Department of Life Sciences, University of Mumbai, Vidyanagari, Kalina,
Santacruz East, Mumbai, Maharashtra, 400 098 India.
Corresponding author email: indu.george@lifesciences.mu.ac.in
Article Publishing History
Received: 25/11/2021
Accepted After Revision: 29/03/2022
The natural benzoquinone, embelin, fromthe Embelia species has therapeutic benefits in a wide range of diseases.Although several extraction methods and solvents have been explored, consensus on the economic use of material and time was ambiguous. The purpose of this study was to devise a protocol for the rapid estimation of embelin. Chloroform, ethyl acetate and acetone extracts were prepared using soxhlet, microwave, sonication and cold extraction methods.The bioactivity of the chloroform extracts was assayed using the DPPH radical scavenging and the Reducing Power Assays. The embelin content in chloroform and ethyl acetate extracts were better in some extraction methods. The chloroform extracts exhibited antioxidant activity which remained unaffected regardless of the extraction technique. The microwave extraction technique yielded quick and accurate results. This technique could be adopted for rapid screening of samples with limited availability of biomass.
Antioxidant Activity,Embelia sp., Embelin, Extraction Methods, Microwave Extraction.
Dandekar S. S, George* I. A. An Accurate Embelin Extraction Method for Limited Biomass of Embelia Species. Biosc.Biotech.Res.Comm. 2022;15(1).
Dandekar S. S, George* I. A. An Accurate Embelin Extraction Method for Limited Biomass of Embelia Species. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3hIENZK“>https://bit.ly/3hIENZK</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Embelia basaal is a large shrub and Embelia ribes is a rare woody climber or shrub of the family Primulaceae. E.ribes is located up to an altitude of 1750 meters in the western and eastern ghats of India, Sri Lanka, Singapore, China and Malaysia (Annapurna et al. 2013; Patwardhan et al. 2014). The brittle pericarp of the dried berries of E. ribes encloses a reddish, single seed with spots of embelin on its surface and is covered by a thin membrane(Sudhakaran 2016). The dried berries of E.basaal appear reddish-black with vertical striations on its surface and can be easily differentiated from the grey to black, wrinkled berries of E. ribes(Fig. 1) (Nayak et al. 2009).Traditionally Embelia has been used extensively for impaired digestion, colon diseases, ulcers, skin diseases, as an anthelmintic, for the treatment of abdominal pain, mental disorders, jaundice, heart diseases and bronchitis(Bhishagratna 1911; Atal et al. 1984; Choudhary et al. 2021).
Embelin has clinically proven its value as an antimicrobial, antioxidant, anti-tumour, analgesic, anti-inflammatory, anti-androgenic, anti-hyperglycaemic, anthelmintic agent and in healing wounds (Radhakrishnan and Gnanamani 2014). Different parts of the plant were used in the form of paste, decoction, oil and powder for treating various illnesses. Embeliaribes(Vidanga) hasbeen used in the preparation of about 75 ayurvedic formulations (Bhishagratna 1911; Patwardhan et al. 2014). Pharmacological applications aside, it has also been used as a dyeing agent for cotton, nylon, wool and silk (Radhakrishnan et al. 2011a). A recent study of embelin indicated an antiviral property against Covid-19 (Caruso et al. 2020b). The medicinal value of E. basaal and E. ribes can be ascribed to its active component embelin a yellow-orange crystalline compound (2,5-dihydroxy-3-undecyl-1,4-benzoquinone)that constitutes about 1 to 5% of the berries (Lu et al. 2016; Prabhu et al. 2018; Ferreria and Laddha 2013).
Bioprospecting or evaluation of the best medium composition for desired results would demand rugged and accurate protocols with an economical investment of biomass and time. The plant biomass has been extracted using various solvents and extraction techniques and the determination of the biochemical activities of phytochemicals in the crude extracts have been regularly explored for better alternatives to the existing options (Altemimi et al. 2017; Tlili et al. 2019; Kamble et al. 2020; Vijayan and Raghu 2021).
Extraction of embelin has been reported using several extraction methods and solvents. However, these studies did not actively explore their efficacy in embelin from small samples. The present study aims to optimize the embelin extraction from small samples of the berries of two species of Embelia using different solvents and extraction methods while retaining their bioactivity.
MATERIAL AND METHODS
The analytical grade solvents were procured from SD Fine-Chem (India) and Merck. The other reagents used for analysis were sourced from Sigma-Aldrich, HiMedia (India), SRL (India) and SD Fine-Chem (India).Embelia basaal berries were collected from Supegaon village, Raigad District, Maharashtra. A specimen was deposited in Blatter Herbarium, St. Xaviers College, Mumbai and was identified as Embelia basaal (Roem & Schult) A. DC. The berriesofEmbelia ribes were collected from Shimoga District, Karnataka. A specimen was submitted to ‘Botanical Survey of India’, Pune and was identified as Embelia ribes Burm f.
The berries of both species were air-dried and stored at room temperature for further analysis.The berries of E. basaal and E. ribeswere crushed, using a mortar and pestle, enough to separate the pericarp from the seeds and expose sufficient seed surface for extraction of the entire embelin content (Ferreria and Laddha 2013). The pericarp and the seeds were subjected to extraction using the solvents chloroform, ethyl acetate, acetone and four extraction methods. The sample to solvent ratio was maintained at 2% (w/v) in each method. The extractions were carried out in triplicate and the embelin content was expressed as mean ± standard error of the mean (SEM).
For Soxhlet Extraction (SE),Coarse ground berries (1 g) of Embelia species were refluxed in 50 ml of extraction solvent in a soxhlet apparatus (J-SIL company, India) for 1 h by which time the extract in the siphon arm of the apparatus was colourless.Microwave extraction (ME): The sample (0.2 g) was extracted with 5 ml of solvent for 3 min in a microwave (LG, India) set at 180 W. The sample was heated for 50 s, three times, in a cycle. A resting period of 10 s was given between each spurt of 50 s heating. The solvent was then filtered through Whatman filter paper no. 1 and collected in a beaker. Fresh solvent (5 ml) was added to the sample residue and a second extraction was carried out for 50 s twice, with a resting period of 10 s as described above,followed by filtration. The filtrates obtained were pooled together.
Ultrasonication extraction (UE): The sample (0.2 g) in 5 ml of solvent was subjected to sonication using a water bath sonicator (Pure Enterprises, India) for 10 min, followed by filtration. Fresh solvent (5 ml) was added to the sample which was sonicated for another 5 min and filtered. The filtrates obtained were pooled together.For cold extraction (CE), the sample (0.2 g) with 10 ml of solvent was placed on an orbital shaker (Remi RS 12 PLUS) at 200 rpm for 17 h. The samples were then filtered.The extracts obtained by the four extraction methods using various solvents were oven-dried at 40°C and the residues were weighed. The dried extracts were dissolved in chloroform for quantification by UV-VIS spectrophotometer (Shimadzu Scientific Instruments Inc.)
Standard embelin was procured from Sigma-Aldrich and a stock solution (1 mg/ml) was prepared in chloroform. Dilutions were prepared such that the final concentrations ranged between 4 to 24 µg/ml. The UV absorbance at 290 nm of each solution was recorded using a UV-VIS spectrophotometer. A standard graph of absorbance vs concentration of embelin was plotted and the resultant equation was used for calculating the concentration of embelin in the samples.The embelin from samples was also quantified by High Performance Liquid Chromatography (HPLC) for the bioactivity experiments.
Four concentrations of standard embelin in methanol (10, 20, 30 and 40 µg/ml) were run through an HPLC column (Shim-pack GIST C18, 5µm, 20 x 250 of the Shimadzu HPLC system). The mobile phase, 0.1% formic acid in acetonitrile: deionized water (90:10; v/v), was degassed for 30 min. The flow rate was maintained at 0.5 ml/min in isocratic elution mode and the chromatogram was run for 20 min. The volume of sample injected each time was 20 µl. The area under the peak was considered to generate a standard graph for the estimation of embelin in samples. The dried chloroform extracts of the Embelia berries, obtained by the four extraction methods were reconstituted in methanol (1 mg/ml) by sonication for 5 min and filtered through a 0.22 µm nylon filter. These extracts would be further referred to as reconstituted extracts. The embelin content in these samples was determined by the HPLC analysis described above and the antioxidant activity was confirmed by the following DPPH and RPA assays.
The DPPH assay method described by Brand-Williams et al. (1995) was adopted with some modifications. The assays included ascorbic acid as a reference standard, a methanol control and the test samples. The DPPH reagent (100 µM) was prepared in chilled methanol and the initial U.V. absorbance of the reagent was approximately 1 (1.0542). Two-fold dilutions of the test samples dissolved in methanol were prepared with final concentrations that ranged from 800 µg/ml to 25 µg/ml. Similarly, standard ascorbic acid solutions were prepared in the range between 40 µg/ml to 1.25 µg/ml. Aliquots (500 µl of standard/ sample) were added to 2.5 ml of DPPH solution and incubated in dark for 30 min before noting the U.V. absorbance at 517 nm. The percentage of radical scavenging activity was calculated by the formula:
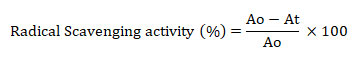
Where Ao = absorbance of the control and At= absorbance of the test sample.
EC50 values (50% effective concentration value – the value obtained by plotting percentage radical scavenging activity vs concentration of extract) were calculated for each sample and standard using GraphPad Prism 6.0 software.The reducing power assay (RPA)method described by Muruhan et al. (2013) was adopted with some modifications. A range of two-fold dilutions of standard solution (80 µg/ml to 1.25 µg/ml) and sample (800 µg/ml to 25 µg/ml) was prepared. Each concentration of the standard or sample was reacted individually with 1.25 ml of sodium phosphate buffer (pH 6.6) and 1.25 ml potassium ferricyanide.
This mixture was incubated at 50°C for 20 min.The reaction was stopped with the addition of 10% trichloroacetic acid (TCA). The solutions were then centrifuged at 3000 rpm for 10 min. The resultant supernatants (2.5 ml) were mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride reagent. The U.V. absorbance of these reaction mixtures was recorded at 700 nm and its reducing potential was expressed as ascorbic acid equivalents.All data obtained were subjected to appropriate statistical treatment and analysis. Two-way analysis of variance (ANOVA) followed by Tukey’s test was performed using GraphPad Prism 6.0 software wherever required.
RESULTS AND DISCUSSION
Figure 1: The dried berries of E. basaal(A) and E. ribes (B); The brittle testa removed to
expose the seeds with spots of embelin of E. basaal (C) and E. ribes (D).

Yield of Extract:The residue obtained after the evaporation of solvent from the extract (yield) varied across the different extraction methods and the solvent used. This ranged from 5% to 7.8% (w/w) for E. basaal and 3.5% to 6.2% (w/w) for E. ribes. Two-way ANOVA followed by Tukey’s test revealed that the yield of the ME – chloroform extract was significantly higher than that of the other methods (E. basaal) whereas, for E. ribes, the CE – chloroform extract showed maximum yield. The yields obtained by different extraction methods tested in this study were variable but inconsistent with their embelin content (Table 1). Similarly, the Supercritical carbon dioxide extraction resulted in a lower yield than the SE but had a higher flavonoid content (Bimakr et al. 2011).
Table 1. The yield, embelin content and time required for extraction from berries of E. basaaland E. ribes
| E. basaal | E. ribes | ||||||
| Yield (mg/g) | Embelin content (mg/g) | Time (min) | Yield (mg/g) | Embelin content (mg/g) | Time (min) | ||
| SE | SE | ||||||
| CH | 71.37 ± 3.76ф | 54.69 ± 0.96A | 60 | CH | 39.50 ± 1.50αβ | 18.17 ± 0.25ab | 60 |
| EA | 59.90 ± 2.47ф | 57.96 ± 1.52A | 60 | EA | 41.13 ± 1.86αβ | 19.34 ± 0.69abcd | 60 |
| AC | 64.37 ± 0.67ф | 44.94 ± 1.84ABC | 60 | AC | 40.13 ±0.68αβ | 17.05 ± 1.03a | 60 |
| ME | ME | ||||||
| CH | 78.83 ± 3.90Ѱ | 54.22 ± 2.65A | 5 | CH | 53 ± 0.0.29γ | 21.28 ± 0.57bcd | 5 |
| EA | 55.33 ± 5.18ф | 53.06 ± 5.30AB | 5 | EA | 40 ± 1.32αβ | 21.94 ± 0.14cd | 5 |
| AC | 65.83 ± 5.73ф | 40.30 ± 2.53ABC | 5 | AC | 43.33 ± 1.67β | 18.78 ± 0.18abc | 5 |
| UE | UE | ||||||
| CH | 63.33 ± 3.03ф | 53.30 ± 2.08AB | 15 | CH | 55 ± 0.76γ | 21.18 ± 0.76bcd | 15 |
| EA | 50.67 ± 2.89ф | 49.45 ± 2.46AB | 15 | EA | 37.50 ± 2.25αβ | 21.25 ± 0.57bcd | 15 |
| AC | 54.50 ± 7.01ф | 35.32 ± 7.31BC | 15 | AC | 35.50 ± 1.00α | 18.63 ± 0.48ab | 15 |
| CE | CE | ||||||
| CH | 75.67 ± 7.86ф | 47.20 ± 1.34AB | 1020 | CH | 62.50 ± 0.50δ | 22.06 ± 0.60d | 1020 |
| EA | 63.50 ± 5.20ф | 49.41 ± 6.02AB | 1020 | EA | 42.67 ± 0.88β | 22.51 ± 0.89d | 1020 |
| AC | 63 ± 7.25ф | 28.88 ± 1.67C | 1020 | AC | 44 ± 1.32β | 19.81 ±0.64abcd | 1020 |
Legend Table (1): The letters andsymbols across the cells for each species indicates the statistical significance at p ≤ 0.05. CH: chloroform; EA: ethyl acetate; AC: acetone; The equation and the regression coefficient (squared) of embelin standard graph is as follows: y = 0.063x – 0.059 and r2 = 0.9963.
Estimation of embelin content by spectrophotometry: Variations in the embelin content in berries could be attributed to its geographical location (1.2 to 4.9%)and/or stage of maturation (1 to 5.2%)(Pandey and Ojha 2011; Nagamani et al. 2013). The embelin content of extracts would also depend on the extraction method and the solventwhich was significant in this study of both Embelia species. The embelin content in Embeliaribes extracts, reported using different extraction methods, ranged from 1.9% to 3.8% (SE), 5% (ME), 0.84% to 23.71 % (UE) and 1.77% (CE) (Madhavan et al. 2011; Radhakrishnan et al. 2011b; Alam et al. 2015; Sathe and Dixit 2015; Kamble et al. 2020).
The embelin content of the various extracts in the current study of E. ribes was determined as 1.7% to 1.93% (SE), 1.87% to 2.19% (ME), 1.86% to 2.12% (UE) and 1.98% to 2.25% (CE) with different solvents. These values for E. basaal ranged from 4.49% to 5.79% (SE), 4.03% to 5.42% (ME), 3.53% to 5.33% (UE) and 2.88% to 4.94% (CE). The highest extracted embelin content in this study was 5.79% from E. basaal berries.E. ribes was reported to be the best source of embelin when compared to the species tested in a previous study(Vijayan and Raghu 2021).
A selection of conventional (SE and CE) and modern (ME and UE) methods of extraction were comparedin this study. The SE and ME embelin contents were better in E. basaal extracts whereas the SE of E. ribes was comparatively inefficient than the other extraction methods tested.Solvents used for extraction of secondary metabolites may include a pure organic solvent, a mixture of solvents, water or a percentage of a solvent in water (Gupta et al. 2012).
Embelin has a polar benzoquinone ring and a nonpolar alkyl saturated chain. The isolated and purified embelin from E. ribes is insoluble in water, freely soluble in organic solvents such as DMSO, slightly soluble in methanol and ethanol (Kaur et al. 2015; Caruso et al. 2020a). Previous studies preferred hexane, diethyl ether, chloroform, ethyl acetate, acetone and methanol for extraction of embelin (Madhavan et al. 2011; Alam et al. 2015; Pundarikakshudu et al. 2016;(Vijayan and Raghu 2021)). Even so, the results of these experiments failed to reach a consensus on the best solvent for embelin extraction.
Chloroform extraction by maceration, ethyl acetate extraction by sonication and acetone extraction by microwave-assisted extraction method of E. ribesyielded maximum embelin (Latha 2007; Alam et al. 2015; Pundarikakshudu et al. 2016). The most promising solvents were investigated for the development of the rapid analysis protocol. The statistical analysis of this study however indicated that the solvents chloroform and ethyl acetate were found to be marginally more efficient in the extraction of embelin (Table 1). Chloroform was also found to be suitable for the extraction of benzoquinone in Ficus foveolata (Meerungrueang and Panichayupakaranant 2015). Methanol was discontinued after the initial extraction process as the residue obtained was sticky, which was also observed by (Pundarikakshudu et al. 2016).Another factor that should be considered is the efficacy of the bioactive principle(s) following an extraction method. The chloroform extracts using all four extraction methods from both species were further analysed for their bioactivity.
Estimation of embelin in reconstituted extracts using HPLC:The current solvent system was inspired by the existing literatureand modified for better resolution(Ferreria and Laddha 2013). The standard embelin chromatogram exhibited one sharp peak at a retention time that ranged from 7.082 to 7.16. The spectrophotometric analysis of this peak showed a λmax at 286 nm. Hence the chromatograms of the samples were monitored at 286 nm and all samples showed one peak with retention times between 6.66 and 7.17 with the λmax that ranged between 277 to 283 nm (Fig. 2).
The embelin content of the reconstituted E. basaal and E. ribes extracts ranged between 602 to 807 µg/ml and 269 to 509 µg/ml, respectively. A significant difference was noticed between the embelin content of E. basaal and E. ribes extracts. However, all extraction methods were comparatively efficient (Table 2).Contrary to the current observation, a study described that the embelin content varied with different extraction methods (Kamble et al. 2020). The differences in embelin content among species of Embelia have been reported in previous studies(Vijayan and Raghu 2021).
Figure 2: The HPLC chromatogram of standard embelin (A), λmax of the standard peak from the chromatogram (B), HPLC chromatogram of Sample (E. basaal – UE) (C), and λmax of the sample peak obtained in the chromatogram (D).
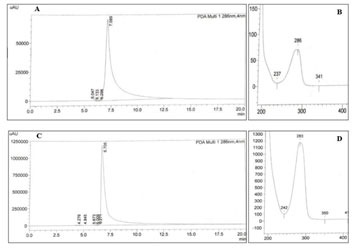
Table 2. Embelin content of reconstituted extracts by HPLC
| Embelin content by HPLC µg/ml | ||||
| SE | ME | UE | CE | |
| E. basaal | 807.12a | 621.58ab | 617.07ab | 601.55ab |
| E. ribes | 508.77ab | 450.58ab | 374.3ab | 268.96b |
Legend: SE: Soxhlet extraction; ME: Microwave extraction; UE: Ultrasonication extraction; CE: Cold extraction.The equation determined for the embelin estimation by the HPLC method was y = 69697x –38593 with the r2 value 0.9972.
Antioxidant Assays: Antioxidants are chemical substances that halt the chain reaction of free radical species generation and protect the cells from cellular damage. The antioxidant activity of plants is generally attributed to the flavonoid, phenolic and tannin content in the extract (Mosquera et al. 2009). Earlier reports of the antioxidant activity of extracts from E. basaal and E. ribes prompted testing for their antioxidant activity(Ansari et al. 2008; Kamble et al. 2011).
DPPH assay;Antioxidant activity of the chemically synthesized embelin, a benzoquinone, was studied by Joshi et al. (2007) and its activity determined by the DPPH assay was found to be 2.55 × 10-4 mol dm-3. Purified embelin extracts displayed an IC50 of 27.92 µg/ml and an EC50 of 27 µg/ml(Mahendran et al. 2011; Mohapatra and Basak 2017).Ascorbic acid (standard) was reported to have an IC50 that ranged from 3.028 to 4.92 µg/ml(Kamble et al. 2011; Mahendran et al. 2011). The IC50 of crude embelin extracts from various Embelia species ranged from 9.87 to 50 µg/ml whereas the EC50 reported from E. tsjeriamcottam was 11 µg/ml(Kamble et al. 2011;Barbade and Datar 2015; Mohapatra and Basak 2017).
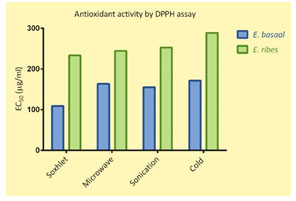
The radical scavenging activity (the EC50) of the ascorbic acid in the present study was 12.15 µg/ml and that of the samples were 109 to 171 µg/ml (E.basaal) and 233 to 289 µg/ml (E. ribes). Statistical evaluation of the calculated EC50 values indicated that the antioxidant activity of E. basaal extracts were significantly higher than those of E. ribes and this was more pronounced in the SE and CE extracts of the species. The variation between the extraction methods was insignificant (Fig. 3 and 4).
Figure 3: Percentage of DPPH radical scavenging activity of E. basaal (A) and E. ribes (B) extracts at different concentrations (µg/ml) obtained using selected extraction techniques.SE: Soxhlet extraction; ME: Microwave extraction; UE: Ultrasonication extraction; CE: Cold extraction.
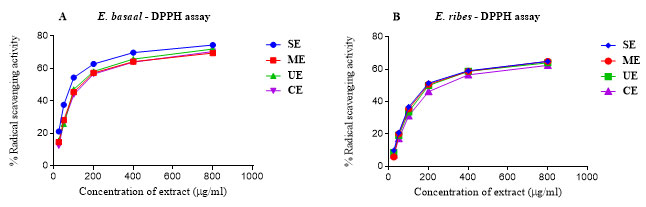
Figure 4: The EC50 values of the reconstituted extracts of E. basaal and E. ribes, using various extraction methods.
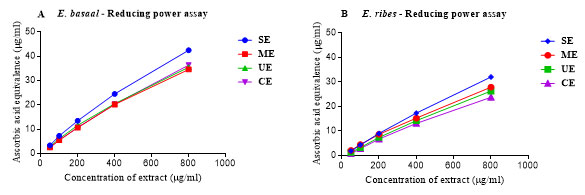
Reducing power assay:RPA is another standard assay to confirm the antioxidant activity of extracts. High values of UV absorbance indicate high antioxidant capacity. The results of the RPA have been shown in Fig. 5. The average antioxidant activity of the reconstituted extracts (1 mg/ml) expressed as the equivalence of ascorbic acid ranged from 64.33 to 50.52 µg/ml and 41.85 to 27.84 µg/ml for E. basaal and E. ribes respectively. The statistical analysis of this data (normalized to 1 mg/ml) revealed that there is a significant difference between the reducing power of the E. basaal and E. ribes extracts.The reducing power of extracts obtained using different extraction methods was insignificant within each species. This is the first report as yet that compared the antioxidant activities of different Embelia species.Genotypes of the plants collected from different localities and solvents have influenced variations in the antioxidant activities of E. ribes (Kamble et al. 2020).
Figure 5: The Reducing Power Assay using the reconstituted extracts of E. basaal (A) and E. ribes(B), at different concentrations (µg/ml) obtained using different extraction techniques and expressed as the equivalence of antioxidant activity of ascorbic acid. SE: Soxhlet extraction; ME: Microwave extraction; UE: Ultrasonication extraction; CE: Cold extraction. The equation obtained for the reducing power assay using ascorbic acid as standard was Y = 0.0174 X + 0.0204 (r2= 0.9975) and was used to calculate the ascorbic acid equivalence antioxidant activity of samples.
CONCLUSION
The findings of the present study indicated that the preferred solvents for extraction of embelin would be chloroform or ethyl acetate. The embelin content of ME extracts was comparable to conventional methods. Antioxidant studies with the reconstituted chloroform extracts confirmed that the bioactivity was retained regardless of the extraction method. Thus, ME has the added advantage of the speed and economic use of biomass. This protocolwould be valuable for the screening and selection of plants or callus with high embelin content. This protocol could be adopted for the screening of other important phytoconstituents as well.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Ashutosh Srivastava, Scientist-E (Rtd; VRS), Institute of Wood Science and Technology, Bangalore, India for providing berries and samples of E. ribes. Ms. Dandekar is grateful for the Non-NET fellowship and DST-PURSE fellowship received.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and/or analyzed during in the current study are not publicly available but are available from the corresponding author on responsible request.
Author Contributions: Shraddha Dandekar and Indu George designed the study. Shraddha Dandekar collected laboratory data. Both authors participated equally in the analysis of the data and writing the manuscript.
REFERENCES
Alam, S., Damanhouri, Z.A., Ahmad, A.,et al. (2015). Development of response surface methodology for optimization of extraction parameters and quantitative estimation of embelin from Embelia ribes Burm by high performance liquid chromatography. Pharmacognosy Magazine, Vol. 11 No. 42, pp. 166–172.doi: 10.4103/0973-1296.157722.
Altemimi, A., Lakhssassi, N., Baharlouei, A., et al. (2017). Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, MDPI AG, Vol. 6 No. 4, p. 42.doi: 10.3390/plants6040042.
Annapurna, D., Srivastava, A. and Rathore, T.S. (2013). Impact of population structure, growth habit and seedling ecology on regeneration of Embelia ribes Burm. f. —approaches toward a quasi in situ conservation strategy. American Journal of Plant Sciences, Vol. 4 No. 6, pp. 28–35.doi: http://dx.doi.org/10.4236/ajps.2013.46A005.
Ansari, N.N., Bhandari, U., Islam, F.,et al. (2008). Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundamental and Clinical Pharmacology, Vol. 22 No. 3, pp. 305–314.doi: 10.1111/j.1472-8206.2008.00580.x.
Atal, C.K., Siddiqui, M.A., Zutshi, U., et al. (1984). Non-narcotic orally effective, centrally acting analgesic from an ayurvedic drug. Journal of Ethnopharmacology, Vol. 11 No. 3, pp. 309–317.doi: 10.1016/0378-8741(84)90076-X.
Barbade, K.D. and Datar, A.G. (2015). Antibacterial activity, free radical scavenging potential,phytochemical investigation and in-vivo toxicity studies of medicinal plant Embelia basaal (R. & S.) A. DC. Asian Journal of Pharmaceutical and Clinical Research, Vol. 8 No. 2, pp. 171–177.https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.917.1539&rep=rep1&type=pdf
Bhishagratna, K.L. (1911). In: An English Translation of the Sushruta Samhita Vol 2, (Edited by Kaviraj Kunja Lal Bhishagratna, M.R.A.S.) Published by the author, Calcutta, pp. 265-361.https://rarebooksocietyofindia.org/book_archive/196174216674_10154367293811675.pdf
Bimakr, M., Rahman, R.A., Taip, F.S., et al. (2011). Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food and Bioproducts Processing, Institution of Chemical Engineers, Vol. 89 No. 1, pp. 67–72.doi: 10.1016/j.fbp.2010.03.002.
Brand-Williams, W., Cuvelier, M.E. and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT – Food Science and Technology, Vol. 28 No. 1, pp. 25–30.doi: 10.1016/S0023-6438(95)80008-5.
Caruso, F., Rossi, M., Kaur, S., et al. (2020a). Antioxidant properties of embelin in cell culture. Electrochemistry and theoretical mechanism of scavenging. Potential scavenging of superoxide radical through the cell membrane. Antioxidants, Vol. 9 No. 5, p. 382.doi: 10.3390/antiox9050382.
Caruso, F., Rossi, M., Pedersen, J. Z., et al. (2020b). Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. Journal of Infection and Public Health, Vol 13 No. 12, pp. 1868–1877. doi: 10.1016/j.jiph.2020.09.015
Choudhary S., Kaurav H., Chaudhary, G. (2021). Vaibidang (Embelia ribes): A Potential Herbal Drug in Ayurveda with Anthelmintic Property. International Journal for Research in Applied Sciences and Biotechnology Vol. 8, pp. 237–243. doi:10.31033/ijrasb.8.2.31
Ferreria, G.M. and Laddha, K.S. (2013). Histochemical localization of embelin in fruits of Embelia ribes Burm and its quantification. International Journal of Pharma Bioscience and Technology, Vol. 1 No. 1, pp. 16–19.https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.404.1065&rep=rep1&type=pdf
Gupta, A., Naraniwal, M. and Kothari, V. (2012). Modern extraction methods for preparation of bioactive plant extracts. International Journal of Applied and Natural Sciences, Vol. 1 No. 1, pp. 8–26.https://www.researchgate.net/publication/236229645_Modern_extraction_methods_for_preparation_of_bioactive_plant_extracts
Joshi, R., Kamat, J.P. and Mukherjee, T. (2007). Free radical scavenging reactions and antioxidant activity of embelin: biochemical and pulse radiolytic studies. Chemico-Biological Interactions, Vol. 167 No. 2, pp. 125–134.doi: 10.1016/j.cbi.2007.02.004.
Kamble, G.S., Torane, R.C., Mundhe, K.S., et al. (2011). Evolution of free radical scavenging potential of Embelia basal. Journal of Chemical and Pharmaceutical Research, Vol. 3 No. 2, pp. 465–471.https://www.jocpr.com/articles/evolution-of-free-radical-scavenging-potential-of-embelia-basal.pdf
Kamble, V., Attar, U., Umdale, S., et al. (2020). Phytochemical analysis, antioxidant activities and optimized extraction of embelin from different genotypes of Embelia ribes Burm f.: a woody medicinal climber from western ghats of India. Physiology and Molecular Biology of Plants, Vol. 26 No. 9, pp. 1855–1865.doi: 10.1007/s12298-020-00859-2.
Kaur, V., Hallan, S.S., Nidhi, et al. (2015). Isolation of embelin from Embelia ribes and evaluation of its anti-cancer potential in breast cancer. Asian Journal of Pharmacy and Pharmacology, Vol. 1 No. 1, pp. 33–39.http://ajpp.in/uploaded/p5.pdf
Latha, C. (2007). Microwave-assisted extraction of embelin from Embelia ribes. Biotechnology Letters, Vol. 29 No. 2, pp. 319–322.doi: 10.1007/s10529-006-9243-z.
Lu, H., Wang, J., Wang, Y., et al. (2016).In: Advances in Experimental Medicine and Biology,Embelin and its role in chronic diseases, Vol. 928 (Edited by Gupta, S.C. et al.), Springer International Publishing Switzerland, pp. 397–418.doi: 10.1007/978-3-319-41334-1_16.
Madhavan, S.N., Arimboor, R. and Arumughan, C. (2011). RP-HPLC-DAD method for the estimation of embelin as marker in Embelia ribes and its polyherbal formulations. Biomedical Chromatography, Vol. 25 No. 5, pp. 600–605.doi: 10.1002/bmc.1489.
Mahendran, S., Badami, S., Ravi, S., et al. (2011). Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of embelin – a structure-activity relationship. Chemical and Pharmaceutical Bulletin, Vol. 59 No. 8, pp. 913–919.doi: 10.1248/cpb.59.913.
Meerungrueang, W. and Panichayupakaranant, P. (2015). Quantitative HPLC analysis and extraction of 2,6-dimethoxy-1,4-benzoquinone from Ficus foveolata stems. Natural Product Sciences, Vol. 21 No. 3, pp. 192–195.https://www.researchgate.net/publication/283477984_Quantitative_HPLC_analysis_and_extraction_of_26-dimethoxy-14-benzoquinone_from_Ficus_foveolata_stems
Mohapatra, M. and Basak, U.C. (2017). Quantitization of antioxidant potency in various plant parts of Embelia tsjeriam-cottam, an important medicinal plant. Journal of Medicinal Plants Studies, Vol. 5 No. 3, pp. 241–249.https://www.plantsjournal.com/archives/2017/vol5issue3/PartD/5-3-5-464.pdf
Mosquera, O.M., Correra, Y.M. and Niño, J. (2009). Antioxidant activity of plant extracts from Colombian flora. Brazilian Journal of Pharmacognosy, Vol. 19 No. 2A, pp. 382–387.doi: 10.1590/S0102-695X2009000300008.
Muruhan, S., Selvaraj, S. and Viswanathan, P.K. (2013). In vitro antioxidant activities of Solanumsurattense leaf extract. Asian Pacific Journal of Tropical Biomedicine, Vol. 3 No. 1, pp. 28–34.doi: 10.1016/S2221-1691(13)60019-2.
Nagamani, V., Rani, A.S., Satyakala, M. et al. (2013). High performance liquid chromatography (HPLC) analysis of embelin in different samples of Embelia ribes Burm. f. – a threatened medicinal plant of India. Journal of Medicinal Plants Research, Vol. 7 No. 24, pp. 1761–1767.doi: 10.5897/JMPR2013.4447.
Nayak, S.U., Joshi, V.K., Maurya, S.,et al. (2009). Analysis of Phenolic acids in Different Market Samples of Vidanga (False black pepper). AYU, Vol. 30 No. 2, pp. 181–187.https://www.researchgate.net/publication/284789418_Analysis_of_phenolic_acids_in_different_market_samples_of_vidanga_False_black_pepper
Pandey, A.K. and Ojha, V. (2011). Estimation of embelin in Embelia tsjeriam-cottam fruits by HPLC to standardize harvesting time. Indian Journal of Pharmaceutical Sciences, Vol. 73 No. 2, pp. 216–219.doi: 10.4103/0250-474X.91563.
Patwardhan, A., Mhaskar, M., Joglekar, A., et al. (2014).In: Future Crops, Propagation and cultivation techniques of Embelia ribes (Vidanga), Vol 2 (Edited by K. V. Peter) Daya publishing house, New Delhi, pp. 237–256.https://www.researchgate.net/publication/274084630_Propagation_and_Cultivation_Techniques_of_Embelia_ribes_Vidanga
Prabhu, K.S., Achkar, I.W., Kuttikrishnan, S., et al. (2018). Embelin: a benzoquinone possesses therapeutic potential for the treatment of human cancer. Future Medicinal Chemistry, Vol. 10 No. 8, pp. 961–976.doi: 10.4155/fmc-2017-0198.
Pundarikakshudu, K., Joshi, H. and Panchal, S. (2016). A simple, facile method for isolation of embelin from fruits of Embelia ribes Burm.f (Vidang). Indian Drugs, Vol. 53 No. 2, pp. 23–27.doi:10.53879/id.53.02.10337
Radhakrishnan, N. and Gnanamani, A. (2014). 2, 5-dihydroxy-3-undecyl-1,4-benzoquinone (embelin)- a second solid gold of India – a review. International Journal of Pharmacy and Pharmaceutical Sciences, Vol. 6, pp. 23–30.https://www.researchgate.net/publication/260640191_2_5-dihydroxy-3-undecyl-1_4-benzoquinone_Embelin-a_second_solid_gold_of_India-_A_review
Radhakrishnan, N., Gnanamani, A. and Mandal, A.B. (2011b). A potential antibacterial agent embelin, a natural benzoquinone extracted from Embelia ribes. Biology and Medicine, Vol. 3 No. 2, pp. 1–7.http://repository.ias.ac.in/83861/1/25-PUB.pdf
Radhakrishnan, N., Kavitha, V., Raja, S., et al. (2011a). Embelin – a natural potential cosmetic agent. Journal of Applied Cosmetology, Vol. 29, pp. 99–107.http://www.iscd.it/files/Embelin-A-natural-potential-cosmetic-agent.pdf
Sathe, V.P. and Dixit, A.P. (2015). High performance thin layer chromatography (HPTLC) analysis of embelin in different organs/ parts of Embelia ribes Burm F. A threatened medicinal plant of western ghats of Maharashtra. International Journal of Pharma and Bio Sciences, Vol. 6 No. 4, pp. 662–669.https://www.researchgate.net/publication/283764170_High_performance_thin_layer_chromatography_HPTLC_analysis_of_embelin_in_different_organs_parts_of_embelia_ribes_burm_f_a_threatened_medicinal_plant_of_western_ghats_of_maharashtra
Sudhakaran, MV. (2016). Botanical Pharmacognosy of the Fruit of Embelia ribes Burm. F. Journal of Pharmacognosy & Natural Products, Vol 1. doi:10.4172/2472-0992.1000103
Tlili, H., Hanen, N., BenA.A., et al. (2019). Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing in southern Tunisia. PLoS ONE, Vol. 14 No. 9, p. e0213049.doi: 10.1371/journal.pone.0213049.
Vijayan, K.P.R. and Raghu, A. V. (2021). Embelin: an HPTLC method for quantitative estimation in five species of genus Embelia Burm. f. Future Journal of Pharmaceutical Sciences, Vol. 7 No. 55.doi:10.1186/s43094-021-00210-w.


