1Key Laboratory of Biomedical Engineering & Technology of Shandong High School, Qilu Medical University, Zibo, 255213, China
2School of clinical medicine, Binzhou Medical University, Yantai, 264003, China
Corresponding author email: wql_zcq@126.com
Article Publishing History
Received: 10/12/2020
Accepted After Revision: 21/03/2021
Bisphenol A (BPA) is an endocrine disrupting chemical that is widely used in the synthesis of polycarbonate and epoxy resins and plays A role in the manufacture of small plastic bottles, food packaging, water bottles, medical devices and dental sealants. In recent years, studies have shown that BPA has different harms on various human systems, including nervous system, urinary system, reproductive and immune system, digestive system, behavioral development, etc., and the harm of BPA to human body may last for several generations. In recent years, studies have shown that BPA has different harms on various human systems, including nervous system, urinary system, reproductive system, digestive system, and behavioral development, etc. the harm of BPA to human body may last for several generations.
So we critically reviewed the recent literature on the role of BPA in the nervous system. At the same time, the role of BPA in nervous system is reviewed. This paper reviews the effects of bisphenol A on the nervous system from three aspects: molecular, cellular and behavioral development. It provides a material basis for the subsequent study of bisphenol A and nervous system.
Bisphenol A, Nervous system, Neuron, Brain estrogen, Neural stem cells
Wang R, Li N, Dong Y, Cai Y, Ye G, Wang Q. An Updated Review on Harmful Effects of Bisphenol a on Human Nervous System from Molecular, Cellular and Behavioral Aspects. Biosc.Biotech.Res.Comm. 2021;14(1).
Wang R, Li N, Dong Y, Cai Y, Ye G, Wang Q. An Updated Review on Harmful Effects of Bisphenol a on Human Nervous System from Molecular, Cellular and Behavioral Aspects. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3vG5EeO“>https://bit.ly/3vG5EeO</a>
Copyright © Wang et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The nervous system is composed of the central part and the peripheral part, which plays a leading role in regulating the physiological functions of the human body. The functions of various organs and systems are directly or indirectly controlled and coordinated by the nervous system, making the human body into a unified whole (Jalilian, et al. 2019).
The nervous system can accept all kinds of information about changes in the internal and external environment, analyze and integrate them, and make the body respond to stimuli accordingly, so as to maintain the unity between the body and the internal and external environment (Heuckeroth, Schafer 2016).
In addition, the high development of cerebral cortex makes the brain become the highest center and the higher organ of thought and consciousness. Accordingly, nervous system has crucial effect to human body, substances that do damage to nervous system can produce adverse effect to many aspects of the human body. Bisphenol A inhibits the proliferation of neurons and neural stem cells (Liang, et al. 2020).
BPA has estrogen-like effects, and its exposure affects the synthesis of endogenous estrogen, leading to endocrine disorders and interfering with the human metabolic process (Spackova, et al. 2019). In this review, we mainly summarized the effects of BPA on the nervous system and the possible mechanism, which can provide a reference for future studies on the effects of BPA on human body.
Effect of BPA on estrogen in the brain: Aromatase is the rate-limiting enzyme in the process of estrogen synthesis, which can catalyze the conversion of androgen to estrogen. Bisphenol A increases the level of aromatase by enhancing the expression of brain-specific aromatase, thus increasing the endogenous estrogen level in the developing brain (Chung, et al. 2011).
So BPA, a form of estrogen, interferes with estrogen dependent processes by binding to estrogen receptors (ERS) (Adewale, et al. 2011). As A powerful estrogen analogue, bisphenol A not only interferes with normal hormonal regulation in synaptic plasticity and memory in female mice but also shows estrogen-damaging effects at different concentrations of circulating estrogen (Xu, et al. 2015).
BPA exposure interferes with the development of signaling pathways in the brain such as estrogen, oxytocin and vasopressin that are critical for synaptic organization and delivery (Arambula, et al. 2018). Corticosterone and its role in the brain are easily influenced by the programming effects of current acceptable doses of BPA. Corticosterone levels rose in body exposed to low doses of BPA (Poimenova, et al. 2010).0
In addition, bisphenol A enhanced mRNA expression levels of extracellular signal regulated kinase (ERK), fatty acid amide hydrolase (FAAH) and sodium gated channel (NAV 1.8), leading to changes in the expression of estrogen and pain-related genes and increased migraine in mouse models (Vermeer, et al. 2014).
Environmental BPA exposure enhanced the biosynthesis of local estrogen in the brain and further inhibited the ER-β signaling pathway in the nervous system. Perinatal exposure to BPA not only significantly inhibited the expression of Nr1, Nr2A, and 2B of the NMDAR subunits in the developing hippocampus, but also decreased the expression of ER-Beta (Xu, et al. 2010, Xu, et al. 2010). Prenatal exposure to low doses of bisphenol A alters estrogen receptor expression levels in newborns of both sexes (Cao, et al. 2013).
Fahrenkopf and others in the maternal exposure to bisphenol a mouse fetal estrogen receptor (ERalpha) dependence were used in this study the effects of progesterone receptor (PR) expression bioassay method, the results show that the gestation maternal exposure to bisphenol A (BPA) side inside the offspring pronucleus (MPN) of PR has enhanced the role of immune responses (PRR) level, and this effect is done through activation of estrogen to ERalpha, instead of ERbeta ( Fahrenkopf, et al.2020).
The results of Tonini, C et al showed that prenatal BPA exposure did not affect ERα phosphorylation in female fetuses but did affect ERα phosphorylation in male fetuses. In conclusion, the effect of BPA on the induction of estrogen receptor alpha is sex-dependent and more significant in males than females (Tonini, C, et al. 2020).
Effect of BPA on neurotransmitters: Perinatal exposure to low doses of BPA disrupts overall metabolism and brain function in CD-1 mice (Cabaton, et al. 2013). Low doses of BPA disrupt the serotonin system in prenatal and lactating mice and significantly increase dopamine, serotonin, and metabolites in the putamen, thalamus, and plasma of mice at 3 weeks (3 weeks) and/or 14 — 15 weeks (14 weeks) postpartum (Sarrouilhe, Dejean 2017, Nakamura, et al. 2010).
When the perinatal female mice exposed to bpa, like the 3-4-2 hydroxy benzene acetic acid (DA metabolites), temperature, acid (HVA DA metabolites), 5 – hydroxy indole acetic acid (5 – hydroxy indole acetic acid) and 5 – hydroxy ethyl amine acid ester (5 – glycolic acid) and many other chemicals in its descendants in the brain or blood increased, however, the proportion of HVA/DA only in certain areas of the brain (Honma, et al. 2006). So, not only are the effects of BPA different in different areas of the brain but the effects of BPA exposure are different in different genders.
Exposure to low doses of bpa causes of male mice, the amygdala and hippocampus of GABA levels drop, but some areas of Glu levels rising, however for perinatal and lactation female mice low dose of bisphenol A is not only the brain almost all areas of GABA (gamma-aminobutyric acid) and Glu (glutamic acid) levels, and lead to the cerebral cortex and the hypothalamus NE levels (Ogi, et al. 2015). Therefore, the effect of bisphenol A on females and males is different.
Perinatal exposure to low doses of BPA resulted in reduced levels of neurotransmitters such as gamma-aminobutyric acid and glutamate in serum and brain samples of neonates with PND21 (21 days postpartum) (Cabaton,et al 2013). BPA may affect monoamine (which increases serotonin and dopamine) levels in newborns, although the dose of BPA is lower than the prescribed environmental level, 28 days after injection and 2 days after birth (Matsuda, et al. 2010).
Therefore, in the Tang and others with blood clam (Tegillarca granosa) as the research object, using exposure to accord with the actual concentrations of BPA and environment MPs to observe BPA and MPs alone or with the effects of exposure on the immune and nervous biomarkers of this study found that BPA not only has obvious immune toxicity, but also lead to treatment of BPA and MP NFkappaB signaling pathways in four immune related gene expression level serious inhibition ( Tang, et al. 2020).
Exposure to BPA and/or DEHP induces apoptosis and histopathological changes in hippocampal cells. In addition, the mechanism of neurotoxicity induced by BPA and DEHP may be changes in oxidative/antioxidant status as well as neurotransmitters and related enzymes ( Yirun, et al.2021). Maternal exposure to BPA reduced the levels of hippocampal neurotransmitters such as Glu/GABA in F1 offspring. The adverse neurodevelopmental effects of maternal exposure to environmental doses of BPA persisted into the offspring ( Zhang, et al.2020).
BPA affects the dopamine system: BPA has an effect on the dopamine system. Studies have shown that when mice are exposed to BPA, the dopamine system of mice changes, and the changes are more evident during organ development and lactation (Narita, et al. 2007, Suzuki, et al. 2005). Long-term chronic exposure to BPA during organogenesis or lactation enhances the function of dopamine D1 receptors and activates G proteins in the peripheral brain (Suzuki, Mizuo, Miyagawa, Narita 2005, Suzuki, et al. 2003). Prenatal and neonatal exposure to BPA in rats results in enhanced central dopaminergic system, hypersensitivity to the abuse of reward-acting drugs, and hyperactivity (Suzuki, Mizuo, Miyagawa, Narita 2005).
BPA reduces the expression of the dopamine transporter gene in adult mice (Ishido, et al. 2007). Castro et al. found that BPA, BPF, and BPS had different effects on the expression of genes related to the 5alpha-R and DA/5-HT systems in female PFC (Castro, et al. 2015).
The results show that BPA causes behavioral changes in zebrafish and the mechanism of these changes is the high accumulation and dysregulation of the neurotransmitter systems of serotonin, globulinergic, dopaminergic, cholinergic and GABAergic (Kim, et al. 2020).
Toxic effect of bisphenol A on DNA methylation: Environmental chemicals can affect human health and disease in ways that affect DNA modification. The epigenetic effect of bisphenol A was sufficiently demonstrated by the reduction of CpG methylation upstream of the acanthopteris gene, and in female acanthopteris the epigenetic effect is multigenerational (Singh, Li 2012). Prenatal exposure to low doses of BPA can cause long-term epigenetic damage to the brain (Kundakovic, et al. 2013).
Early exposure to BPA causes epigenetic dysfunction and neurodevelopmental disorders by altering the brain’s epigenetic mechanisms and gene expression levels (Kubota 2016). Exposure to BPA during the early stages of development leads to the continued accumulation of fat by reducing the methylation of fat genes (Shimpi, et al. 2017). Exposure to bisphenol A in the fetal period can damage the naturally occurring bifeng DNA that is associated with obesity-related DNA methylation (Taylor, et al. 2018).
2.6- dibipa, tripipa and tibipa had the effect of increasing lipid accumulation and the expression of specific protein 2 in adipocytes, which increased dose dependence of PPAR gamma transcriptional activity. In addition, TeBBPA, debromide and bromide accumulating in breast milk play an important role in promoting adipocyte differentiation (Akiyama, et al. 2015).
In addition, exposure to BPA resulted in reduced DNA methylation of germ cell imprinted genes IGF2R, PEG3, and H19 in fetal mice (Zhang, et al. 2012). Exposure to BPA altered the expression level of microRNA in human placental cell lines and the treatment of bisphenol A had a strong induction effect on miR-146a in particular (Singh, Li 2012).
Reactive oxygen species (ROS) are closely related to oxidative damage and carcinogenesis of cells, and bisphenol A can cause the production of ROS (Lei, et al. 2018). Low-dose BPA significantly promoted DNA damage (Pfeifer, et al. 2015). When human breast epithelial cells are exposed to BPA, it results in increased methylation of genes associated with the development of most or all tumor types, such as BRCA1, CCNA1, THBS1, TNFRSF10C, and TNFRSF10D (Qin, et al. 2012).
After mothers were exposed to BPA, their offspring showed brain cell DNA damage, and this damage was only seen in the F1 generation (Zhang, et al. 2020). In addition to influencing the methylation patterns of genes such as those that encode proteins associated with reproductive physiology, BPA can have a direct effect on genes responsible for DNA methylation (Cariati, et al. 2020).
Effect of bisphenol A on neurons: BPA had no effect on nerve survival and nerve cell size in postperinatal mice, but the apoptosis of dopaminergic neurons in midbrain of weaned mice and even reduced motor neuron pool volume in adult mice (Lin, et al. 2006, Jones, et al. 2016).
Fetal exposure to low doses of BPA inhibits the release of excitatory neurons in offspring and disrupts cortical neurogenesis and neuromorphic development during neuronal migration in the hippocampus, thus disrupting the localization of mouse offspring neurons and forming between the thalamus and cerebral cortical networks. The damage may even continue into adulthood (Mathisen, et al. 2013, Ling, et al. 2016, Kimura, et al. 2016). BPA is not an anti-androgen mechanism but acts through A non-androgen receptor-dependent mechanism (Jones et al 2016).
Bisphenol A phosphorylates NR2B of the NMDAR subunit through the estrogen receptor mediated pathway, and thus rapidly increases the activity and density of cultured hippocampal neurons (Xu, et al. 2011, Xu, et al. 2010). Bisphenol A reduces the differentiation of dopaminergic neurons by inhibiting the expression of IGF-1 (Huang, et al. 2017). After local injection of bisphenol A into primary visual cortex A17, the targeting selectivity of neurons was significantly increased, while the activity of neurons was rapidly inhibited and the activity of other neurons decreased (Xu, Ye, Li, Chen, Tian, Luo, Lu 2010). Exposure of Hess-derived embryoids to bisphenol A resulted in A decrease in the number of neural precursor cells (NPC) and Hess-C-derived large neurons (Huang, Ning, Zhang, Chen, Jiang, Cui, Hu, Li, Fan, Qin, Liu 2017).
The effect of bisphenol A on the number of different neurons is different. Studies have shown that 50 mg/ kg or 50 μg /kg (BW) BPA can increase the number of oxytocin immune response neurons in PVN, but hardly change the serotonin fiber density or the number of eralphair neurons (Adewale, et al 2011).
BPA affected an area of the MPFC (medial prefrontal cortex) associated with neurological disorders, but only in men and not in women (Sadowski, et al. 2014). Acute exposure to BPA, not through cortical interactions but through altered projections of the lateral geniculate nucleus, leads to limited visual perception in cats (Xu, et al. 2018).
BPA may interfere with the normal development of the cerebellum by affecting the developing cerebellum granule neurons (Mathisen, et al 2013). Maternal exposure to BPA resulted in a decrease in the number of hippocampal neurons and spinal density in the offspring, and this effect was observed in both F1 and F2 (Zhang, et al. 2020). Tang, C et al. showed that long-term exposure to low doses of BPA could inhibit the activation of AVPV-kisspeptin neurons, which were induced by Eralpha and prolonged the oestrus period and reduced ovulation in adult female mice ( Tang, et al. 2020).
Effect of BPA on the proliferation of neural stem cells (NSC): NSC proliferation and differentiation are changed by BPA in vivo and in vitro studies (Tiwari, et al. 2015). Chronic exposure to BPA impairs autophagy-mediated mitochondrial transformation and leads to apoptosis of hippocampal neural stem cells (NSC) (Agarwal, et al. 2016). BPA had adverse effects on NSC proliferation and neuronal differentiation in hippocampus and SVZ. BPA inhibits the proliferation and differentiation of rat NSC through Wnt/ β-catenin signaling pathway, and enhances neurodegeneration. (Tiwari, et al 2015) (Tiwari, et al. 2016).
Exposure to different concentrations of BPA has different effects on neural stem cells. High concentrations (> 400 microns) of BPA have cytotoxic effects on neural stem cells, while low concentrations of BPA have estrogenic activity and stimulate the proliferation or differentiation of NSC (Kim, et al. 2009, Kim, et al. 2007).
Estrogen mediated the proliferation of NS/PCs through nuclear cell mediated induction and had a positive effect on the proliferation or differentiation of neural stem cells (NS/PC) (Okada, et al. 2010). Bisphenol A enhanced the proliferation or differentiation of NS/PCs when the cells are poorly supplied with mitogens or differentiation factors such as FGF-2 in the early stages of neurogenesis (Okada, et al. 2008).
Exposure to bisphenol A had positive effects on the cell cycle outlet of irradiated glial cells and iPS cells, and significantly reduced the proliferation caused by prolonged iPS cell cycle length in SVZ (Komada, et al. 2012). BPAF showed the strongest cytotoxicity on hesc and hesc derived neural stem cells (NSCs), while BPS showed the least cytotoxicity. Exposure to BPA and its derivatives causes lengthening of neurite length in neuron-like cells (Liang, et al. 2020).
Effect of BPA exposure on oligodendrocytes: The proportion of oligodendrocytes generated by neural stem cells/progenitor cells in total cells increased after treatment with E2 or BPA (Okada, Murase, Makino, Nakajima, Kaku, Furukawa, Furukawa 2008). Both in vivo and in vitro BPA exposure at postnatal days 21 and 90 altered the proliferation and differentiation potential of OPCs, and reduced the gene expression and protein levels associated with myelination (Tiwari, et al. 2015). Bisphenol inhibits OPCS differentiation, which is caused by thyroid hormone exposure (Seiwa, et al. 2004).
The proliferation and differentiation of central nervous system astrocyte progenitor cells and non-serum rat embryonic cells were not affected by low levels of BPA in serum-free environment. However, at a dose of 1-100 pg/ mL, low levels of BPA resulted in overactivation of signal transduction, transcriptional activator, and anti-decapida-paralysis homologue 1 (Smad1) mother cells, and significantly increased GFAP expression in SME cells (Yamaguchi, et al. 2006).
Figure 1: Possible mechanism of BPA on dendritic morphology and developmen
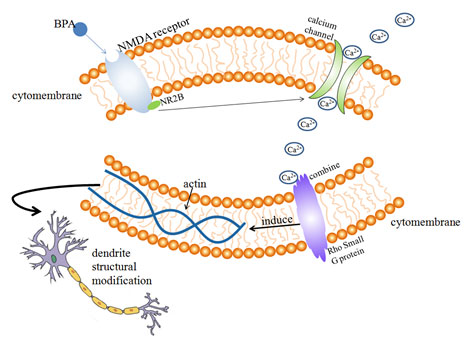
Effects of BPA on dendritic filament and dendritic spine: Dendritic spines are spinous processes on dendrites of neurons. The postsynaptic area of excitatory synapses receives external stimuli and regulates synaptic transmission by changing its shape and size (Koshida, et al. 2018). Actin cytoskeleton plays an important role in the morphological development of dendritic spines, actin is one of the main components of dendritic spine cytoskeleton ( Hlushchenko, et al. 2016).
Rho small G protein is an important cytoskeletal regulator of actin, which is involved in the regulation of neuronal morphological changes. The ability of dendritic filaments and dendritic spines to move rapidly in a short period of time is largely dependent on the regulation of actin cytoskeleton by environmental factors (Bryan, et al. 2004).
After the activation of NMDA receptor, with the participation of Ca2+, actin localization in dendritic filament and dendritic spine is regulated, resulting in of the morphological changes in dendritic spines (Furuyashiki, et al. 2002). According to the previous experimental studies ( Figure 1), it can be speculated that the possible mechanism of BPA’s influence on dendritic morphology is that acute exposure of BPA for 30 min increases the phosphorylation level of NMDA receptor subunit NR2B by means of extracellular estrogen receptor mediation, after the activation of NMDA receptor, Ca2+ concentration increases and binds to Rho small G protein (actin cytoskeleton regulator) on the surface of the cytoskeleton, Rho GTPase regulates the cytoskeleton structure, so that the positioning and dynamics of actin in the dendritic filament can be changed, resulting in rapid movement of dendritic filament dendritic spine and ultimately affecting dendritic morphology, (Bryan, et al 2004, Furuyashiki et al 2002).
Waters et al. found that chronic or acute BPA exposure is mediated by ERs inside or outside the nucleus of neurons, leading to changes in density of dendritic spines in hippocampal neurons, synaptic number and hippocampal dependent cognitive function (Waters, et al. 2009). The morphology of dendrites in neurons is closely related to Rho A Rac1 and Cdc42. Inhibition of Rac1 or Cdc42 expression can lead to decreased density of dendritic spines and dendritic filaments, while inhibition of Rho A expression leads to increased density and length of dendritic spines and dendritic filaments (Tashiro, Yuste 2004).
BPA can promote the Rac1/Cdc42 hippocampal neuron to inhibit the expression of Rho A, and also greatly increase the expression of f-actin in dendritic filaments, so as to increase the length of dendritic branches and the density of dendritic filaments and promote the mobility of dendritic filaments. These changes are related to ERs mediated erk1/2 signaling pathway (Guangxia, et al. 2013).
According to the previous experimental studies (Xu, et al 2011, Waters, et al 2009, Guangxia, et al 2013), it can be speculated that the possible mechanism of BPA on dendritic morphology and development of hippocampal neurons is that chronic or acute BPA exposure promotes Rac1/Cdc42 inhibition of Rho A expression in hippocampal neurons through erk1/2 signaling pathway mediated by ERs inside and outside the nucleus of neurons, and increases the density and length of dendritic branch lengths and dendritic filament dendritic spines.
At the same time, the expression of f-actin in the dendritic filament of hippocampal neurons was also up-regulated, which increased the number of synaptic spines density and hippocampal dependent cognitive function changes caused by the kinematics of dendritic filament, thus affecting the morphological development of hippocampal neuron dendrites and interfering with the normal development of the nervous system (Figure 2).
Figure 2 : Possible mechanism of BPA on the morphological development of hippocampal neurons
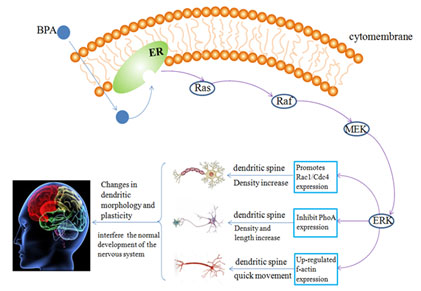
Effect of bisphenol A on behavioral development: Even below the current reference safe daily limit of 50 µg/kg day set by the USEPA, some behaviors and neuronal morphology are also changed by the exposure of BPA in puberty and these changes can continue into adulthood (Bowman, et al. 2015). Perinatal exposure to BPA during brain development is obvious damage to the gender recognition memory space, resulting in female rats’ spatial memory impairment and passivity male mice from memory, and this kind of behavior change is permanent (Poimenova, et al 2010, Jardim, et al. 2017).
The disruption of sexually dimorphic behaviors is related to persistent, sex-specific social and anxiety-like behavior of the BPA exposure (Kundakovic, Gudsnuk, Franks, Madrid, Miller, Perera, Champagne 2013). During exposure of rodents during perinatal period, long-term anxiety behavior occurred in adulthood (Zhou, et al. 2013, Xu, et al. 2012). Exposure to bisphenol A in pregnancy and lactation enhanced anxiety and depression in both genders. However, the difference was that exposure to bisphenol A in pregnancy had a stronger effect on women’s anxiety, ( Xu et al 2012).
Paternal exposure to bisphenol A strengthened the anxiety behaviors of F1 female as well as depression behaviors in both sexes of F1 rats (Fan, et al. 2018). Myelination in the hippocampus of the rat brain can be altered by exposure to bisphenol A during fetal and postnatal periods, which leads to cognitive deficits (Tiwari,et al 2015). When the mice were chronically exposed to low doses of bisphenol A, the brain cells numbers were damaged, and adolescent mice had lower learning and memory skills (Zhou, et al. 2017).
One of the factors leading to the development of neurobehavioral disorders such as autism spectrum disorder, is thought to be exposure to bisphenol a during gestation (Harris, et al. 2017). Among children in the United States, there was a link between higher urinary BPA concentrations and ADHD, and these associations were particularly evident among boys (Tewar, et al. 2016).
Rats exposed to bisphenol A showed a range of behavioral changes, for example, the decrease in locomotion, the increase in the dislike of light and sound, the change of grooming habits and the enhancement of startle reflex (Vermeer, Gregory, Winter, McCarson, Berman 2014). During the development of zebrafish, the exposure of BPA changed the spontaneous movement, and significantly reduced touch response and swimming speed in response to light stimulation (Wang, et al. 2013).
Rat fetuses exposed to BPA led to adult-onset obesity, this adult-onset obesity phenotype may be caused by the destruction of the physiological bimodal nature of epigenetic regulation of fggy in mouse WATs by prenatal exposure to bisphenol A (BPA), (Taylor, et al 2018).
When animals are exposed to low doses of bisphenol A, the development of their reproductive organs are disrupted and the effects on the brain’s physiology are long-lasting (Panagiotidou, et al. 2014, Kawai, et al. 2003). During courtship, the treatment of bisphenol reduced the male locomotion, and was related to the decrease of female courtship behavior but more aggressive toward mating with rivals (Wang, et al. 2017). When female offspring are exposed to 25 weight/kg/day bisphenol A daily, the brain revealed masculinization (Hass, et al. 2016).
Because of bisphenol A’s estrogenic activity and endocrine disruptor capabilities, prenatal exposure to bisphenol A (BPA), even at very low doses, can have an impact on vertebrates in terms of neurological and behavioral sex differences ( Ponzi, et al. 2020). Studies have shown that even low dose maternal BPA exposure can produce sex dependent learning and memory ability of F1 male mice, but has no significant effect on learning and memory ability of F2 generation male mice (Zhang, et al. 2020).
CONCLUSION AND OUTLOOK
From the existing research results, it can be found that it has different effects on the nervous system due to the differences in the exposure period, dose, time, location, sex, age and population of BPA. The effect of BPA on the nervous system is very complicated. BPA is an exogenous estrogen, which has the role of estrogen and antiestrogen. BPA acts as analogue to the estrogen receptors and interferes with normal levels of hormones in the body, leading to endocrine disorders. Bisphenol A has a high lipid solubility, which is easy to pass through the blood-brain barrier and placental barrier.
BPA accumulates in the brain and damages the development of the brain, leading to some abnormal behaviors. BPA can also enter the fetus and affect the growth and development of the fetus. In addition, BPA also reduces the proliferation and differentiation of neural stem cells and oligodendrocytes. Numerous studies have shown that BPA may cause diseases such as obesity, birth defects, breast cancer and so on.
Disclosure : The authors declare that there are no conflicts of interest in this work.
Author contributions: Cheng, J and R Wang and N Li conceived and wrote the manuscript while Y Dong, Y Cai and G Ye revised the manuscript critically with substantial intellectual input. Q Wang supervised the development of the work, critically evaluated the manuscript with intellectual input. All authors approved the final version. J Cheng?
ACKNOWLEDGEMENTS
This work was supported by grants from the Natural Scientific Foundation of Shandong Province, China (ZR2018MH038); the National Natural Scientific Foundation of China (31701042) and the Technology Development Project Plan of Shandong Education Department (J17KB090); and Zibo Platform for Gene Editing and Cell Application (2018ZBXC010, 2018ZBXC008).
REFERENCES
Adewale H.B., Todd K.L., Mickens J.A., Patisaul H.B. (2011) The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology.32(1): 38-49.
Agarwal S., Yadav A., Tiwari S.K., Seth B., Chauhan L.K., et al. (2016) Dynamin-related Protein 1 Inhibition Mitigates Bisphenol A-mediated Alterations in Mitochondrial Dynamics and Neural Stem Cell Proliferation and Differentiation. The Journal of biological chemistry.291(31): 15923-15939.
Akiyama E., Kakutani H., Nakao T., Motomura Y., Takano Y., et al. (2015) Facilitation of adipocyte differentiation of 3T3-L1 cells by debrominated tetrabromobisphenol A compounds detected in Japanese breast milk. Environmental research.140(157-164.
Arambula S.E., Jima D., Patisaul H.B. (2018) Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: a CLARITY-BPA consortium study. Neurotoxicology.65(207-220.
Bowman R.E., Luine V., Diaz Weinstein S., Khandaker H., DeWolf S., et al. (2015) Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Hormones and behavior.69(89-97.
Bryan B., Kumar V., Stafford L.J., Cai Y., Wu G., et al. (2004) GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J Biol Chem.279(44): 45824-45832.
Cabaton N.J., Canlet C., Wadia P.R., Tremblay-Franco M., Gautier R., et al. (2013) Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environmental health perspectives.121(5): 586-593.
Cao J., Rebuli M.E., Rogers J., Todd K.L., Leyrer S.M., et al. (2013) Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicological sciences : an official journal of the Society of Toxicology.133(1): 157-173.
Cariati, F., Carbone, L., Conforti, A., Bagnulo, F., et al. (2020) Bisphenol A-Induced Epigenetic Changes and Its Effects on the Male Reproductive System. Front Endocrinol (Lausanne). 11: 453.
Castro B., Sanchez P., Torres J.M., Ortega E. (2015) Bisphenol A, bisphenol F and bisphenol S affect differently 5alpha-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environmental research.142(281-287.
Chung E., Genco M.C., Megrelis L., Ruderman J.V. (2011) Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proceedings of the National Academy of Sciences of the United States of America.108(43): 17732-17737.
Fahrenkopf, A., Wagner, C. K. (2020) Bisphenol A (BPA) induces progesterone receptor expression in an estrogen receptor alpha-dependent manner in perinatal brain. Neurotoxicol Teratol. 78: 106864
Fan Y., Tian C., Liu Q., Zhen X., Zhang H., et al. (2018) Preconception paternal bisphenol A exposure induces sex-specific anxiety and depression behaviors in adult rats. PloS one.13(2): e0192434.
Furuyashiki T., Arakawa Y., Takemoto-Kimura S., Bito H., Narumiya S. (2002) Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A.99(22): 14458-14463.
Guangxia Z., Xiaohong X., Lei C., Yang L., Yanling Y., et al. (2013) Bisphenol A Promotes Dendritic Development and Changes the Expressions of RhoA and Rac1/Cdc42 of Hippocampal Neurons. Acta Biophysica Sinica.29(10): 759-768.
Harris E.P., Allardice H.A., Schenk A.K., Rissman E.F. (2017) Effects of maternal or paternal bisphenol A exposure on offspring behavior. Hormones and behavior.
Hass U., Christiansen S., Boberg J., Rasmussen M.G., Mandrup K., et al. (2016) Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology.4(4): 594-607.
Heuckeroth R.O., Schafer K.H. (2016) Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention. Dev Biol.417(2): 188-197.
Hlushchenko I., Koskinen M., Hotulainen P. (2016) Dendritic spine actin dynamics in neuronal maturation and synaptic plasticity. Cytoskeleton (Hoboken).73(9): 435-441.
Honma T., Miyagawa M., Suda M., Wang R.S., Kobayashi K., et al. (2006) Effects of perinatal exposure to bisphenol A on brain neurotransmitters in female rat offspring. Industrial health.44(3): 510-524.
Huang B., Ning S., Zhang Q., Chen A., Jiang C., et al. (2017) Bisphenol A Represses Dopaminergic Neuron Differentiation from Human Embryonic Stem Cells through Downregulating the Expression of Insulin-like Growth Factor 1. Molecular neurobiology.54(5): 3798-3812.
Ishido M., Yonemoto J., Morita M. (2007) Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicology letters.173(1): 66-72.
Jalilian H., Zamanian Z., Gorjizadeh O., Riaei S., Monazzam M.R., et al. (2019) Autonomic Nervous System Responses to Whole-Body Vibration and Mental Workload: A Pilot Study. Int J Occup Environ Med.10(4): 174-184.
Jardim N.S., Sartori G., Sari M.H.M., Muller S.G., Nogueira C.W. (2017) Bisphenol A impairs the memory function and glutamatergic homeostasis in a sex-dependent manner in mice: Beneficial effects of diphenyl diselenide. Toxicology and applied pharmacology.329(75-84.
Jones B.A., Wagner L.S., Watson N.V. (2016) The Effects of Bisphenol A Exposure at Different Developmental Time Points in an Androgen-Sensitive Neuromuscular System in Male Rats. Endocrinology.157(8): 2972-2977.
Kawai K., Nozaki T., Nishikata H., Aou S., Takii M., et al. (2003) Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environmental health perspectives.111(2): 175-178.
Kim K., Son T.G., Kim S.J., Kim H.S., Kim T.S., et al. (2007) Suppressive effects of bisphenol A on the proliferation of neural progenitor cells. Journal of toxicology and environmental health. Part A.70(15-16): 1288-1295.
Kim K., Son T.G., Park H.R., Kim S.J., Kim H.S., et al. (2009) Potencies of bisphenol A on the neuronal differentiation and hippocampal neurogenesis. Journal of toxicology and environmental health. Part A.72(21-22): 1343-1351.
Kim, S. S., Hwang, K. S., Yang, J. Y., Chae, J. S. , et al. (2020) Neurochemical and behavioral analysis by acute exposure to bisphenol A in zebrafish larvae model. Chemosphere.239: 124751.
Kimura E., Matsuyoshi C., Miyazaki W., Benner S., Hosokawa M., et al. (2016) Prenatal exposure to bisphenol A impacts neuronal morphology in the hippocampal CA1 region in developing and aged mice. Archives of toxicology.90(3): 691-700.
Komada M., Asai Y., Morii M., Matsuki M., Sato M., et al. (2012) Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology.295(1-3): 31-38.
Koshida R., Tome S., Takei Y. (2018) Myosin Id localizes in dendritic spines through the tail homology 1 domain. Exp Cell Res.367(1): 65-72.
Kubota T. (2016) Epigenetic Effect of Environmental Factors on Neurodevelopmenal Disorders. Nihon eiseigaku zasshi. Japanese journal of hygiene.71(3): 200-207.
Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R.L., et al. (2013) Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences of the United States of America.110(24): 9956-9961.
Lei B., Sun S., Xu J., Feng C., Yu Y., et al. (2018) Low-concentration BPAF- and BPF-induced cell biological effects are mediated by ROS in MCF-7 breast cancer cells. Environmental science and pollution research international.25(4): 3200-3208.
Liang X., Yin N., Liang S., Yang R., Liu S., et al. (2020) Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length. Food Chem Toxicol.135(111015.
Liang, X., Yin, N., Liang, S., Yang, R., et al. (2020) Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length. Food Chem Toxicol.135: 111015.
Lin Y., Zhang H., Wang W.D., Wu D.S., Jiang S.H., et al. (2006) [Effects of perinatal exposure to bisphenol A inducing dopaminergic neuronal cell to apoptosis happening in midbrain of male rat offspring]. Sichuan da xue xue bao. Yi xue ban = Journal of Sichuan University. Medical science edition.37(4): 570-573.
Ling W., Endo T., Kubo K., Nakajima K., Kakeyama M., et al. (2016) In Utero Bisphenol A Exposure Induces Abnormal Neuronal Migration in the Cerebral Cortex of Mice. Frontiers in endocrinology.7(7.
Mathisen G.H., Yazdani M., Rakkestad K.E., Aden P.K., Bodin J., et al. (2013) Prenatal exposure to bisphenol A interferes with the development of cerebellar granule neurons in mice and chicken. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience.31(8): 762-769.
Matsuda S., Saika S., Amano K., Shimizu E., Sajiki J. (2010) Changes in brain monoamine levels in neonatal rats exposed to bisphenol A at low doses. Chemosphere.78(7): 894-906.
Nakamura K., Itoh K., Yoshimoto K., Sugimoto T., Fushiki S. (2010) Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neuroscience letters.484(1): 66-70.
Narita M., Miyagawa K., Mizuo K., Yoshida T., Suzuki T. (2007) Changes in central dopaminergic systems and morphine reward by prenatal and neonatal exposure to bisphenol-A in mice: evidence for the importance of exposure period. Addiction biology.12(2): 167-172.
Ogi H., Itoh K., Ikegaya H., Fushiki S. (2015) Alterations of neurotransmitter norepinephrine and gamma-aminobutyric acid correlate with murine behavioral perturbations related to bisphenol A exposure. Brain & development.37(8): 739-746.
Okada M., Makino A., Nakajima M., Okuyama S., Furukawa S., et al. (2010) Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. International journal of molecular sciences.11(10): 4114-4123.
Okada M., Murase K., Makino A., Nakajima M., Kaku T., et al. (2008) Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomedical research.29(3): 163-170.
Panagiotidou E., Zerva S., Mitsiou D.J., Alexis M.N., Kitraki E. (2014) Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. The Journal of endocrinology.220(3): 207-218.
Pfeifer D., Chung Y.M., Hu M.C. (2015) Effects of Low-Dose Bisphenol A on DNA Damage and Proliferation of Breast Cells: The Role of c-Myc. Environmental health perspectives.123(12): 1271-1279.
Poimenova A., Markaki E., Rahiotis C., Kitraki E. (2010) Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience.167(3): 741-749.
Ponzi, D., Gioiosa, L., Parmigiani, S., Palanza, P.(2020)Effects of Prenatal Exposure to a Low-Dose of Bisphenol A on Sex Differences in Emotional Behavior and Central Alpha2-Adrenergic Receptor Binding. Int J Mol Sci.21(9): 3269.
Qin X.Y., Fukuda T., Yang L., Zaha H., Akanuma H., et al. (2012) Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer biology & therapy.13(5): 296-306.
Sadowski R.N., Wise L.M., Park P.Y., Schantz S.L., Juraska J.M. (2014) Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience.279(122-131.
Sarrouilhe D., Dejean C. (2017) [Autism spectrum disorders and bisphenol A: Is serotonin the lacking link in the chain?]. L’Encephale.43(4): 402-404.
Seiwa C., Nakahara J., Komiyama T., Katsu Y., Iguchi T., et al. (2004) Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells. Neuroendocrinology.80(1): 21-30.
Shimpi P.C., More V.R., Paranjpe M., Donepudi A.C., Goodrich J.M., et al. (2017) Hepatic Lipid Accumulation and Nrf2 Expression following Perinatal and Peripubertal Exposure to Bisphenol A in a Mouse Model of Nonalcoholic Liver Disease. Environmental health perspectives.125(8): 087005.
Singh S., Li S.S. (2012) Epigenetic effects of environmental chemicals bisphenol A and phthalates. International journal of molecular sciences.13(8): 10143-10153.
Spackova J., Oliveira D., Puskar M., Durovcova I., Gaplovska K., et al. (2019) Endocrine-independent Cytotoxicity of Bisphenol A is Mediated by Increased Levels of Reactive Oxygen Species and Affects Cell Cycle Progression. J Agric Food Chem.
Suzuki T., Mizuo K., Miyagawa K., Narita M. (2005) [Exposure to bisphenol-A affects the rewarding system in mice]. Nihon shinkei seishin yakurigaku zasshi = Japanese journal of psychopharmacology.25(3): 125-128.
Suzuki T., Mizuo K., Nakazawa H., Funae Y., Fushiki S., et al. (2003) Prenatal and neonatal exposure to bisphenol-A enhances the central dopamine D1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience.117(3): 639-644.
Tang, C., Zhang, J., Liu, P., Zhou, Y., et al. (2020) Chronic exposure to low dose of bisphenol A causes follicular atresia by inhibiting kisspeptin neurons in anteroventral periventricular nucleus in female mice. Neurotoxicology. 79: 164-176.
Tang, Y., Zhou, W., Sun, S., Du, X., Han, Y., Shi, W., Liu, G. (2020) Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species. Tegillarca granosa. Environ Pollut. 265(Pt A): 115115.
Tashiro A., Yuste R. (2004) Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci.26(3): 429-440.
Taylor J.A., Shioda K., Mitsunaga S., Yawata S., Angle B.M., et al. (2018) Prenatal Exposure to Bisphenol A Disrupts Naturally Occurring Bimodal DNA Methylation at Proximal Promoter of fggy, an Obesity-Relevant Gene Encoding a Carbohydrate Kinase, in Gonadal White Adipose Tissues of CD-1 Mice. Endocrinology.159(2): 779-794.
Tewar S., Auinger P., Braun J.M., Lanphear B., Yolton K., et al. (2016) Association of Bisphenol A exposure and Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Environmental research.150(112-118.
Tiwari S.K., Agarwal S., Chauhan L.K., Mishra V.N., Chaturvedi R.K. (2015) Bisphenol-A impairs myelination potential during development in the hippocampus of the rat brain. Molecular neurobiology.51(3): 1395-1416.
Tiwari S.K., Agarwal S., Seth B., Yadav A., Ray R.S., et al. (2015) Inhibitory Effects of Bisphenol-A on Neural Stem Cells Proliferation and Differentiation in the Rat Brain Are Dependent on Wnt/beta-Catenin Pathway. Molecular neurobiology.52(3): 1735-1757.
Tiwari S.K., Agarwal S., Tripathi A., Chaturvedi R.K. (2016) Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway. Molecular neurobiology.53(5): 3010-3029.
Tonini, C., Segatto, M., Gagliardi, S., Bertoli, S., Leone, A., Barberio, L., Mandala, M., Pallottini, V. (2020) Maternal Dietary Exposure to Low-Dose Bisphenol A Affects Metabolic and Signaling Pathways in the Brain of Rat Fetuses. Nutrients.12(5): 1448.
Vermeer L.M., Gregory E., Winter M.K., McCarson K.E., Berman N.E. (2014) Exposure to bisphenol A exacerbates migraine-like behaviors in a multibehavior model of rat migraine. Toxicological sciences : an official journal of the Society of Toxicology.137(2): 416-427.
Wang H., Ding Z., Shi Q.M., Ge X., Wang H.X., et al. (2017) Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology.387(10-16.
Wang X., Dong Q., Chen Y., Jiang H., Xiao Q., et al. (2013) Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquatic toxicology.142-143(104-113.
Waters E.M., Mitterling K., Spencer J.L., Mazid S., McEwen B.S., et al. (2009) Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res.1290(1-11.
Xu G., Hu F., Wang X., Zhang B., Zhou Y. (2018) Bisphenol A exposure perturbs visual function of adult cats by remodeling the neuronal activity in the primary visual pathway. Archives of toxicology.92(1): 455-468.
Xu X., Gu T., Shen Q. (2015) Different effects of bisphenol-A on memory behavior and synaptic modification in intact and estrogen-deprived female mice. Journal of neurochemistry.132(5): 572-582.
Xu X., Hong X., Xie L., Li T., Yang Y., et al. (2012) Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Hormones and behavior.62(4): 480-490.
Xu X., Li T., Luo Q., Hong X., Xie L., et al. (2011) Bisphenol-A rapidly enhanced passive avoidance memory and phosphorylation of NMDA receptor subunits in hippocampus of young rats. Toxicology and applied pharmacology.255(2): 221-228.
Xu X., Ye Y., Li T., Chen L., Tian D., et al. (2010) Bisphenol-A rapidly promotes dynamic changes in hippocampal dendritic morphology through estrogen receptor-mediated pathway by concomitant phosphorylation of NMDA receptor subunit NR2B. Toxicology and applied pharmacology.249(2): 188-196.
Xu X.H., Wang Y.M., Zhang J., Luo Q.Q., Ye Y.P., et al. (2010) Perinatal exposure to bisphenol-A changes N-methyl-D-aspartate receptor expression in the hippocampus of male rat offspring. Environmental toxicology and chemistry.29(1): 176-181.
Xu X.H., Zhang J., Wang Y.M., Ye Y.P., Luo Q.Q. (2010) Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Hormones and behavior.58(2): 326-333.
Yamaguchi H., Zhu J., Yu T., Sasaki K., Umetsu H., et al. (2006) Low-level bisphenol A increases production of glial fibrillary acidic protein in differentiating astrocyte progenitor cells through excessive STAT3 and Smad1 activation. Toxicology.226(2-3): 131-142.
Yirun, A., Ozkemahli, G., Balci, A., Erkekoglu, P., Zeybek, N. D., Yersal, N., Kocer-Gumusel, B.(2021) Neuroendocrine disruption by bisphenol A and/or di(2-ethylhexyl) phthalate after prenatal, early postnatal and lactational exposure. Environ Sci Pollut Res Int.
Zhang X.F., Zhang L.J., Feng Y.N., Chen B., Feng Y.M., et al. (2012) Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Molecular biology reports.39(9): 8621-8628.
Zhang, H., Wang, Z., Meng, L., Kuang, H., Liu, J., Lv, X., Pang, Q., Fan, R. (2020) Maternal exposure to environmental bisphenol A impairs the neurons in hippocampus across generations. Toxicology. 432: 152393.
Zhou R., Chen F., Chang F., Bai Y., Chen L. (2013) Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. Journal of psychiatric research.47(10): 1535-1544.
Zhou Y., Wang Z., Xia M., Zhuang S., Gong X., et al. (2017) Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: Implications for children exposed to environmental levels of BPA. Environmental pollution.229(40-48.


