the extracts. The bacteria tested, displaying varied MAR resistance phenotypes, had resistance to 8 – 14 antibiotics, and the MAR indices for the bacterial isolates ranged 0.46 – 0.93. The PEE and APE both showed antibacterial activities, with respective ZDI (zone diameter of the inhibition) values of 14.7±5.32 mm and 17.53±5.72 mm (at 1 mg/disc), and 13.3±5.69 mm and 16.65±7.55 mm (at 2 mg/disc). The PEE and APE MICs ranged 2.5 – 3.3 mg/ml and 5 – 20 mg/ml, respectively, for the test bacteria. Thus, fruit peel of pomegranate might be useful in the preparation of antibacterial therapeutic agents, alternative to antibiotics, in order to combat the life-threatening infections of multiple antibiotic resistant gram-negative bacteria.
Pomegranate Fruit Peel, Antibacterial Activity, Minimum Inhibitory Concentration, Mar Indices, Gram-Negative Pathogenic Bacteria
Banu T. N, Mandal S. Antibacterial Activity of Pomegranate (Punica granatum; Family: Punicaceae) Fruit Peel Extracts Against Antibiotic Resistant Gram-Negative Pathogenic Bacteria. Biosc.Biotech.Res.Comm. 2019;12(4).
Banu T. N, Mandal S. Antibacterial Activity of Pomegranate (Punica granatum; Family: Punicaceae) Fruit Peel Extracts Against Antibiotic Resistant Gram-Negative Pathogenic Bacteria. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2R0gSc4
Copyright © Banu and Mandal This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The medicinal and food plants have been in use, for centuries, in treating infectious diseases, and have been considered as important source of antimicrobial agents, and for decades, their (plants) antimicrobial properties have been investigated in curing a variety of bacterial infections, and combating bacterial antibiotic resistances, as well (Alanis et al. 2005; Nozohour et al.2018, Matjuda and Aiyegoro (2019).
The Punica granatum (pomegranate; family: Punicaceae; Bedana in Bengali) fruit peel is an important inedible part, possessing an enormous amount of flavonoids, tannins and other phenolic compounds (Khan et al. 2017; Janani et al. 2019) and thus displaying various kinds of bioactivities including antioxidative and antimicrobial properties (Devatkal et al. 2013; Voravuthikunchai et al. 2005; Reddy et al. 2007). Devatkal et al. (2013) reported the antibacterial activity of aqueous extract of pomegranate peel against poultry meat isolates of Pseudomonas stutzeri. Navidinia and Goudarzi (2017) demonstrated the MICs of aqueous and ethanolic extracts of P. granatum seeds that ranged 9.37- 150 mg/ml and 9.37- 75 mg/ml, respectively, for various gram-negative potential bacterial pathogens. The pomegranate edible and non-edible parts have been reported to be excellent antibacterial as well as antioxidative agents containing rich amount of polyphenolics (Rummun et al. 2013).
The pomegranate fruit parts: peel, aril, seeds, and juice, have been reported to be rich in different bioactive components, as has been demonstrated by Jurenka et al. (2008).
The gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, are top listed WHO (World Health Organization) priority pathogens, and some of the members are included in the ESKAPE (Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp.) group (Rice, 2008; Smith et al. 2018; Perovic et al. 2018).
For such bacterial pathogens, the gram-negative bacteria, in particular, having the capacity to cause severe nosocomial infections (and non-responsive to currently available antibiotics), newly developed effective therapies are required (Tacconelli et al. 2018). Both edible and non-edible parts of pomegranate plant have been reported to treat different pathological conditions in different traditional medicine (Derakhshan et al. 2018). Therefore, the current study was undertaken to authenticate the antibacterial capacity P. granatum fruit peel (available in the local niches: West Bengal, India) against E. coli, A. baumannii, P. aeruginosa, K. pneumoniae, and Proteus spp. (P. mirabilis and P. vulgaris) showing resistance to multiple antibiotics.
MATERIAL AND METHODS
Bacterial Strain And Media
A total of 17 clinical bacterial isolates: Escherichia coli (n=5), Proteus spp. (n=4), K. pneumoniae (n=2), P. aeruginosa (n=3), A. baumannii (n=3), which were maintained in the laboratory in cystine tryptone agar stabs, were utilized in the current study. The media (Hi-Media, India) used in the study were nutrient broth (for bacterial subculture and inoculums preparation) and nutrient agar (for antibiotic susceptibility and antibacterial activity testing).
Antibiotic Susceptibility
The antibiotic susceptibility testing, for the bacterial isolates, was done following disc diffusion (Bauer et al., 1966), using 15 antibiotics (Hi-Media, India): ampicillin (Am; 10-μg), amikacin (Ak; 30-μg), cefoxitin (Cx; 30-μg), cefotaxime (Cf; 30-μg), cefpodoxime (Cpd; 10-μg), chloramphenicol (Cm; 30-μg), ciprofloxacin (Cp; 10-μg), gentamycin (Gm; 30-μg), imipenem (Ip; 10-μg), kanamycin (Km; 30-μg), methicillin (Mc; 5-μg), nalidixic acid (Nx; 30-μg), tetracycline (Tc; 30-μg), trimethoprim (Tr; 5-μg), and vancomycin (Vm; 30-μg). The ZDI (zone diameter of inhibition) values from the antibiotic action against the test bacteria were recorded, and interpreted according to the CLSI protocol (CLSI, 2015). The MAR indices for the bacteria tested were calculated following the formula as stated by Nandi and Mandal (2016), and the results were interpreted according to the criteria published earlier (Krumperman, 1983). The MAR phenotypic profiles were determined for the bacterial isolates displaying resistance to three or more antibiotics (Adefisoye and Okoh, 2017).
Plant Extract Preparation
The indigenous variety fruits of pomegranate, Punica granatum (family: Punicaceae) were collected from Rajapur village of Malda district (West Bengal, India), washed properly with distilled water, and the peels were separated and sliced for shade drying. The dried plant materials were granulated by electrical grinding machine and stored in airtight containers at room temperature for extract preparation. The pomegranate fruit peel ethanolic extract (PEE) and pomegranate fruit peel aqueous extract (APE), were prepared in line with a little modification of the protocol depicted by Sircar and Mandal (2016). Briefly, for PEE preparation, 5 g of dried pomegranate fruit peel granules was extracted by soaking with 100 ml of ethanol shaking at regular interval, for 96 h at room temperature, and sieved through cheese-cloth and Whatman No. 1 filter paper. For the preparation of APE, 5 gm granulated sample was dissolved in 100 ml of double distilled water, and boiled for 30 min in water bath, and filtered as mentioned above, after cooling. The concentration of each of the extracts (APE and PEE) in stock solution was 50-µg/μl. The extracts prepared were stored at 4°C until further use.
Antibacterial Property
The antibacterial activity of PEE and APE extracts were evaluated employing disc diffusion technique (in order to get the zone diameter of the inhibition; ZDI), as explained earlier by Das and Mandal (2016). Agar dilution method, the details of which was mentioned in previous publication (Mandal et al. 2007), was followed for the determination of MIC (minimum inhibitory concentration) values, using nutrient agar medium mixed with varied concentration of the extracts, ranging from 2.5 to 3.3 mg/ml and 5 to 20 mg/ml. The all incubations were done at 37°C for 24 h, and the testing was at once completed in triplicate. The antibacterial activity was recorded based on the ZDIs obtained around the plant extract impregnated discs on the agar plates inoculated with test bacteria, and the ZDI values ≥7 mm accounted sensitivity of the test extracts to the bacterial isolates (Nascimento et al. 2000). The lowest extract concentration that inhibited the visible growth of the test bacteria were defined as MICs (Mandal et al. 2007).
Statistical Analysis
To compare the antibacterial activity (in terms of ZDIs) by disc diffusion technique, and MICs of plant extracts: APE and PRE, against the gram-negative pathogenic bacteria tested, the data were expressed as the mean ± SD (standard deviation), and were evaluated by ‘t’-test , using MS Excel 2010 software; the statistical significance was projected by ‘p’ value of ≤ 0.05 .
RESULTS AND DISCUSSION
The current research explores the antibacterial activity of pomegranate fruit peel ethanolic and aqueous extracts against antibiotic resistant gram-negative pathogenic bacteria (Figure 1).
 |
Figure 1: Flow diagram for antibacterial activity analysis of pomegranate fruit peel ethanolic and aqueous extracts |
The multiple antibiotic resistance phenotypes for the test bacterial pathogens are represented in Table 1; the isolates showed 7-drug to 14-drug resistances, displaying the respective resistance patterns: ‘Vm-Am-Mc-Km-Tr-Cz-Nx’, for A. baumannii AB1 strain, and ‘Gm-Cm-Cx-Cf-Vm-Tc-Cp-Am-Mc-Ak-Km-Tr-Cz-Nx’ for E. coli EC1, P. aeruginosa PA1 and P. aeruginosa PA2 strains. As per the report of Matjuda and Aiyegoro (2019), among a total of 74 resultant MAR phenotypes (ranging from 3-drug to 12-drug resistances), the predominant patterns noted included “penicillin-sulphamethaxazole-Vm-Am-amoxicillin-apramycin-neomycin-tilmicosin-oxytetracycline-spectinomycin-linomycin-Tr” and“
penicillin-sulphamethaxazole-Vm-amoxicillin-neomycin-tilmicosin-oxytetracyclin-spectinomycin-linomycin”, for 15 and 6 test bacterial isolates, respectively. The MAR index for the test clinical bacteria ranged from 0.46 to 0.93 (Table 1). As has been reported earlier by Matjuda and Aiyegoro (2019), the MAR indices of pathogenic bacteria tested ranged from 0.2 to 1.
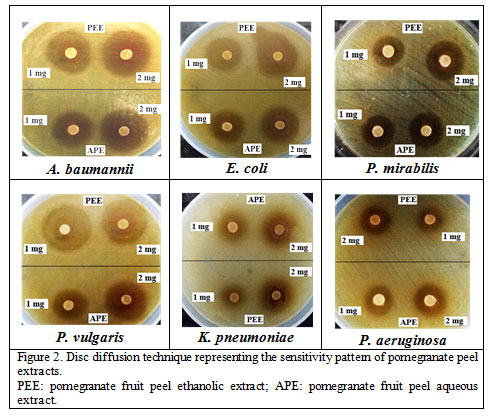 |
Figure 2: Disc diffusion technique representing the sensitivity pattern of pomegranate peel extracts. PEE: pomegranate fruit peel ethanolic extract; APE: pomegranate fruit peel aqueous extract. |
0Das et al., (2018), reported different MAR resistance phenotypes, among gram-negative clinical bacteria, which ranged up to 10-drug resistance, displaying the pattern: ‘Am-Ce-Cp-Ct-Cx-Mp-Nx-Pc-PT-Tc’ by E. coli CSD2 strain and in that study the MAR indices for the test bacteria ranged 0.15 – 0.77. The earlier authors (Tambekar et al. 2005; Kaneene et al. 2007) explained that the bacteria demonstrating MAR indices >0.4 might be originated from niches with human-faecal contamination, while the bacteria displaying MAR indices >0.2, have been regarded to be derived from niches with high antibiotic pollution (Krumperman, 1983; Matjuda and Aiyegoro, 2019).
Table 1: Multiple antibiotic resistance (MAR) phenotypes and MAR indices for clinical bacterial isolates (n=17)
| Bacteria | Resistance | MAR phenotypes | MAR index |
| A. baumannii AB1 | 7-drug | Vm-Am-Mc-Km-Tr-Cpd-Nx | 0.46 |
| E. coli EC3 | 8-drug | Cx-Vm-Am-Ip-Mc-Tr-Cpd-Nx | 0.53 |
| E. coli EC4 | 9-drug | Cx-Cf-Vm-Am-Ip-Mc-Tr-Cpd-Nx | 0.6 |
| P. vulgaris PV2 | 9-drug | Cf-Cp-Am-Ip-Mc-Km-Tr-Cpd-Nx | 0.6 |
| E. coli EC2 | 10-drug | Cx-Vm-Tc-Cp-Am-Ip-Mc-Tr-Cpd-Nx | 0.66 |
| A. baumannii AB2 | 11-drug | Cm-Cx-Cf-Vm-Am-Mc-Ak-Km-Tr-Cpd-Nx | 0.73 |
| K. pneumoniae KP2 | 11-drug | Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Ak-Km-Cpd | 0.73 |
| A. baumannii AB3 | 12-drug | Gm-Cm-Cx-Cf-Tc-Am-Ip-Mc-Ak-Km-Tr-Cpd | 0.8 |
| K. pneumoniae KP1 | 12-drug | Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Ak-Tr-Cpd-Nx | 0.8 |
| P. aeruginosa PA3 | 12-drug | Cm-Cx-Cf-Vm-Tc-Cp-Am-Mc-Km-Tr-Cpd-Nx | 0.8 |
| P. mirabilis PM1 | 12-drug | Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Km-Tr-Cpd-Nx | 0.8 |
| E. coli EC5 | 13-drug | Cm-Cx-Cf-Vm-Tc-Cp-Am-Ip-Mc-Km-Tr-Cpd-Nx | 0.86 |
| P. mirabilis PM2 | 13-drug | Gm-Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Km-Tr-Cpd-Nx | 0.86 |
| P. vulgaris PV1 | 13-drug | Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Ak-Km-Tr-Cpd-Nx | 0.86 |
| E. coli EC1 | 14-drug | Gm-Cx-Cf-Vm-Tc-Cp-Am-Ip-Mc-Ak-Km-Tr-Cpd-Nx | 0.93 |
| P. aeruginosa PA1 | 14-drug | Gm-Cm-Cx-Cf-Vm-Tc-Am-Ip-Mc-Ak-Km-Tr-Cpd-Nx | 0.93 |
| P. aeruginosa PA2 | 14-drug | Gm-Cm-Cx-Cf-Vm-Tc-Cp-Am-Mc-Ak-Km-Tr-Cpd-Nx | 0.93 |
| Ak: amikacin; Am: ampicillin; Cf: cefotaxime; Cx: cefoxitin; Cpd: ceftazidime; Cp: ciprofloxacin; Cm: chloramphenicol; Gm: gentamycin; Ip: Imipenem; Km: kanamycin; Mc: methicillin; Nx: nalidixic acid; Tc: tetracycline; Tr: trimethoprim; Vm: vancomycin | |||
The high MAR indices (0.46 – 0.93) among the test gram-negative clinical bacteria demonstrated, in the current study, their origin from human-faecal contaminated niches with high antibiotic pollution.The antibacterial activity of pomegranate peel extracts against gram-negative pathogenic bacteria, following disc diffusion method, is shown in Figure 2. The ZDIs from the action of APE and PEE, against the test bacteria, are represented in Table 2. The PEE and APE had ZDIs 10 – 22 mm and 8 – 20 mm, respectively (at 1.0 mg/well), and 12 – 25 mm and 11 – 26 mm, respectively (at 2.0 mg/well), against E. coli isolates. The PEE was active against all the test A. baumannii isolates (ZDIs 10 – 28 mm), while the APE showed activity against A. baumannii AB1 isolate only. The pomegranate fruit peel showed anti-Proteus spp. activity with ZDIs 15 – 26 mm, for PEE, and 15 – 27 mm, for APE. The PEE had growth inhibitory activity against all the K. pneumoniae isolated tested (n=3; ZDIs: 8 – 14 mm). The pomegranate peel extract had ZDIs of 10 – 18 mm against P. aeruginosa; however, for P. aeruginosa PA1 the APE had no activity (ZDI: 6 mm). As per the report of Algurairy (2018) the pomegranate fruit peel ethanolic extract (10 – 100 %) had ZDIs of 22 – 36 mm, for Staphylococcus aureus clinical isolates. The respective ZDIs of pomegranate fruit peel methanolic and aqueous extracts (50 mg/ml) for Enterobacter cloacae were 14 mm and 10 mm, and for Salmonella enterica serovar Typhi, 20 mm and 10 5mm, while for the gram-positive (S. aureus and Bacillus subtilis) bacteria, the ZDIs ranged 22 – 26 mm and 24 – 28 mm, respectively (Kanoun et al. 2014).
The methanolic extract of pomegranate fruit peel showed antibacterial activity against food-borne bacteria, such as, Listeria monocytogenes, S. aureus, E. coli and Yersinia enterocolitica (Al-Zoreky, 2009). As has been reported by Kunte et al. (2018), the pomegranate fruit peel aqueous extract had antibacterial activity against potential cariogenic Streptococcus mutans isolates, displaying ZDIs of 15 – 17 mm. The pomegranate peel fresh aqueous extract showed growth inhibitory activity against Pseudomonas stutzeri isolates from poultry meat displaying ZDIs of 21 – 26 mm (Devatkal et al. 2013). The pomegranate peel extract showed antibacterial activity against S. mutans and Streptococcus mitis having ZDIs of 20 mm and 25 mm, respectively, while the leaf extract had ZDIs of 16 mm and 18 mm, respectively, for the bacterial isolates (Rummun et al. 2013). The P. granatum seed ethanolic extract showed antibacterial activity against gram-positive bacteria: S. aureus, with ZDIs 22 – 42 mm as well as gram-negative bacteria: E. coli, having ZDIs 27 – 42 mm, while the respective ZDIs of petroleum ether extract for the isolates ranged 16 – 34 mm and 15 – 27 mm (Bora et al. 2018). The two extracts, APE and PEE, by disc diffusion (Table 2), displayed growth inhibition activities against the test bacteria, wherein there was no significant difference between the antibacterial properties of APE and PEE (p values: 0.47 – 0.71).
Table 2: The ZDI (zone diameter of inhibition) values of pomegranate fruit peel extracts for clinical bacterial isolates (n=17)
| Bacteria | ZDI (mm) | |||
| PEE
(1 mg/disc) |
APE
(1 mg/disc) |
PEE
(2 mg/disc) |
APE
(2 mg/disc) |
|
| E. coli EC1 | 10 | 14 | 13 | 22 |
| E. coli EC2 | 13 | 15 | 14 | 23 |
| E. coli EC3 | 10 | 8 | 12 | 11 |
| E. coli EC4 | 13 | 18 | 15 | 26 |
| E. coli EC5 | 22 | 20 | 25 | 22 |
| A. baumannii AB1 | 22 | 20 | 28 | 25 |
| A. baumannii AB2 | 14 | 6 | 18 | 6 |
| A. baumannii AB3 | 10 | 6 | 12 | 6 |
| P. mirabilis PM1 | 19 | 16 | 22 | 20 |
| P. mirabilis PM2 | 15 | 15 | 20 | 18 |
| P. vulgaris PV1 | 22 | 24 | 26 | 27 |
| P. vulgaris PV2 | 25 | 18 | 26 | 24 |
| K. pneumoniae KP1 | 10 | 6 | 10 | 6 |
| K. pneumoniae KP2 | 8 | 8 | 13 | 11 |
| P. aeruginosa PA1 | 10 | 6 | 12 | 6 |
| P. aeruginosa PA2 | 12 | 10 | 14 | 12 |
| P. aeruginosa PA3 | 15 | 16 | 18 | 18 |
| Mean | 14.7 | 17.53 | 13.3 | 16.65 |
| SD | 5.32 | 5.72 | 5.69 | 7.55 |
| p value | 0.47 | 0.71 | ||
The MICs of PEE ranged 2.5 – 3.3 mg/ml for all the gram-negative pathogenic bacteria tested, while the APE MICs ranged from 5 mg/ml (for E. coli EC1, E. coli EC2, E. coli EC3 and E. coli EC4) to 20 mg/ml (for E. coli EC5), and the APE MICs for the remaining bacterial pathogens ranged 6.6 – 16.66 mg/ml (Table 3). The earlier authors also have demonstrated antibacterial activity of pomegranate extracts against clinically relevant bacteria, from different parts of the globe. The pomegranate peel methanolic and aqueous extract had MICs 0.39 and 0.195 for S. aureus, 1.56 and 0.78 mg/ml, for B. subtilis, 3.125 and 1.56 mg/ml, for E. cloacae, and 12.5 and 6.25 for S. enterica serovar Typhi (Kanoun et al. 2014). The respective MICs of aqueous and ethanolic pomegranate seed extracts for the bacteria tested were: E. coli (75 and 37.5 mg/ml), Shigella sonnei (37.5 and 18.75 mg/ml), Shigella flexneri (18.75 and 9.37 mg/ml), Shigella dysentery (18.75 and 9.37 mg/ml), P. vulgaris (18.75 and 9.37 mg/ml), P. mirabilis (9.37 and 9.37 mg/ml) and Citrobacter fraundii (150 and 75 mg/ml), as demonstrated by Navidinia and Goudarzi (2017).
Prashanth et al. (2001) reported antibacterial activity of pomegranate fruit extract against grem-negative bacteria: E. coli, K. pneumoniae, P. vulgaris and S. enterica serovar Typhi, in terms of MICs, which were 12 mg/ml, 12 – 25 mg/ml, 1.5 – 12 mg/ml, 12 – 50 mg/ml, for petroleum ether extract, chloroform extract, methanol extract and aqueous extract. The pomegranate fruit methanol and aqueous extracts had MICs, against gram-negative bacterial strains: E. coli ATCC 25922, S. dysantriae PTCC 1188 and S. enterica serovar Typhi ATCC 19430, of 6.25 – 12.5 and 3.12 – 12.5 mg/ml, respectively, as has been reported by Mahboubi et al. (2015). On the basis of the MIC determination (Table 3), the pomegranate fruit peel extracts, APE and PEE, were well active against the gram-negative multiple antibiotic resistant pathogenic bacteria; the APE, however, displayed greater activity compared to the PEE (p value: 0.00012).
Table 3: The MIC (minimum inhibitory concentration) values of pomegranate fruit peel extracts for clinical bacterial isolates (n=17)
| Bacteria | MIC (mg/ml) | |
| PEE | APE | |
| E. coli EC1 | 2.5 | 5 |
| E. coli EC2 | 3.3 | 5 |
| E. coli EC3 | 3.3 | 5 |
| E. coli EC4 | 3.3 | 5 |
| E. coli EC5 | 3.3 | 20 |
| A. baumannii AB1 | 2.5 | 6.6 |
| A. baumannii AB2 | 2.5 | 6.66 |
| A. baumannii AB3 | 2.5 | 16.66 |
| P. mirabilis PM1 | 2.5 | 6.6 |
| P. mirabilis PM2 | 2.5 | 6.6 |
| P. vulgaris PV1 | 2.5 | 6.6 |
| P. vulgaris PV2 | 2.5 | 6.6 |
| K. pneumoniae KP1 | 3.3 | 20 |
| K. pneumoniae KP2 | 3.3 | 20 |
| P. aeruginosa PA1 | 2.5 | 11.6 |
| P. aeruginosa PA2 | 2.5 | 6.6 |
| P. aeruginosa PA3 | 3.3 | 13.33 |
| Mean | 2.83 | 9.65 |
| SD | 0.41 | ±5.8 |
| p value | 0.00012 | |
CONCLUSION
The fruit peel ethanolic as well as aqueous extracts of pomegranate displayed antibacterial activity against gram-negative bacteria having high multiple antibiotic resistance indices, suggesting the usefulness of the of the plant parts in the preparation of antibacterial bio-therapeutics that might be utilized in the treatment of diseases caused due to the infection of multiple antibiotic resistant gram-negative bacteria. Further, phytochemical analysis and pharmacokinetic studies are required to explore the bioactive components responsible for antibacterial activity, and to determine the effective dosage.
REFERENCES
Adefisoye, M. A., and Okoh, A. I. (2017). Ecological and public health implications of the discharge of multidrug-resistant bacteria and physicochemical contaminants from treated wastewater effluents in the Eastern Cape, South Africa. Water 2017, 9(8), 562.
Algurairy, A. T. M. (2018). Assessing the antibacterial activity of pomegranate against Staphylococcus aureus obtained from wound infections. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(4), 1602-1606.
Alanis, A. D., Calzada, F., Cervantes, J. A., Torres, J., and Ceballos, G. M. (2005). Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Journal of Ethnopharmacology, 100, 153-157.
Al-Zoreky, N. S. (2009). Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology, 134(3), 244-248.
Bauer, A. W., Kirby, W. M., Sherris, J. C., and Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 45(4), 493-496.
Bora, A. J., Dash, B., Paul, S., and Gupta, B. (2018). Preliminary phytochemical screening and in vitro antimicrobial activity of ethanolic extracts of seeds of Punica granatum against standard pathogenic strains. International Journal of Current Pharmaceutical Research, 10(4), 55-58.
Chen, C. Y., Chen, Y. H., Lu, P. L., Lin, W. R., Chen, T. C. and Lin, C.Y. (2012). Proteus mirabilis urinary tract infection and bacteremia: Risk factors, clinical presentation, and outcomes. Journal of Microbiology, Immunology and Infection, 45(3), 228-236.
Das, M. K., and Mandal, S. (2016). Syzygium cumini and Mangifera indica seed extracts: In vitro assessment for antibacterial activity alone and in combination with antibiotics against clinical bacteria. Journal of Infectious Diseases and Preventive Medicine, 4, 1-6.
Das, S. N., Mandal, M., and Mandal, S. (2018). Heavy metal tolerance in association with plasmid mediated multiple antibiotic resistances among clinical bacterial isolates. Bioscience Biotechnology Research Communications, 11(4), 612- 618.
Derakhshan, Z., Ferrante, M., Tadi, M., Ansari, F., Heydari, A., Hosseinei, M. S., Conti, G. O., and Sadrabad, E. K. (2018). Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food and Chemical Toxicology, 114, 108-111.
Devatkal, S. K., Jaiswal, P., Jha, S. N., Bharadwaj. R., and Viswas, K. N. (2013). Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. Journal of Food science and Technology, 50(3), 555–560.
Janani, J., Rajiv, P., Gopalan, R., and Lakshmanapermalsamy, P. (2019). An overview of phytochemical and pharmacological potentials of Punica granatum L. Pharmacognosy Journal, 11(5), 1167-1171.
Jurenka, J. (2008). Therapeutic applications of pomegranate (Punica granatum L.): A review. Alternative Medicine Review, 13, 128–144.
Kaneene, B. J., Miller, R., Sayah, R., Johnson, Y. J., Gilliland, D., and Gardiner, J. C. (2007). Considerations when using discrimination function analysis of antimicrobial resistance profiles to identify sources of faecal contamination of surface water in Michigan. Applied and Environmental Microbiology, 73, 2878–90.
Kanoun, K., Abbouni, B., Gabbés, S., Dellani, S. and Zizi, N. (2014). In-vitro Antibacterial activity of algerian pomegranate (Punica granatum linn) peels on some antibiotic resistant gram-negative and positive bacterial strains. Middle-East Journal of Scientific Research, 21, 1579-1589.
Khan I et al. (2017). Punica granatum peel extracts: HPLC fractionation and LC MS analysis to quest compounds having activity against multidrug resistant bacteria. BMC Complementary and Alternative Medicine, 17, 247.
Krumperman, P. H. (1983). Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Applied and Environmental Microbiology, 46, 165-70.
Kunte, S., Kadam, N., Patel A., Shah P., Lodaya, R., and Lakde, L. (2018). Comparative evaluation of antimicrobial properties of pomegranate peel extract against Streptococcus mutan s and Lactobacillus – An in vitro study. International Dental and Medical Journal of Advanced Research, 4, 1-6.
Mahboubi, A., Asgarpanah, J., Sadaghiyani, P. N., and Faizi, M. (2015). Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. pleniflora flowers (Golnar) against bacterial strains causing food borne diseases. BMC Complementary and Alternative Medicine, 15, 366.
Mandal, S., Mandal, MD., and Pal, N. K. (2007). Antibacterial potential of Azadirachta indica seed and Bacopa monniera leaf extracts against multidrug resistant Salmonella enterica serovar Typhi isolates. Archives of Medical Science, 3, 14-18.
Matjuda, D.S., Aiyegoro, O.A. (2019). Analysis of bacteriological pollution and the detection of antibiotic resistance genes of prevailing bacteria emanating from pig farm see page. Microbiology Open, 8, 737.
Nandi S, Mandal S. (2016). Bacteriological profiling of commercially available eye cosmetics and their antibiotic susceptibility pattern. Translational Biomedicine, 7, 3: 80.
Nascimento, G.G.F., Locatelli, J., Freitas, P.C., Silva, G.L. (2000). Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian Journal of Microbiology, 31, 247-56.
Navidinia, M., Goudarzi, M. (2017). The antibacterial properties of aqueous and ethanolic extracts of Punica granatum seeds on bacterial infectious diarrhea. Journal of Paramedical Sciences, 8, 6-13.
Nozohour, Y., Golmohammadi, R., Mirnejad, R., and Fartashvand, M. (2018). Antibacterial activity of pomegranate (Punica granatum L.) seed and peel alcoholic extracts on Staphylococcus aureus and Pseudomonas aeruginosa isolated from health centers. Journal of Applied Biotechnology Reports, 5(1), 32-36.
Prashanth, D., Asha, M.K., Amit, A. (2001). Antibacterial activity of Punica granatum. Fitoterapia, 72(2), 171 – 173.
Perovic, O., Ismail, H., Schalkwyk, E.V., Lowman, W., Prentice, E., Senekal, M., and Govind, C.N. (2018). Antimicrobial resistance surveillance in the South African private sector report for 2016. Southern African Journal of Infectious Diseases, 33(4), 114-117.
Reddy, M. K., Gupta, S. K., Jacob, M. R., Khan, S. I., and Ferreir, D. (2007). Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Medca, 73(5), 461–467.
Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. The Journal of Infectious Diseases, 197(8), 1079–1081.
Rummun, N., Somanah, J., Ramsaha, S., Bahorun, T., Vidushi, S., and Bhujun, N. (2013). Bioactivity of Nonedible Parts of Punica granatum L.: a potential source of functional ingredients. International Journal of food Science, http://dx.doi.org/10.1155/2013/602312
Sircar, B., Mandal, S. (2016). Antibacterial Activity of Mimusops elengi Leaf. Seed and Bark Extracts Alone and in combination with Antibiotics against Human Pathogenic Bacteria. Translational Medicine (Sunnyvale), 6, 188.
Smith P A et al. (2018). Optimized arylomycins are a new class of Gram-negative antibiotics. Nature, 561, 189–194.
Tacconelli E et al. (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infectious Diseases, 18(3), 318-327.
Tambekar, D.H., Hirulkar, N.B., Waghmare, A.S. (2005). MAR indexing to discriminate the source of faecal contamination in drinking water. Nature Environment and Pollution Technology, 4, 525–528.
Voravuthikunchai, S. P., Sririrak, T., Limsuwan, S., Supawita, T., Iida, T., and Honda, T. (2005). Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohaemorrhagic Escherichia coli O157:H7. Journal of Health Science, 51(5), 590–596.


