1Ph D Scholar, K S Hegde Medical Academy, NITTE University, Mangalore, India
2Department of Radiotherapy & Oncology, K S Hegde Medical Academy, NITTE University, Mangalore
3Central Research Laboratory, SDM College of Medical Sciences & Hospital, Dharwad, India
4Department of Biochemistry, SDM College of Medical Sciences & Hospital, Dharwad, India
5Department of Biochemistry, K S Hegde Medical Academy, Mangalore, NITTE University, Mangalore
6Department of Microbiology, SDM College of Medical Sciences & Hospital, Dharwad, India
7Cancer Biology division, National Centre for Cell Science, Pune
Corresponding author Email: anymh9832@rediffmail.com
Article Publishing History
Received: 12/01/2019
Accepted After Revision: 27/03/2019
BRCA1 is a nuclear phosphoprotein involved in genome integrity by regulating cell cycle checkpoints, DNA repair and apoptosis. BRCA1 down regulation occurs in sporadic breast cancer (BC). Posttranscriptional regulation of gene expression has evolved as a means of fine-tuning of protein levels. There are several posttranscriptional regulatory motifs, including CU-rich, U-rich and AU-rich elements, which are usually located in the 5’ and 3’ UTR’s. Post-transcriptional regulation of BRCA1 is poorly characterized and underappreciated. To elucidate the molecular mechanism of AnxA2 mediated posttranscriptional regulation of BRCA1 mRNA in sporadic breast cancer. In order to investigate BRCA1 mRNA levels in MDAMB-231 & MCF-7 cells, we conducted DRB chase experiment. The BRCA1 mRNA destabilization is significantly increased upon AnxA2 induction in MCF-7 cells. Further we knocked down AnxA2 in MDAMB-231 cells we found, BRCA1 mRNA stabilization which proves AnxA2 destabilizes BRCA1 mRNA. In RIP-CHIP experiment we found BRCA1 immunoprecipitated with AnxA2. This supports our finding that AnxA2 has role in the regulation of BRCA1 at mRNA level. Luciferase reporter assay showed decrease in luciferase activity with BRCA1 3’UTR. On treating with AnxA2 binding oligo from BRCA1 3’UTR showed decrease in cell viability.
Anxa2, Brca1, Rna Binding Protein, Renilla Luciferase
Bargale A. B, Shetty K. J, Kumari N. S, Patil V. S, Kulkarni R. D, Manne R. K, Kumar E. S, Shetty P. Annexin A2 Mediated Posttranscriptional Destabilization of BRCA1 Mrna in Sporadic Breast Cancer. Biosc.Biotech.Res.Comm. 2019;12 (1).
Bargale A. B, Shetty K. J, Kumari N. S, Patil V. S, Kulkarni R. D, Manne R. K, Kumar E. S, Shetty P. Annexin A2 Mediated Posttranscriptional Destabilization of BRCA1 Mrna in Sporadic Breast Cancer. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2Mm1Cpq
Copyright © Bargale et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Breast Cancer (BC) is one of the leading causes of death among women and has 10% life time risk of developing malignancies in many countries in the western world (Newman 1988). Hereditary BC Syndrome accounts for 5-10% of all BC cases, while the other 90-95% of BC is “Sporadic. According to Population Based Cancer Registry (PBCR); In India, Breast cancer accounts for 25 – 35% of all female cancers in metro cities like; Mumbai, Delhi, Bengaluru, Kolkata, Chennai, Ahmadabad, Bhopal, etc. and it is second common cancer in rural areas. This implies, practically, one fourth of all female cancer cases are breast cancers, (Adnan 2018). Several studies have been reported the evidences, suggesting involvement of BRCA1 in the etiology of sporadic breast and ovarian cancer through reduced expression. Decreased levels of BRCA1 mRNA are frequently observed in breast tumors, (George 1998, Adnan, 2018, Nicolas 2018, Harahap 2018, Huszno 2019).
Lower or undetectable levels of expression of the BRCA1 protein have been observed in sporadic BC (McCoy 2003). Different mechanisms have been shown to be responsible for the reduced expression of BRCA1; epigenetic silencing of the BRCA1 gene at the transcriptional level by means of promoter methylation is another mechanism involved (Das 2004, Choic 2009, Robertson 2002, Baylin 2006). The BRCA1 gene was identified and cloned in 1994. BRCA1 known for its multiple vital functions such as tumor suppressor activity, including roles in cell cycle progression, DNA damage repair processes DNA damage responsive cell cycle check point regulation of a set of specifi c transcriptional pathways and apoptosis (Rice 1998). Since, BRCA1 gene is rarely mutated in sporadic breast cancer. It has been suggested that, dysregulation of BRCA1 expression causes reduced mRNA or protein levels in BC (Thompson 2011).
Post-transcriptional regulation of gene expression has evolved as a mean of fi ne tuning protein levels and generating rapid temporal or spatial changes in protein expression following an environmental stimulus (Saunus 2008). Regulation of the dynamics of mRNA molecule is a wide spread phenomenon and there are many such examples of genes regulated in this way including tumor suppressor genes (Rosen 2003, Maity 1997). The mechanisms often involve RNA-binding proteins and non-coding RNAs (transacting factors) which recognizes sequence motif in their target transcripts and ‘tag’ them for recognition by macromolecular complexes involved in RNA metabolism such as mRNA transport granules, RNA induced silencing complexes (RISC), the exosome, GW bodies and stress granules(Thanin 2018, Cok 2001, Fu 1997, anderson 2006). There are many examples of post-transcriptional regulatory motifs, including CU CUrich, U-rich and AU-rich elements which are usually located regions of regulated transcripts (Moore 2005, Kedeersha 2002, Engels 2006). It can be diffi cult to predict the regulatory functions of individual motif based on primary sequence alone, as they often associate with multiple transacting factors with different and sometimes opposing activities.
BRCA1 is post-transcriptionally regulated by mRNA binding proteins, the identity of which is unknown. HuR is an RNA binding protein already proven to play role in the event of post-transcriptional regulation of BRCA1 by Jodi M Saunus et al. HuR is ubiquitously expressed RNA binding protein that regulates the stability and translation of transcripts that function in multiple cellular pathways, such as p21WAFI, COX, TP24, Cyclin A and B and P27. As like HuR other mRNA binding proteins like Annexin A2 (AnxA2) expression increases in basal breast cancer where BRCA1 expression is very less or null. AnxA2 is a multifunctional calcium dependent phospholipid binding protein. AnxA2 shows functional diversity like it regulates membrane traffi c and cytoskeleton organization, extracellular activities and targeted gene disruption (Lopez 2005). AnxA2 is highly expressed in the surface of human tumor cells and promotes cell migration and invasion by activating plasminogen and cleaving extracellular matrix25. AnxA2 stimulates cell proliferation, angiogenesis and invasion (Wein 2003, Wiklund 2002, Maji 2016, Volker 2002). This study demonstrates the hypothesis of AnxA2 mediated post-transcriptional regulation of tumor suppressor BRCA1 gene contributes to the development and progression of sporadic breast cancer.
Materials and Methods
Cell Culture
Human breast cancer cell lines, MDA-MB-231 and MCF-7 were obtained from National Center for cell Sciences (NCCS), Pune and grown in respective media as prescribed by the supplier.
Real Time RT-PCR
Total RNA was isolated using TRIZOL (Invitrogen). cDNA was synthesized using RevertAid First strand cDNA synthesis kit by Thermo Fischer scientifi c with following primers; For BRCA1 (F: 5’-GGTTCTGATGACTCACATGATGGG- 3’&R: 5’-TCTGTGGCTCAGTAACAAATGCTC- 3’),AnxA2(5’TAACTTTGATGCTGAGCGGG-3’&R:5’- TAATTTCCTGCAGCTCCTGG-3’) and for GAPDH (5’-GAGCGAGATCCCTCCAAA-3’ & R: 5’-ACTGTGGTCATGAGTCCTTC- 3’. BRCA1, AnxA2 & GAPDH were amplifi ed by DyNAmo Color Flash SYBR Green q-PCR kit (Thermo Fischer scientifi c) on Cepheid Smart cycler. PCR set up for BRCA1 was 2 minutes for 950 C for one cycle, followed by 30 second 940C, 550C & 720C for 35 cycles. PCR reaction set up for AnxA2 & GAPDH used was 2 minutes at 950C and 40 cycles of 30 s at 950C, 30 s at 540C, and 30 s at 680C. The comparative ΔCt method was used to determine relative BRCA1, AnxA2 mRNA expression.
RNA stability
RNA stability was analyzed by transcription-chase experiment. The cells were stimulated to over express BRCA1 & AnxA2 by selected agonists respectively and then treated with DRB to inhibit ongoing transcription, after which total RNA was isolated at selected time points and cDNA was synthesized and amplified as described earlier.
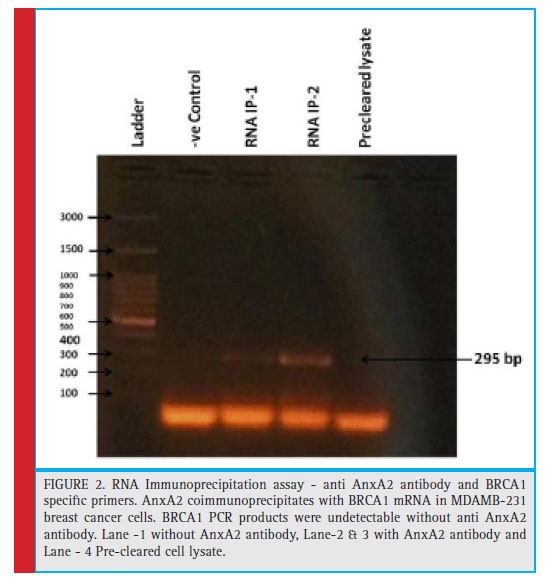
RNA Immunoprecipitation: Formaldehyde was added to cosslink protein-RNAs in MDAMB-231 Cells grown in specific medium, at RT after 15 minutes stopped by addition of 2M glycine and incubated for 5 minutes. Cells were collected in 500μl of RIPA lysis buffer containing RNase inhibitor. The lysate was centrifuged at 12000g. Annexin A2 specifi c primary antibody added and incubated at 4ºC overnight on shaker. 50 μl of Protein A/G beads added to the lysate and incubated at 4ºC for 4 hours. The lysate was washed twice with RIPA lysis buffer. The crosslinking reversed by 6μl of 5M NaCl and 20 μg of proteinase k added to the lysate and incubated 1 hour at 42ºC and 1 hour at 65ºC. RNA was extracted by following TRIZOL RNA extraction protocol. RNA was reverse transcribed and amplifi ed for BRCA1 by specific primers as described earlier. PCR products were resolved on ethidium bromide–stained 1.5% agarose gel.
Luciferase reporter assays
Luciferase reporter used was BRCA1 3’UTR luciferase reporter construct, made by ligating the full-length BRCA1 3’UTR downstream of the luciferase of pLuc. MDAMB-231 cells in 12 well plates were transiently transfected with equimolar amounts of luciferase reporter constructs. After 24 h of luciferase expression, Firefl y luciferase reporter activity was determined relative to Renilla using a Dual Luciferase Reporter Assay kit (Promega) and Promega Luminometer.
MTT assay
The MTT (3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl- 2H-tetrazolium bromide) assay conducted to check cell proliferation by transfecting the MDAMB-231 cells by 50 oligo nucleotide specifi c binding to BRCA1 3’UTR analyzed by NCBI BLAST software and read by Epoch analyzer.
Results and Discussion
A detailed understanding of the mechanisms regulating BRCA1 expression is very much essential to identify the regulatory abnormalities in breast cancer (Diaz 2004, Blasi 1999). Reduction in BRCA1 expression in sporadic breast cancer is well documented (Tarui 2002). In our earlier data, we have demonstrated that the expressions of BRCA1 and AnxA2 protein are reciprocally regulated, which indicate the possibility of AnxA2 involvement in BRCA1 posttranscriptional regulation. The posttranscriptional regulation of BRCA1 in the sporadic breast cancer is underappreciated and poorly studied. This study suggests BRCA1 mRNA stability is regulated by AnxA2 in sporadic breast cancer (Wein 2003, Wiklund 2002, Huszno 2019).
BRCA1 & AnxA2 mRNA expression in different cells
Levels of BRCA1 & AnxA2 mRNA were analyzed in BT-549, MCF-7 & MDAMB-231 cells. We found abundant AnxA2 mRNA in MDAMB-231 & BT-549 cells than MCF-7. On the other hand MCF-7 cells showed abundant BRCA1 mRNA as compared to BT-549 & MDAMB-231 (Figure 1).
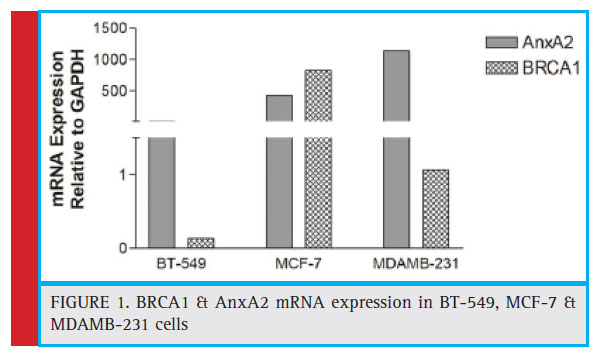 |
Figure 1: BRCA1 & AnxA2 mRNA expression in BT-549, MCF-7 & MDAMB-231 cells |
Endogenous AnxA2 and BRCA1 mRNA are binding partners
To determine the association between endogenous AnxA2 and BRCA1 mRNA transcripts, we performed an IP-RT-PCR assay using an anti AnxA2 antibody and BRCA1 specifi c primers. We are able to show that AnxA2 coimmunoprecipitates with BRCA1 mRNA in MDAMB- 231 breast cancer cells (figure 2). Conversely, BRCA1 PCR products were undetectable without anti AnxA2 antibody. The results provide clear evidence that AnxA2 associates with BRCA1 mRNA TNBC cells.
AnxA2 Regulates BRCA1 Post-transcriptionally
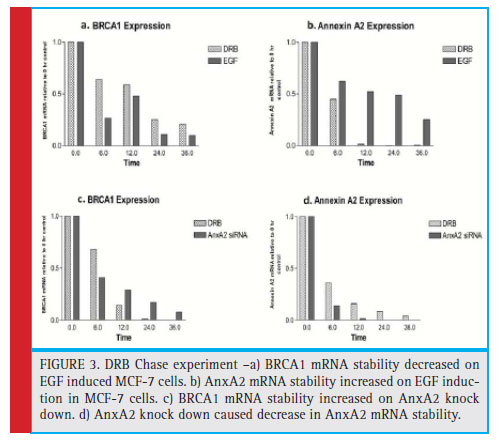
To address AnxA2 associated with BRCA1 mRNA, we investigated the effect of AnxA2 over expression on the regulation of BRCA1 mRNA stability. MCF-7 cells were induced to over express AnxA2 and studied the effect on BRCA1 mRNA stability. We observed decreased BRCA1 mRNA stability with time dependent manner, which shows AnxA2 overexpression degrades BRCA1 mRNA. To validate, AnxA2 associated in the regulation of BRCA1 mRNA. We knocked down AnxA2 by specific siRNA in MDAMB-231 cells and analyzed for BRCA1 mRNA stability. We found increase in BRCA1 mRNA stability in time dependent manner (fi gure 3). This shows AnxA2 is involved in regulation of BRCA1 mRNA. To study whether post-transcriptional regulation of BRCA1 occurs in sporadic breast cancer, we investigated BRCA1 expression and mRNA stability by DRB chase experiment. BRCA1 mRNA was normalized to control mRNA. We observed decrease in BRCA1 mRNA stability when we over expressed AnxA2 in TNBC cells. Interestingly, when we knocked down AnxA2 by AnxA2 specifics iRNA we found increase in BRCA1 mRNA stability (figure 3). This data suggests that AnxA2 may be involved in the post transcriptional processes as well as in the destabilization of BRCA1 mRNA (Tali 2017, Harahap 2018, Zhang 2018).
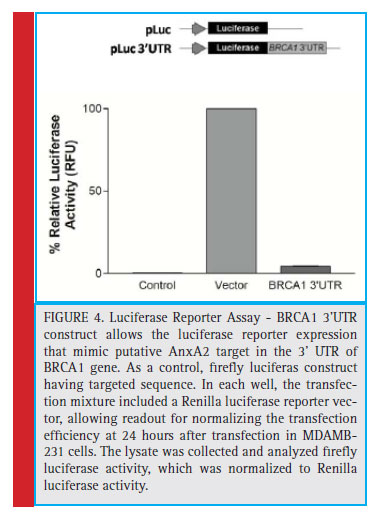
To investigate, AnxA2 is involved in the regulation of BRCA1 mRNA through the binding of BRCA1 3’UTR region. We designed pLuc vector having multiple cloning site downstream to a luciferase reporter gene in the coding region. The cloning of BRCA1 3’UTR construct allows the luciferase reporter expression that mimic putative AnxA2 target in the 3’ UTR of BRCA1 gene. As a control, firefly luciferase construct having targeted sequence was tested. In each well, the transfection mixture included a Renilla luciferase reporter vector, allowing readout for normalizing the transfection efficiency at 24 hours after transfection in MDAMB-231 cells. The lysate was collected and analyzed fi refl y luciferase activity, which was normalized to Renilla luciferase activity. We found reduced luciferase expression containing BRCA1 3’ UTR as compared to the control and pLuc vector control (fi gure 4). We identifi ed that AnxA2 interacts with BRCA1 3’UTR, leading to decreased luciferase activity (Maji 2016).
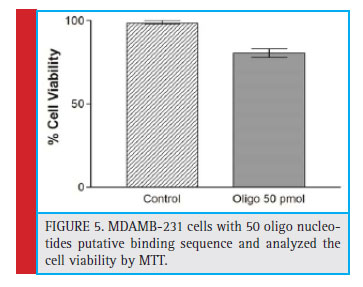 |
Figure 5: MDAMB-231 cells with 50 oligo nucleotides putative binding sequence and analyzed the cell viability by MTT. |
Effect of AnxA2 putative binding oligo on cell viability
To study the similarity or association of AnxA2 protein sequence with BRCA1 3’UTR, with the help of Vienna RNA web service for secondary structure prediction and BLAST of NCBI software we selected 50 nucleotide oligo having affinity of AnxA2 to bind BRCA1 3’UTR. We transfected MDAMB-231 cells with 50 oligo nucleotides and analyzed cell viability by MTT experiment. We found decrease in cell viability of oligo treated cells than without treatment (Figure 5). Finally, putative 50 oligo nucleotide transfected MDAMB-231 cells showed increased apoptosis upon performing cell viability assay. This suggests that, putative oligo nucleotide spares BRCA1 3’UTR from AnxA2 to accelerate tumor suppressor activity in turn promoting the cells to undergo apoptosis (Shetty 2012).
In summary, we demonstrate that AnxA2 is involved in BRCA1 gene expression at the posttranscriptional level as well as destabilization on BRCA1 in sporadic breast cancer.
Conclusion
In the present study, we demonstrate that AnxA2 has a role in posttranscriptional regulation of BRCA1 expression.
Acknowledgement
The authors would like to acknowledge the help of Harikrishna Ellanki, Avinash Kedari, Clinton D’Souza & Vishwas Kaveeshwar, SDM college of Medical Sciences & Hospital, Dharwad, Karnataka for their help.
References
Adnan M, Saldanha E, Palatty PL, Naik T, Monteiro AD, Manjeshwar, et al. (2018) Incidence of Breast Cancer in Women from Agriculture based Areas of Costal Karnataka, India: A Twenty Year Observations from a Tertiary Care Hospital. 3: ClinOncol.; 3: 1437
Anderson, P. and Kedersha, N. (2006). RNA granules. J. Cell. Biol. 172 (6): 803–808.
Baylin SB, Ohm JE. (2006). Epigenetic gene silencing in cancer- a mechanism for early oncogenic pathway addiction. Nat Rev Cancer. 6:107–116. doi: 10.1038/nrc1799.
Blasi F, Stoppelli MP. (1999).Proteases and cancer invasion: From belief to certainty. AACR meeting on proteases and protease inhibitors in cancer, nyborg, denmark, BiochimBiophys- Acta. 1423(1):R35-44.
Choi JY, James SR, Link PA, et al. (2009). Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 30:1889–1897. doi: 10.1093/carcin/ bgp143.
Cok, S.J. and Morrison, A.R. (2001). The 30-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational effi ciency. J. Biol. Chem. 276 (25): 23179–23185
Das PM, Singal R. (2004). DNA methylation and cancer. J Clin Oncol., 22: 4632–4642. doi: 10.1200/JCO.2004.07.151.
Diaz VM, Hurtado M, Thomson TM, Reventos J, Paciucci R. (2004).Specifi c interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut.;53(7):993-1000.
Engels, B.M. and Hutvagner, G.(2006). Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene 25 (46): 6163–6169.
Fu, L. and Benchimol, S. (1997). Participation of the human p53 30UTR in translational repression and activation following gamma-irradiation. EMBO J. 16 (13): 4117–4125
Harahap, W. A., Sudji, I. R., &Nindrea, R. D. (2018). BRCA1 Promoter Methylation and Clinicopathological Characteristics in Sporadic Breast Cancer Patients in Indonesia. Asian Pacifi c journal of cancer prevention: APJCP, 19(9), 2643-2649. doi:10.22034/APJCP.2018.19.9.2643
Huszno, J., Kołosza, Z., &Grzybowska, E. (2019). BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. ncology Letters, 17, 1986-1995.
Jodi M Saunus, Juliet D Freanch et al, (2008). post-transcriptional regulation of the breast cancer susceptibility gene BRCA1 by the RNA binding protein HuR. Cancer Res; 68(22); 9469-9478
Kedersha, N. and Anderson, P. (2002). Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30 (6): 963–969.
Lopez-de-Silanes, I. et al. (2005).Identifi cation and functional outcome of mRNAs associated with RNA-binding protein TIA- 1. Mol. Cell. Biol. 25 (21):9520–9531.
Maity, A., McKenna, W.G. and Muschel, R.J. (1997). Cyclin A message stability varies with the cell cycle. Cell Growth Differ. 8 (3):311–318.
Maji Sayantan, Pankaj Chaudhary, Irina Akopova, et al. (2016). ExosomalAnnexin A2 Promotes Angiogenesis and Breast Cancer Metastasis. Mol Cancer Res, DOI:101158/1541-7786.MCR-160163.
McCoy, M. L., Mueller, C. R., & Roskelley, C. D. (2003). The role of the breast cancer susceptibility gene 1 (BRCA1) in sporadic epithelial ovarian cancer. Reproductive biology and endocrinology: RB&E, 1, 72. doi:10.1186/1477-7827-1-72
Moore, M.J. (2005). From birth to death: the complex lives of eukaryotic mRNAs. Science 309 (5740):1514–1518
Newman Beth, A Melissa. Austin, Lee Ming and KingMary- Claire. (1988). Inheritance of human breast cancer: evidence for autosomal dominant transmission in high-risk families. 85(9): 3044-3048
Nicolas, E., Bertucci, F., Sabatier, R., &Gonçalves, A. (2018). Targeting BRCADefi ciency in Breast Cancer: What are the Clinical Evidences and the Next Perspectives?. Cancers, 10(12),506. doi:10.3390/cancers10120506
Rice, J. C., Massey-Brown, K. S., and Futscher, B. W. (1998). Oncogene 17: 1807–1812
Robertson KD. (2002). DNA methylation and chromatinunraveling the tangled web. Oncogene. 21:5361–5379. doi:10.1038/sj.onc.1205609.
Rosen, E.M., Fan, S.J., Pestell, R.G. and Goldberg, I.D. (2003). BRCA1 gene in breast cancer. J. Cell. Physiol. 196 (1):19–41.
Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK. (2012). Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS ONE. 7(9): e44299. DOI10.1371/journal.pone.0044299
Spiros Miyakis George, Sourvinos Demetrios A. Spandidos. (1998). Differential Expression and Mutation of the ras Family Genes in Human Breast Cancer. Biochem. Biophys. Res. Commun, 20(2), Pages 609-612
Tali Cohen Sinai, Zoya Cohen, Haim Werner, Raanan Berger. (2017). Identifi cation of BRCA1 as a potential Biomarker for Insulin like growth factor-1 receptor targeted therapy in breast cancer. Front Endocrinol. 8:10.3389/Fendo.2017.00148
Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. (2002).Plasmin- induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J Biol Chem. 277(37): 33564-70.
Thanin Chantarachot and Julia Bailey-Serres. (2018). Polysomes, Stress Granules, and Processing Bodies: A Dynamic Triumvirate Controlling Cytoplasmic mRNA Fate and Function. Plant Physiology. Vol. 176, pp. 254–269
Thompson, C., MacDonald, G., & Mueller, C. R. (2011). Decreased expression of BRCA1 in SK-BR-3 cells is the result of aberrant activation of the GABP Beta promoter by an NRF-1-containing complex. Molecular cancer, 10, 62. doi:10.1186/1476-4598-10-62
Volker Gerke, Stephen E, ( 2002).Annexins: From structure to function. Physiol Rev: 82; :331-371.
Wein, G., Rossler, M., Klug, R. and Herget, T. (2003).The 30-UTR of the mRNA coding for the major protein kinase C substrate MARCKS contains a novel CU-rich element interacting with the mRNA stabilizing factors HuD and HuR. Eur. J.Biochem.270 (2): 350–365.
Wiklund, L., Sokolowski, M., Carlsson, A., Rush, M. and Schwartz, S. (2002).Inhibition of translation by UAUUUAU and UAUUUUUAU motifs of the AU-rich RNA instability element in the HPV-1 late 30 untranslated region. J. Biol. Chem. 277 (43): 40462–40471.
Zhang Y, Meng L, Xiao L, Liu R, Li Z, Wang Y: (2018). The RNABinding Protein PCBP1 Functions as a Tumor Suppressor in Prostate Cancer by Inhibiting Mitogen Activated Protein Kinase 1. Cell Physiol Biochem. 48:1747-1754. doi: 10.1159/000492315