1Department of Microbiology, Bidhannagar College, Kolkata, West Bengal, India
2Department , HMM College for Women, Kolkata, West Bengal, India
Corresponding author email: zoologist.rehan@gmail.com
Article Publishing History
Received: 10/12/2020
Accepted After Revision: 23/03/2021
Anti-metabolites are produced by the microorganisms and have the highest potential as the agents of bio-preservation. The aim of the present research work was to study the antimicrobial activities of some selected antimicrobial and anti-metabolites producing microorganisms, against the microorganisms responsible for food spoilage. In addition to this, we tried to extract and isolate these microorganisms from natural sources available. In the present research work, a total of 75 bacterial cultures were extracted and isolated from different food samples and later they were purified and screened to record their antimicrobial activity against some food spoiling standard bacterial cultures and fungi which were isolated from spoiled vegetables and fruits. The isolated bacteria were kept and maintained on MRS medium which is a fast growing mesophile with low generation time.
Czapec-Dox agar media was used to keep and maintained the fungi isolated from several spoiled vegetables after purification. In order to test the antimicrobial activity of the supernatants from the isolates after the span of 18 hours of incubation against the fungi isolated from rotten vegetable like Tomato (Solanum lycopersicum), Cucumber (Cucumis sativus), Brinjal (Solanum melonngena) and rotten fruits like Orange (Citrus x sinensis), Grape (Vitis vinifera) and Apple (Malus domestica) the paper disk assay method was used. The antimicrobial activity of the supernatant was evidenced by the clear zone of inhibition ranging from 1.9 -3.5 cm by using 50 μl soup. It was found that out of seventy-five (75) isolates, three isolates, IP-1 (isolated from rotten peach), IVP-2 (isolated from vermicompost) and IRF-1(isolated from a teleost fish Rohu, Labeo rohita ) have most prominent and potent activity against standard bacterial cultures and fungi isolated from spoiled vegetable and fruits. It is evident from the present research that bio-control can be a potent method for food preservation.
Bio-Preservative, LAB, Rotten Vegetable And Rotten Fruits.
Kalam A, Ahmad S. R. Analysis on the Antifungal Activity of Bacteria Isolated from Rotten Fruits and Vegetables Along with their Partial Morphological and Biochemical Characterization. Biosc.Biotech.Res.Comm. 2021;14(1).
Kalam A, Ahmad S. R. Analysis on the Antifungal Activity of Bacteria Isolated from Rotten Fruits and Vegetables Along with their Partial Morphological and Biochemical Characterization. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3ttkKSP“>https://bit.ly/3ttkKSP</a>
Copyright © Kalam and Ahmad This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Food products that are likely to be perished, require protection from spoilage during the process of their preparation, storage and distribution. One of the biggest concerned and challenge for the food industry is safety and quality of food product, as there is huge demand for processed fresh food products across globe after the food industry being globalised. There is constant threat of contamination of these food products by microbes. Many of these microorganisms can cause undesirable reactions that deteriorate flavor, odor, color, sensory, and textural properties of foods.
The development and survival of common spolage and pathogenic microorganisms such as Clostridium perfringens, Escherichia coli, Staphylococcus aureus, Aspergillus niger, Listeria monocytogenes, Saccharomyces cerevisiae, Salmonella, Bacillus cereus, and Campylobacter are catalysed and affected by a variety of intrinsic factors like presence of oxygen and pH and extrinsic factors and conditions which includes relative humidity, time and temperature (Appendini and Hotchkiss 2002; Brewer, et al., 2002; Davidson et al., 2014).
To prevent growth of spoilage and pathogenic microorganisms in foods, several physical and chemical preservation techniques, such as heat treatment, salting, acidification, and drying have been applied in the food industry (Frakas, 2007; Gálvez et al., 2007). Benzoic acid, sulphur dioxide, sodium nitrite, sorbates, ethylene diamine tetra acetic acid, citric acid and butylated hydroxytoluene (BHT) are some of the examples of synthetic preservatives commonly used for enhancing the shelf life of edible materials, but their full compatibility with the human system is still questionable (Gálvez et al., 2007; Gutierrez et al., 2008; Davidson et al., 2014 Oechslin, 2018).
Moreover, sulphur dioxide causes breathing difficulties; sodium nitrite and BHT are reported to be carcinogenic (Gálvez et al., 2007; Gutierrez et al., 2008; Davidson et al., 2014). A few years back, concern regarding synthetic chemicals, additives were increased because of the great consumer awareness and foods preserved with natural additives became quite popular. Natural preservatives are thought to be better alternatives. Traditionally, herbal drugs were utilized in the form of mixture of different plants. Plant-based preservatives are biodegradable, renewable, and safe for non-target organisms and have diverse biological effects.
They provide less chance of resistance development to microbes. The antimicrobials can be used in different ways in order to control undesirable microorganisms, it can be directly added into the product formulation, coated on its surface or incorporated into the packaging material.The result of direct incorporation of active agents into foods is immediate but short-term reduction of bacterial population, on the other hand the antimicrobial films can control their activity for a longer period of time (Hanušová et al., 2009). The essential oil derived from plants (e.g., basil, thyme, oregano, cinnamon, clove, and rosemary), enzymes obtained from animal sources (e.g., lysozyme, lactoferrin), bacteriocins from microbial sources.
(natamycin, nisin,), organic acids (e.g., citric acid, sorbic, propionic,) and naturally occurring polymers (chitosan) are important natural compounds that can be used for food preservations.Essential oils from plants have started to gain wide interest from food industry as decontaminating agents, as they are Generally Recognized as Safe (GRAS). The active components found in essential oils have wide wide spectrum of antimicrobial activity, against food-borne pathogens and spoilage bacteria (López-Pedemonte et al., 2003; López-Malo, et al., 2005; Davidson et al., 2014). Another method that is widely receiving interest is the lactic acids bacteria, which are bacteriocin producing or somewhat more or less purified form of the same (López-Pedemonte et al., 2003; López-Malo, et al., 2005; Davidson et al., 2014).
This bacteriocin is usually smaller in kinds and show strong anti-microbial potential activities. This bacteriocin is even gram positive, has a larger spectrum of activities present against the various kinds of lactic acids, and has even able to achieve the GRAS standards across 50 countries worldwide as food preservatives (Rydlo, Miltz, and Mor, 2006; Sobrino-López and Martín-Belloso, 2006; Kilcawley and O’sullivan, 2017). The modernization in the lifestyles of the human has also resulted in various consequences that contribute to many kinds of outbreaks being caused by the food diseases. One of the most continuous concerns of the health of the public, economic and social cause is the infectious diseases that are caused in the intestinal of the human body by the various kinds of bacteria. Hence, one of the most promising tools that have emerged is biological preservation of the food (Kilcawley and O’sullivan, 2017).
The use of endolysins is considered to be safe as they do not create gene transduction issues or contribute to the emerging problem of resistant bacteria. Although there are concerns about the application of phages such as the emergence of phage-resistant bacteria and gene transductionendolysins do not create such problems; therefore, endolysins are promising biocontrol agents that could be applied in the field of food safety (Bakhshinejad et. al., 2014; Oechslin, 2018). The sole purpose of current investigation is to isolate the bacteria from spoiled food sample which produces antimicrobial substances and purified them.
There will be study made in the characteristics of these antimicrobial producing microbes and analysis will be made to know the application for the controlling the pathogens of that spoil the vegetable and fruits (Oechslin, 2018). To check the inhibitory effect of antimicrobial producing bacteria against food pathogenic fungi, the pathogenic food fungi will be isolated, purified and preserved for same. It has been revealed by the literature review that there has not been enough research done in this field. Bio-preservation is a promising strategy to control spoilage risk against lightly preserved food industries (Oechslin, 2018).
MATERIAL AND METHODS
For the isolation of the microorganisms (bacteria), which are anti-metabolite producing using the techniques of crowded plate from the natural sources. The first step was to collect various kinds of sample from the market, i.e., the spoiled food like spoiled fish, spoiled products of milk, spoiled dose, and spoiled products of meat. Then sterilized one gram of each of the sample and kept suspended in 10 ml of sterilized distilled water which is kept in a test tube. Now the sample needed for the process of inoculation, was spread on the MRS agar medium and left for incubation at 37o degrees centigrade for around 4-7 days. Soon after the process of incubation, it was seen that several colonies were appearing on the plates of the MSG agar (Table-01). The clear zones were surrounded by the colonies and were isolated and maintained on the slants of the MRS agar as the pure cultures.
Table 1. Composition of MRS Agar Medium
| Ingredients | Grams/Litre |
| Universal peptone | 10.0 |
| Meat extract | 5.0 |
| Yeast extract | 5.0 |
| D (+)-Glucose | 20.0 |
| Dipotassium hydrogen phosphate | 2.0 |
| Diammonium hydrogen citrate | 2.0 |
| Sodium acetate | 5.0 |
| Magnesium sulfate | 0.1 |
| Manganous sulfate | 0.05 |
| Agar | 2% |
For the screening of the anti-microbial activity against the standard cultures of the bacteria, the bacterial colonies which were isolated by previous step were maintained on MRS agar slants and same were inoculated in MRS broth and kept in incubator for overnight at 370C. Broth culture of each isolated organisms amounting 1.5 ml, after overnight incubation was taken in sterilizeeppendorf and centrifuged at 10,000 rmp for 15 min. After centrifugation, 50 μl cell-free culture supernatant was applied into the well on agar plates previously inoculated with indicator organisms (MTCC 3041, Lactococcus lactis subsp. lactis) and then plates were incubated at 37°C for 24 hours. The place was observed for the presence of zone of inhibition after incubation process. Out of 75 isolates (Table -02), bacteria isolatedIP-1 (isolated from rotten peach), IVP-2 (isolated from vermicompost) and IRF-1(isolated from Rohufish) had shown antimicrobial activity against the three indicator organisms. After this the diameters of zone of inhibition were pen downed.
Table 2. Details of 75 Isolated colonies
| Sl. No. | SAMPLES | ISOLATED PURE COLONIES | No. of colonies |
| 01 | Fish intestine | A) F1(white).
B) F2(Yellow) C)F3(Off white) D)F4 (Gummy white) |
4 |
| 02 | Peach | A) Peach1 (cream colour) (IP-1)
B) Peach 2 (gummy) |
2 |
| 03 | Idli | A) Idli1(pure white)
B) Idli2 (Whitish) |
2 |
| 04 | Salted fish (Rohu) | A) Salted fish1(White) | 1 |
| 05 | Salted (3% NaCl)
Cabbage after 3 days |
A) Cab1 (Slight redish)
B) Cab2 (Cream color) |
2 |
| 06 | Guava | A) Guava1
B) Guava2 |
2 |
| 07 | Goat milk | A) Goat milk1 (White)
B) Goat milk2 (Whitish) |
2 |
| 08 | Cucumber | A) Cucumber1 (slight yellow)
B) Cucumber2 (Offwhite) C)Cucumber3 (Cream) |
3 |
| 09 | Apple | A) Apple1(Maroon)
B) Apple2 (White) |
2 |
| 10 | Pineapple | A) Pineapple1
B) Pine apple 2 C)Pineapple 3 |
3 |
| 11 | Sosage | A) Sosage1 (Offwhite)
B) Sosage2 (Yellow) |
2 |
| 12 | Papine | A) Papine1 (Dark yellow)
B) Papine2 (Pure white) C)Papine3 (Cream) |
3 |
| 13 | Salted Cabbage after 9 days of storage | A) Cab1 (Yellow)
B) Cab2 (White) |
2 |
| 14 | Mango | A) Mango1 (White)
B) Mango2 (Faint white) |
2 |
| 15 | Dosachatni | A) Dosa chatni1 (Slight yellow)
B) Dosachatni (White, glossy) |
2 |
| 17 | Vermi compost | A) Vermicompost1 (White)
B) Vermicompost (Slight yellow) (IVP-2) |
2 |
| 18 | Soya sausage | A) Soya sosage1
B) Soya sosage2 |
2 |
| 19 | Salted cabbage after 16 days of storage | A) Cab1 (Pure yellow)
B) Cab2 (Pure White)
|
2 |
| 20 | Papine (3 days old) | A) Pap1 (maroon)
B) Pap2 (Cream) |
2 |
| 21 | Taal (Palm) | A) Taal1 (Gummy white)
B) Taal2 (white, tiny) C)Taa3(Off-white) |
3 |
| 22 | Without salted cabbage | A) Without salted cab1
B) Without salt6ed cab2 (Slight yellow) C)without salted cab3 (Cream) |
3 |
| 23 | Sambar | A) Sambar1
B) Sambar 2 C)Sambar 3 |
3 |
| 24 | Fish | A) Fish1 (Dark yellow)
B) Fish2 (Yellow) C)Fish3 (White) |
3 |
| 25 | Rohu Fish | A) Rohufish (Yellow) (IRF -1)
B) Rohu fish2 (White) |
2 |
| 26 | Food grain | A) Food grain1(yellow)
B) Food grain2 (Whitish) |
2 |
| 27 | Milk | A) Milk1 (White)
B) Milk2 (Tiny white) C)Milk3 (White droplet like) |
3 |
| 28 | Doi | A) Doi1 (White round)
B) Doi2 (White, Gummy) |
2 |
| 29 | Cabbage after 28 days | A) Cab1 (White)
B) Cab2 (Cream) |
2 |
| 30 | Naspati | A) Naspati1 (Yellow)
B) Naspati 2 (White) (IN-1) |
2 |
| 31 | Bamboo chatni | A) Bamboo cahtni1 (White)
B) Bamboo chatni2 (Off white) C) Bamboo chatni3 (Off white) |
3 |
| 32 | Soya sausage (After 14 days of storage) | A) Soya1 (Yellow)
B) Soya2 (White) C)Soya (Gray) |
3 |
| 33 | Soil from vat | A) Soil1 (White)
B) Soil2 (Yellow) C) Soil 3(Gray) |
3 |
| 34 | Donkey Milk | A) Milk (White)
B) Milk (yellowish) |
2 |
| Total number of isolated colonies | 75 | ||
activity against the three indicator organisms. After this the diameters of zone of inhibition were pen downed.
To find the solution of food spoilage fungi from different spoiled fruits and screening of their sensitivity by well diffusion method, three types of rotten vegetables and three types of rotten fruits like Tomato (Solanum lycopersicum), Cucumber (Cucumis sativus), Brinjal (Solanum melonngena) and Orange (Citrus x sinensis), Grape (Vitis vinifera) and Apple (Malus domestica) respectively were collected from local market of Salt Lake, Kolkata, West Bengal. Samples of all six individual items were taken in a test tube per sample 1 gm and suspended in 10 ml sterilized distilled water. This mixture was then taken to be incubated at 370C while being streaked on the Czapec-Dox agar plate for around 3-5 days.
After 3-5 days it was seen that Czapec-Dox agar plates were filled with fungi and these fungi was later maintained and isolated on the slants of the Czapec-Dox agar plate as the pure culture. Furthermore, a culture was prepared in a test tube containing 5 ml of each of MRS broth medium and Czapec-Dox agar. MRS broths were inoculated with the four test isolates and incubated under shaking condition for about 18 hours at 370C.
The supernatant of each sample was then collected by centrifugation at 10000 rpm for 12 min. Six Czapek-Dox agar plates were prepared and six fungal cultures were inoculated on six different plates. Four wells were made on each Czapek-Dox agar plate and filled with 50 ul supernatant of the four isolates. Then the plates were incubated at 370C for 3 to 5 days. Observations were made and the effects of the supernatants on the spoilage organisms were recorded. For the morphological and biochemical characterization of IRF-1 and determining the growth curve, IRF-1 was inoculated in 100 ml MRS broth medium and incubated in a rotary shaker at 370C. At 1-hour intervals, the absorbance of the culture was monitored in a colorimeter at 540 nm
RESULTS AND DISCUSSION
Since long time microorganism like LAB are used in fermentation because of their beneficial properties and major role on nutritional enhancement, organoleptic, and shelf –life characteristic. These organisms help in causing a speedy acidification through the process of producing anti-microbial substances inside the raw materials that results in preserving the nutritional values of the edible products via increasing the shelf life of these products and inhibiting the spoilage that is caused by the pathogenic bacteria. Apart from these, there are several lactic acid bacteria that are known to be food grade found and used in the fermentation of food (Kilcawley and O’sullivan, 2017; Oechslin, 2018).
In the current investigation it was observed that the isolate IRF-1, IP1 and IVP-2 can inhibit the growth of the pathogenic fungi isolated from rotten vegetables/fruits. The supernatant of isolate IRF-I, IP-1 and IVP-1 after 18 hours of growth produced clear zone of inhibition against spoilage fungi isolated from Tomato (Solanum lycopersicum), Cucumber (Cucumis sativus), Brinjal (Solanum melonngena) and rotten fruits like Orange (Citrus x sinensis), Grape (Vitis vinifera) and Apple (Malus domestica). The range of inhibition zone diameter was between 1.9-3.5 cm. Morphological, physiological and biochemical characters of IRF-1 was studied and recorded.
By 16S rRNA sequencing analysis isolate IRF-1 was identified as Bacillus subtilis subsp. inaquosorum (Identified from IMTECH Chandigarh) (Kilcawley and O’sullivan, 2017; Oechslin, 2018).
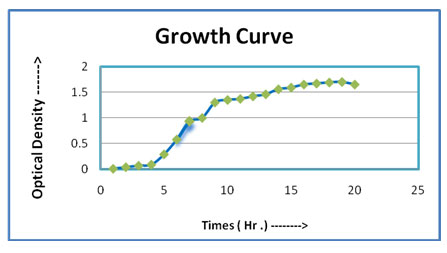
Biochemical, Morphological and Physiological characters of IRF-1: The IRF-1 had its growth curve, which represented that the IRF-1 (Figure -01). Its lag phase occured during the 0-4 hours, it experienced its log phase during the 5-18 hours, experienced the stationary phase during the period of 18-21 hours and later after the 21 hours mark, the death phase started. The Figure – 02 of stain IP-1 showing the growth curve that it experienced its lag phase during the 0-5 hours mark, it experienced its log phase around 5-19 hours mark, experienced the stationary phase at around the 19-22 hours mark and after the 22 hours mark, it experienced the death phase. By 16S rRNA sequencing analysis isolate IRF-1 was identified as one of bacteria belongs to Bacillus group of bacteria. After 16S rRNA sequencing analysis isolate IRF-1 was identified as Bacillus subtilis subsp. inaquosorum (Identified from IMTECH Chandigarh) (Oechslin, 2018).
Figure 1: Growth curve of IRF -1
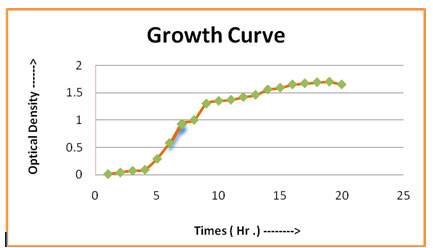
Figure 2: Growth curve of IP -1
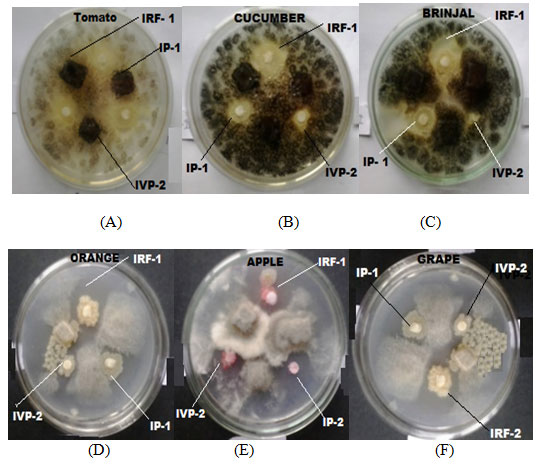
Screening for antimicrobial activity against standard bacterial cultures and against fungi isolated from spoiled fruits: There were seventy-five (75) morphological different bacterial colonies selected from different food samples. Furthermore, the paper disc method was implied in order to screen each individual isolated strain for the antagonistic activity that might have taken place against the cultures of the standard bacteria. Out of those isolated bacteria only IP-1, IRF-1 and of the IVP-2 was able to reflect antimicrobial activities against the standard bacterial cultures (MTCC 3041) (Picture -01).
Figure 3: Anti-microbial activity of 14 hours grown culture of IRF-1, IP-1 and IVP-2 against MTCC 3041(Lactococcus lactis subsp. lactis.)
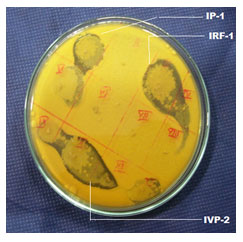
Figure 4: Morphological structure after 12 hrs (A) IRF-1 (B) IP-1
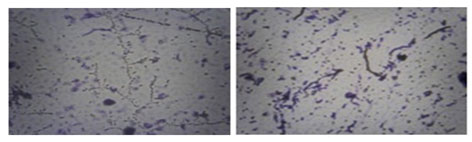
The characterization was done using the biochemical and the morphological processes of the IRF-1 and was performed using the cultures that were kept for 24 hours. The process of Gram straining along with the observation of the microbes aided in identifying that the IRF-1 is gram positive and is a rod-shaped bacterium existing in chain formations. It was observed from the result that the IP-1 and the IRF-1 was able to grow at around 30 0C, 370C and 450C and the maximum growth potential was recorded at around temperature of 370C and they were unable to grow at 40C and 100C. Regarding the tolerance of the pH levels, the IRF-1 and IP-1 were only able to grow at the pH -7 and were not able to grow at the pH – 3, pH – 5, and pH – 9. Regarding the tolerance of salt concentration, it has been observed in case of IRF -1, at 5% the growth went up to 1.16, at 10% went up to 1.01, at 15% negligible growth took place and at 20% there was no growth at all. In case of IP-1, at 5% the growth went up to 0.02, at 10% went up to 0.01, at 15% extremely negligible growth took place and at 20% there was no growth at all (Table -03).
Table 3.Temperature, pH and Salt Concentration of two isolates (IP-1 and IRF-1)
| Sample | Parameter | Variation | After 24 hrs | After 48 hrs |
|
IP -1 |
Temp. |
4oC | – | – |
| 10oC | – | – | ||
| 30oC | + | +++ | ||
| 37oC | ++ | +++ | ||
| 45oC | +++ | +++ | ||
|
pH |
3 | – | – | |
| 5 | – | – | ||
| 7 | + | + | ||
| 9 | – | – | ||
|
Salt Concentration |
5% | – | 0.02 | |
| 10% | – | 0.01 | ||
| 15% | – | 0.0 | ||
| 20% | – | 0.0 | ||
|
IRF-1 |
Temp. |
4oC | – | – |
| 10oC | – | + | ||
| 30oC | ++ | +++ | ||
| 37oC | ++ | +++ | ||
| 45oC | +++ | +++ | ||
|
pH |
3 | – | – | |
| 5 | – | – | ||
| 7 | + | + | ||
| 9 | – | – | ||
|
Salt Concentration
|
5% | – | 1.16 | |
| 10% | – | 1.01 | ||
| 15% | – | 0.5 | ||
| 20% | – | 0.0 |
Testing was also done to indentify the ability of the isolates to utilize various sugars in order to grow using them as the sole carbon sources in the MRS broth medium using various sugars (Mannitol, Sucrose, Lactose, Rhamnose, Cellobiose, Fructose, Galactose, Dextrose, Trehalose, Inositol and Maltose) (Table -04) (Oechslin, 2018).
Table 4. Utilization of different carbon sources was studied of two isolates
| Carbon sources | IRF -1 | IP-1 |
| After 24 hrs | After 24 hrs | |
| Mannitol | + | – |
| Sucrose | + | + |
| Lactose | + | + |
| Rhamnose | + | + |
| Cellobiose | – | – |
| Fructose | + | + |
| Galactose | + | + |
| Dextrose | + | + |
| Trehalose | + | + |
| Inositol | + | + |
| Maltose | + | + |
The findings showed that the IRF-1 was capable to utilize all the sugar but cellobiose and in the case of the IP-1 the exceptions of the sugars, which it was not able to utilize, was the mannitol and the cellubiose. The fungi were isolated from three rotten vegetables namely Tomato (Solanum lycopersicum), Cucumber (Cucumis sativus), Brinjal (Solanummelonngena) and three rotten fruits namely Orange (Citrus x sinensis), Grape (Vitis vinifera) and Apple (Malus domestica). Out of the three isolates that were being observed, IRF-1 was able to reflect the maximum antimicrobial activities against the fungi, which was isolated from the rotten Rohu Fish. The zone of inhabitation ranges from 2.2 cm to 3.5 cm for the IRF-1 and for the IP-1 the ranges is from 2.1cm to 3.3 cm and for the IVP-2 isolates the range is from 1.9 cm to 2.8cm for the Lactobacillus plantarun (MTCC3089, indicator organism) were observed (Table – 5 and Picture from 03- 08) (Kilcawley and O’sullivan, 2017; Oechslin, 2018).
Table 5. The clear zones surrounding the wells (the zone of inhibition) were observed and the results were recorded
| Serial No. | Source of spoilage fungi | Supernatant of the Isolates | Presence of zone of inhibition | Diameter of zone of inhibition in cm |
| 1. |
Tomato |
IRF-I | + | 2.6 |
| IP-1 | + | 2.3 | ||
| IVP-2 | + | 2.2 | ||
| 3089 | + | 2.1 | ||
| 2.
|
Orange |
IRF-I | + | 2.7 |
| IP-1 | + | 2.6 | ||
| IVP-2 | + | 2.5 | ||
| 3089 | + | 2.2 | ||
| 3. |
Cucumber |
IRF-I | + | 2.8 |
| IP-1 | + | 2.9 | ||
| IVP-2 | + | 2.6 | ||
| 3089 | + | 1.9 | ||
| 4. |
Grape |
IRF-I | + | 3.2 |
| IP-1 | + | 3.1 | ||
| IVP-2 | + | 2.8 | ||
| 3089 | + | 2.2 | ||
| 5. |
Apple |
IRF-I | + | 3.5 |
| IP-1 | + | 3.3 | ||
| IVP-2 | + | 2.7 | ||
| 3089 | – | – | ||
| 6. | Brinjal | IRF-I | + | 2.2 |
| IP-1 | + | 2.1 | ||
| IVP-2 | + | 1.9 | ||
| 3089 | – | – |
Figure 5: Graphic representation of Zone of Inhibition against all 6 fungi
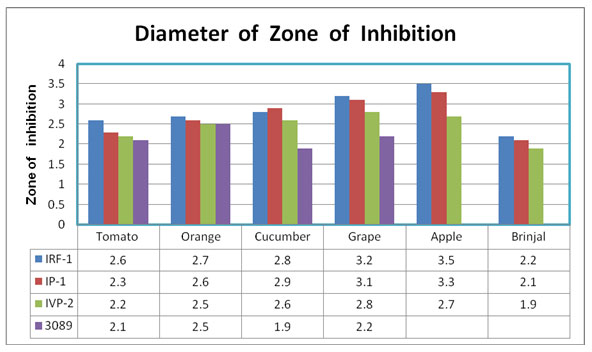
Figure 6: 08 Inhibition zone observed in the plates containing different pathogenic fungus culture, A = Tomato: B = Cucumber: C = Brinjal: D = Orange: E = Apple: F= Grape
CONCLUSION
The present study has found that the isolate IRF-1, IP1 and IVP-2 can inhibit the growth of the pathogenic fungi isolated from rotten vegetables and fruits. The current research also revealed that in future bio-control could be a major part of food industry for preservation of food items, because preservation of food by means of biological ways can be highly accepted as it would be non-toxic in nature.
ACKNOWLEDGEMENTS
The authors would like to thank to Bidhannagar College for providing the infrastructure and West Bengal Dept. of Science and Technology (WBDST), Govt. of West Bengal for funding the project. The author received financial support for the said research project from West Bengal Department of Science and Technology (WBDST), Government of West Bengal, India.
Conflict of Interest: The authors declare that there exist no commercial or financial relationship that could, in any way, lead to potential conflict of interest.
REFERENCES
Appendini, P. and Hotchkiss, J. H. (2002). Review of antimicrobial food packaging, Innovative Food Science and Emerging Technologies, 3(2), pp. 113-126. doi:10.1016/s1466-8564(02)00012-7.
Bakhshinejad, B. and Sadeghizadeh, M. (2014). Bacteriophages as vehicles for gene delivery into mammalian cells: Prospects and problems, Expert Opin. Drug Delivery, 11, pp.1561–1574.
Brewer, R., Adams, M., and Park, S. (2002). Enhanced inactivation of Listeria monocytogenes by nisin in the presence of ethanol, Letters in Applied Microbiology, 34(1), pp. 18-21. doi:10.1046/j.1475-765x.2002.01035.x.
Davidson, P. M., Taylor, T. M., and Schmidt, S. E. (2014). Chemical Preservatives and Natural Antimicrobial Compounds, Food Microbiology, pp. 765-801, doi:10.1128/9781555818463.ch30.
Frakas, J. (2007). Physical Methods of Food Preservation. Food Microbiology: Fundamentals and Frontiers, Third Edition, pp. 685-712, doi:10.1128/9781555815912.ch32.
Gálvez, A., Abriouel, H., López, R. L., and Omar, N. B. (2007). Bacteriocin-based strategies for food biopreservation, International Journal of Food Microbiology, 120(1-2), pp.51-70. doi:10.1016/j.ijfoodmicro.2007.06.001.
Gutierrez, J., Barry-Ryan, C., and Bourke, P. (2009). Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components, Food Microbiology, 26(2), 142-150, doi:10.1016/j.fm.2008.10.008.
Gutierrez, J., Barry-Ryan, C., and Bourke, P. (2008). The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients, International Journal of Food Microbiology, 124(1), pp. 91-97, doi:10.1016/j.ijfoodmicro.2008.02.028.
Hanušová, K., Dobiáš, J., and Klaudisová, K. (2009). Effect of Packaging Films Releasing Antimicrobial Agents on Stability of Food Products, Czech Journal of Food Sciences, 27(Special Issue 1), doi:10.17521/958-cjfs.
Kilcawley, K., and O’sullivan, M. (2017). Cheese Flavour Development and Sensory Characteristics, Global Cheesemaking Technology, pp. 45-70, doi:10.1002/9781119046165.ch0c.
López-Malo, A., Alzamora, S. M., and Palou, E. (2005). Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds, International Journal of Food Microbiology, 99(2), pp.119-128, doi:10.1016/j.ijfoodmicro.2004.08.010.
López-Pedemonte, T. J., Roig-Sagués, A. X., Trujillo, A. J., Capellas, M., and Guamis, B. (2003). Inactivation of Spores of Bacillus cereus in Cheese by High Hydrostatic Pressure with the Addition of Nisin or Lysozyme, Journal of Dairy Science, 86(10), 3075-3081. doi:10.3168/jds.s0022-0302(03)73907-1.
Oechslin, F. (2018). Resistance development to bacteriophages occurring during bacteriophage therapy, Viruses, 10, pp. 351.
Rydlo, T., Miltz, J. and Mor, A. (2006). Eukaryotic Antimicrobial Peptides: Promises and Premises in Food Safety, Journal of Food Science, 71(9), doi:10.1111/j.1750-3841.2006.00175.x.


