1Department of Home Economic, College of Home Economic, King Khalid University, Abha, KSA .
2Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
3Commission for Biotechnology and Genetic Engineering , National Centre for Research, Khartoum, Sudan.
Corresponding author email: abuelhadi@hotmail.com
Article Publishing History
Received: 03/10/2019
Accepted After Revision: 30/11/2019
Seventeen free amino acids from the various parts of watermelon plant, including essential and non-essential amino acids were found by using Automatic Amino Acid Analyzer. In addition, the antimicrobial activity study of the susceptibility of watermelon (Citrus vulgarisL) against tested microorganisms was investigated. The identified amino acids included: phenylalanine, histidine, tryptophan, lysine, ornithine, arginine, aspartic acid, threonine, serine, glutamine, citrulline, alanine, valine, isoleucine and leucine. The essential amino acids (histidine, leucine, lysine, methionine, phenylalanine, tryosine, and valine ranged between 1.8%-3.6%, 5.5-9.1%, 3.7-6.7%, 0.4-1.1%, 4.1, 6.4%, 0.8-7.2% and 5.5-7.6% in the various parts of watermelon plant, respectively. There were notable variations between the various parts of watermelon plants in their contents of amino acids. However, the highest contents of most of the essential amino acids were concentrated in the leaves, while the highest contents of most of the non-essential amino acids were concentrated in the roots. The investigated amino acids had antibacterial and antifungal activities against the tested organisms.
Automatic Amino Acid Analyzer, HPCL, Glutamine, Valine, Isoleucine.
Ibrahim S. E, Sulieman A. M. E, Ali N. A, Bothaina, Hakeem S. A, Amin H. B, Abdelmuhsin A. A, Veettil V. N. Amino acid Profile of the Watermelon, Citrullus vulgaris and Detection of its Antimicrobial Activity. Biosc.Biotech.Res.Comm. 2019;12(4).
Ibrahim S. E, Sulieman A. M. E, Ali N. A, Bothaina, Hakeem S. A, Amin H. B, Abdelmuhsin A. A, Veettil V. N. Amino acid Profile of the Watermelon, Citrullus vulgaris and Detection of its Antimicrobial Activity. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/34xd3P4
Copyright © Ibrahim et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Strains of the significant human pathogen have demonstrated expanded from all accessible traditional antibiotics (Aloush, 2006; Manchanda et al., 2010). A lot of systems have been engaged in bacterial resistance procedure including the mutation or enzymatic activation that results in the modified objective protein, gaining genes from other bacteria to express less susceptible focused on proteins, and furthermore obtain portable elements including transposons or plasmids (Baltzer and Brown 2011). In spite of nonstop endeavors, multiple drug resistance is as yet a genuine worldwide concern, especially a serious restorative danger to creating countries bringing about monetary weight and increment death rate. It is assessed that worldwide death because of antimicrobial resistance would reach to 10 million by 2050 (Wang et al., 2016). Antimicrobial peptides (AMPs have turned into a promising competitor and have gotten outstanding consideration as a novel class of antibiotics (Antimicrobial Resistance Benchmark 2018). They are peptides normally delivered by all organisms including prokaryotes to human beings in response to foreign microbes and have a role in inborn explicit safeguard defense system and provide instant non-specific defense against infections
Amino acids are found either in the free state or as linear chains in peptides and proteins. There are 22 commonly occurring amino acids in proteins. Amino acid analysis has vital for the study of the composition of proteins, foods, and feed stuffs. Free amino acids are additionally determined in organic material, for example, plasma and urine, and in fruit juice and wine. When it is performed on a pure protein, amino acid analysis is equipped for distinguishing the protein (Hobohm et. al., 1994; Schegg et al., 1997), and the analysis is likewise utilized as an essential for Edman degradation and mass spectrometry and to determine the most reasonable enzymatic or chemical digestion technique for further investigation of the protein.Watermelon juice may give a novel source of the essential amino acid, arginine. Arginine is a precursor for nitric oxide, which has been appeared to lower blood pressure lessen blood thickening and ensure against myocardial localized necrosis and strokes (Collins et al., 2003; Cutrufello et.al., 2015; Fall et. al., 2019).
Free amino acids such as phenylalanine, histidine, tryptophan, lysine, ornithine, arginine, aspartic acid, threonine, serine, glutamic acid, glutamine, citrulline, alanine, valine, isoleucine and leucine are accounted to have been extracted from watermelon and quantitatively analyzed (Tedesco et. al., 1984; Perkins-Veazie et. al., 2012; Jordan et al., 2019)). This study aimed to analyze the free amino profile of various parts of watermelon plant and detection of their antimicrobial activity.
MATERIALS AND METHODS
Amino Acid Analysis by Automatic Amino Acid Analyser S-433
Automatic Amino Acid Analyser S-433
The innovative automatic Amino Acid Analyzer S 433 combines the advantages of the classical ion exchange separation method with the modern technique of high performance liquid chromatography. The complete package of sophisticated instrumentation, a wide variety of preplaced and tested separation columns, combined with optimized ready-to-use buffer solutions and chemicals, create the right answer for any amino acid determination
High Performance Liquid Chromatography (HPLC)
HPLC instrument consists of a reservoir of mobile phase, a pump, an injector, a separation column and a detector (Karen et al., 2019). Sykam chromatographs are modular systems optimized for customer’s needs. Every single system can be configured to suit a certain application. Any existing Sykam HPLC can be upgraded by adding additional components.
ION Chromatography System – S 135
Ion Chromatography is one of the most important methods for the determination of alkaline, alkaline earth, transition metals, inorganic anions, sulphuric compound of different oxidation levels, organic acids and various tensides. Sykam S 135 is a compact system with modular setup. Even the basic system is designed for most sensitive anion analysis employing suppression of eluent conductivity.
Reagent organizer S 7131
The S 7131 Reagent Organizer is the optimal solutions for storing your solvents. The integrated gas supply with pressure regulator let you store the eluent under inert-gas pressure or for degassing purposes using Helium. The organizer can hold up to four eluent bottles which can be closed individually with integrated value bottle caps.
Conductivity Detector S 3115
The S 3115 Conductivity Detector‘s outstanding features are high background suppression, baseline stability, and signal linearity over a range of several decades. These characteristics become especially important when single column techniques are employed, e.g. for the determination of alkaline ions and alkaline earths.The HPLC Pump S 2100 is a compact eluent dosing system, upgradable from a high performance isocratic pump to a quaternary gradient pump with outstanding features.
Solvent Delivery System S 2100-Ex
The HPLC Pump S 2100 is a compact eluent dosing system, upgradable from a high performance isocratic pump to a quaternary gradient pump with outstanding features.
Sample preparation
For preparation of sample hydrolysate, 200 milligram of watermelon (roots, stems, leaves and three maturation stages of its fruit and seeds), was weighted in a hydrolysis tube to prepare hydrolysate sample. Then 5 ml of 6N HCl were added and the tube was tightly closed. The sample was incubated for 24 hours at 110o C. The sample was allowed to cool. 125 ml was filtered using 200 micro-liters of the filtrate were taken into another tube and evaporated at 140o C ovens for about 1 hour. 1 ml of diluting buffer was then added to the dried sample and transferred to amino acid analyzer vial and then injected for analysis.
After the separation of the injected sample with a temperate cation separation column, ninhydrin was added continuously to the system. An integrated reagent dosing pump was responsible for the delivery of this reagent, while an external buffer pump was delivering the eluent. After adding ninhydrin, the eluent was lead through a high temperature reactor coil of about 16 m length. With a typical flow rate of 0.7 m1/min the buffer / ninhydrin mixture was stayed in the heated reactor for about 2 min. maximum absorption at 570 nm), while the secondary amino acids (have their maximum at 440 nm). After the reaction the mixture was lead through a dual-channel photometer where both wavelengths (570 nm and 440 nm) were measured continuously.
Tested microorganisms
The standard microorganisms used for this study were received from the Medicinal and Aromatic Plants Research Institute, National Centre for Research, Sudan and from the Department of Microbiology, Beijing University of Chemical Technology, China. They were Bacillus subtilis, B.s. (NCTC 8236, ACCC ), designated as Gram +ve, Staphylococcus aureus, S.a. (ATCC 25923 & CVCC), designated as Gram +ve, Escherichia coli, E. coli (ATCC 25922, ACCC), designated as Gram -ve, and one fungus, Candida albicans, Ca.a. (ATCC 7596, CVCC), designated as Gram +ve.The microorganisms were produced a stock preparation containing a log-phase cell density of approximately 107 colony forming units (CFU)/ml as evaluated initially by measurements of the optical density at 600 nm.
Antibacterial Assay
Determination of antibacterial activity
After separation of amino acids in small pre-sterilized containers, they were taken for determination of antimicrobial activity. The antibacterial activity was assessed by the agar–well diffusion method (Kinsbury and Wagner, 1990). The inoculum size of each tested bacterium was adjusted to a suspension of 106 cells. The inoculum suspension was spread over a Mueller Hinton agar (MHA) plate to achieve confluent growth and allowed to dry. 10 mm-diameter wells were bored in the agar using a sterile cork borer. A 100 μL-aliquot of the reconstituted extract was placed into a well with standard Pasteur pipette and the plate was held for 1 hr at room temperature for diffusion of extract into the agar. Subsequently, the plate was incubated for 18 hr at 37ºC. After incubation, the diameters of the zones of inhibition were measured to the nearest mm. Three replicates were performed and results were recorded.
RESULTS
Amino acid composition
Using the amino acid analyzer, the retention time and the amount of amino acid in the various parts of the watermelon plant as obtained from the chromatograms are indicted in Table (1) and in Figures (1 and 2). 17 essential and non-essential amino acid were identified. The identified amino acids included: phenylalanine, histidine, tryptophan, lysine, ornithine, arginine, aspartic acid, threonine, serine, glutamic acid, glutamine, citrulline, alanine, valine, isoleucine and leucine. The results also indicated that the highest amount of amino acids present were in this order, Glutamine (39.0%) then Ammonia (9.2%) and Arginine (7.7%) in the roots (SP1a).
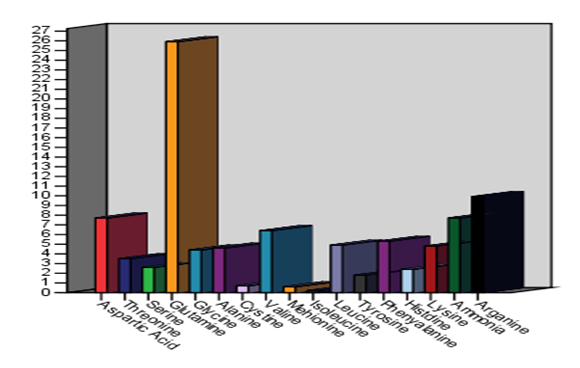 |
Figure 1: Amino acid profile of the various parts of watermelon plant |
Table 1: Essential and non-essential amino acids contents of various parts of watermelon plant
| NO. | Amino acids | SP1a | SP1b | SP1c | SP1d | SP1e | SP1f | SP1h |
| 1 | Aspartic Acid | 6.0 | 9.9 | 9.5 | 8.1 | 6.0 | 6.4 | 8.3 |
| 2 | Threonine | 2.8 | 4.0 | 4.7 | 3.8 | 2.8 | 3.0 | 3.3 |
| 3 | Serine | 1.8 | 2.9 | 3.0 | 3.7 | 1.8 | 2.5 | 2.7 |
| 4 | Glutamine | 39.0 | 21.9 | 19.5 | 20.4 | 39.0 | 18.7 | 22.7 |
| 5 | Glycine | 5.3 | 5.0 | 5.5 | 3.8 | 5.3 | 0.6 | 5.0 |
| 6 | Alanine | 2.6 | 6.6 | 6.1 | 4.0 | – | 7.7 | 5.1 |
| 7 | Cysteine | – | 0.1 | 0.7 | 0.2 | 2.6 | 0.5 | 1.0 |
| 8 | Valine | 5.1 | 7.6 | 7.2 | 6.9 | 5.1 | 7.3 | 5.9 |
| 9 | Methionine | 0.4 | 0.2 | 0.6 | 0.2 | 0.4 | 1.1 | 1.1 |
| 10 | Isoleucine | 4.3 | 5.7 | 5.7 | 5.3 | 4.3 | 5.8 | 4.7 |
| 11 | Leucine | 5.5 | 8.0 | 9.1 | 7.9 | 5.5 | 7.3 | 7.1 |
| 12 | Tyrosine | 0.8 | 0.5 | 7.2 | 0.7 | 0.8 | 1.0 | 1.3 |
| 13 | Phenylalanine | 4.1 | 5.6 | 6.4 | 5.6 | 4.1 | 5.4 | 6.0 |
| 14 | Histidine | 1.8 | 2.4 | 2.6 | 3.6 | 1.8 | 2.1 | 2.7 |
| 15 | Lysine | 3.7 | 6.4 | 6.4 | 6.7 | 3.7 | 2.5 | 4.4 |
| 16 | Ammonia | 9.2 | 8.2 | 4.3 | 6.9 | 9.2 | 11.9 | 4.0 |
| 17 | Arginine | 7.7 | 4.9 | 6.1 | 12.3 | 7.7 | 16.0 | 14.6 |
Watermelon plant = (SP1); SP1a= Roots; SP1b=Stem; SP1c= Leaves; SP1d = Green crust; SP1e = White crust; SP1f = kernel; SP1h = seed
The results indicated in Fig. 2 shows the identification of 17 essential and non-essential amino acids by using the amino acid analyzer to determine the retention time and the amount of amino acid in watermelon various parts. Results revealed that Glutamine has the highest amount among detected amino acids (39.0%) followed by Ammonia (9.2%) and Arginine (7.7%) in the plant roots (SP1a) (Fig. 2 -A). Also the highest amount of amino acids present the stems of watermelon (SP1b) was for Glutamine (21.90%) then Aspartic Acid (9.9%) and the Ammonia (8.2%) as indicated in Fig. 2 (B). On the other hand, Glutamine (19.5%) has the highest amount among amino acids present in the leaves of watermelon (SP1c), followed by Aspartic Acid (9.5%) and Leucine (9.1%) as shown in Fig. 2 (C). The results shown in Fig 2 (D) indicated that Glutamine Acid highest amount (20.4%) among amino acids in the green crust of watermelon (SP1d), followed by Arginine (12.3%) and Aspartic Acid (8.1%).
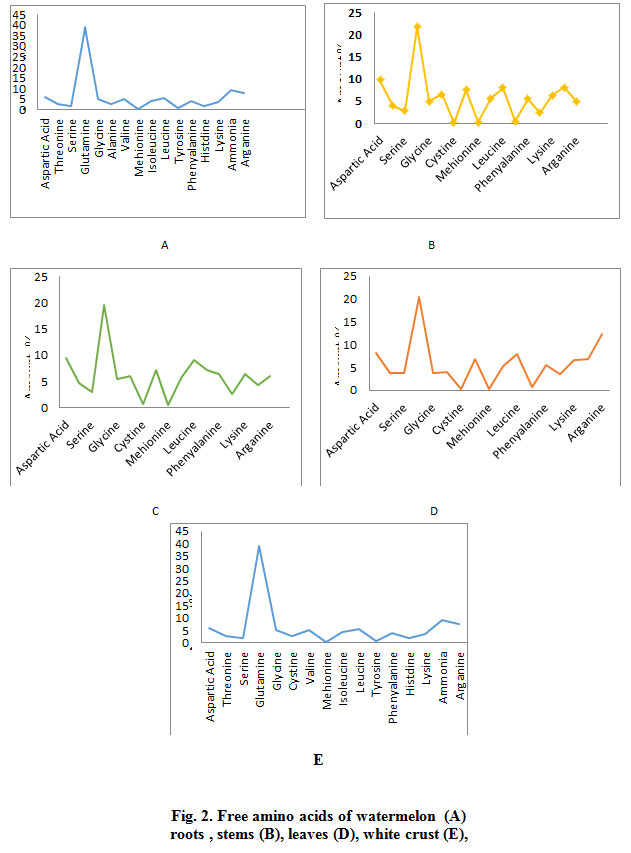 |
Figure 2: Free amino acids of watermelon (A) roots , stems (B), leaves (D), white crust (E), |
Fig. 2 (E) also shows the identification of 17 essential and non-essential amino acids using the amino acid analyzer to determine the retention time and the amount of amino acids in white crust of watermelon. The results showed the that the highest amount found was Glutamic Acid (39.0%) followed by Ammonia (9.2%) and Arginine (7.7%) in the white crust of watermelon (SP1e). As for the watermelon kernel (SP1f), the highest amount present of was that of Glutamine Acid (18.7.0%), Arginine (16.0%) and Ammonia (11%). The results showed that the highest amount of amino acids present in the seeds of watermelon (SP1h) was Glutamic Acid (22.7.0%), Arginine (14.6%) and Aspartic acid (8.3%) .
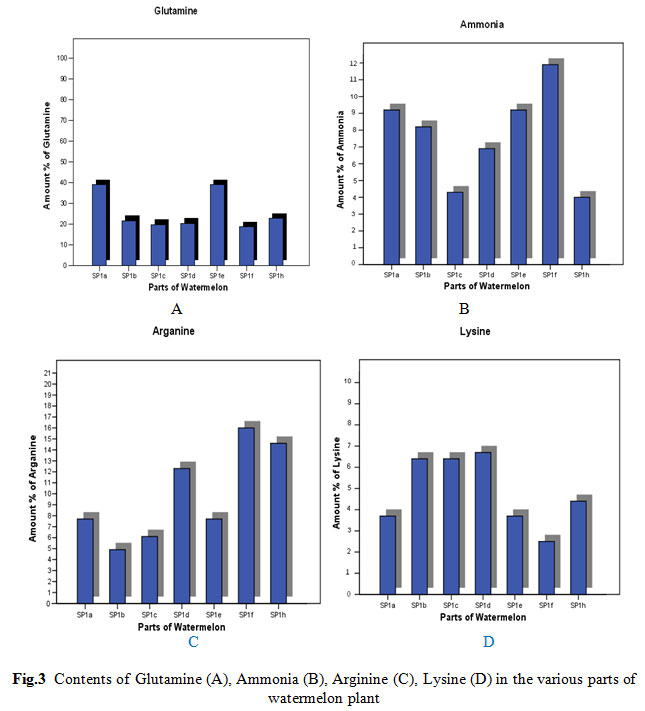 |
Figure 3: Contents of Glutamine (A), Ammonia (B), Arginine (C), Lysine (D) in the various parts of watermelon plant |
Antimicrobial activity of the amino acids
The results of the antimicrobial activity of the free amino acids as shown in Fig. (4) shows that the investigated amino acids had antibacterial and antifungal activities against the tested organisms. However, the antimicrobial varies with the nature of amino acids, and the part of the plant and organisms. Fig. (4) also shows that the amino acid glutamine shows the highest antimicrobial activity against the fungus Candida albican which had the highest inhibition zone diameter (28 mm) followed by the bacteria Staphylococcus aureus, then E.coli and lowest activity was against Bacillus subtilis which had the lowest inhibition zone diameter (13 mm).
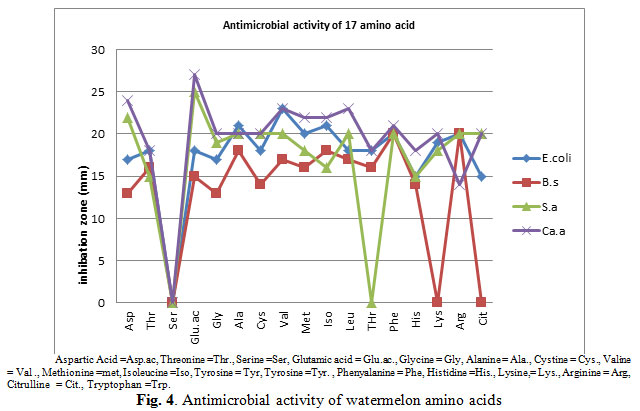 |
Figure 4: Antimicrobial activity of watermelon amino acids |
The bacteria Staphylococcus aureus was highly inhibited by all detected amino acids with exception to serine and threonine. However, E.coli was inhibited by all detected amino acids with exception to serine. Bacillus subtilis was inhibited by all detected amino acids with exception to serine, lysine and cittruline. The fungus Candida albican was highly inhibited by all detected amino acids with exception to serine amino acid.
DISCUSSION
Amino acids contents
Amino acids are the building blocks of peptides and proteins. They possess two functional groups, the carboxylic acid group gives the acidic character, and the amino group provides the basic character. Proteins are composed of different amino acids and hence the nutritional quality of a protein determined by the content, proportion, and availability of its amino acids (Becker, 2007). To date, more than 300 amino acids have been found in nature, of which 20 are engaged in protein synthesis and are known as proteinogenic amino acids. Proteinogenic amino acids exist in two structures: in a free state in physiological liquids (e.g., plasma, urine) and food (e.g., wine, beverage) and bound in peptides or proteins (Yu and Mou 2005).Table 1 shows the amino acid profile of watermelon various parts. This result suggested that the water melon plant parts were rich in amino acids, which might be served as good source of nutrition. Of 22 amino acids, almost 17 amino acids were present. However, some of the amino acids were present in lower concentration in comparison to that reported by FAO pattern (FAO 2018).
The essential amino acids (Histidine, Leucine, Lysine, Methionine, Phenylalanine, Tryosine, and Valine ranged between 1.8%-3.6%, 5.5-9.1%, 3.7-6.7%, 0.4-1.1%, 4.1, 6.4%, 0.8-7.2% and 5.5-7.6% in the various parts of watermelon plant, respectively. Valine essential amino acid was the dominant essential amino acid in all watermelon plant parts. Moreover, watermelon stem contained the highest value, while both the root and white kernel contained the least value. On the other hand, Methionine amino acid was the least in concentration among all essential amino acids in all watermelon plant. Nonessential amino acids (Alanine, Aspartic acid, Glutamine, and Serine) ranged between 1.8 – 39% for all watermelon plant parts. Glutamine was the dominant non-essential amino acids in all watermelon parts. Moreover, it was the most dominant amino acid investigated in this study.
Most of the amino acid values are comparable with those of most vegetable protein determined by many investigators (El- Adawy et. al., 2001; Mune et al., 2011; Ogunladeet al, 2011; Sánchez- Vioque et. al., 1999). Generally the average percentage of nonessential amino acids was higher in concentration than essential amino acids in watermelon plant parts. In comparison, Usman et al. (2010) revealed the prevalence of glutamic acid and aspartic acid in watermelon seeds. This suggests variety happens as indicated by genotype and the geographical and environmental conditions in which watermelons are developed. Glutamine is an important amino acid with numerous capacities in the body. It is a building block of protein and critical part of the immune system. Likewise, glutamine has an extraordinary role in intestinal health. Your body naturally produces this amino acid, and it is also found in numerous foods. There were notable variations between the various parts of watermelon plants in their contents of amino acids (Table 1). However, the highest contents of most of the essential amino acids were concentrated in the leaves (SP1a), while the highest contents of most of the non-essential amino acids were concentrated in the roots (SP1b). Watermelon plant = (SP1); SP1a= Roots; SP1b=Stem; SP1c= Leaves; SP1d = Green crust; SP1e = White crust; SP1f = kernel; SP1h = seed.
A high- quality protein contains essential amino acids in ratios proportionate with human needs. This can be dictated by comparing the amino acid contents of different proteins with the FAO reference pattern. The FAO reference pattern dependent on the essential amino acid requirements of young children (1–2 years) is viewed the preferred reference protein (Andini et. al., 2013). Methionine and Tyrosine were found in limited amount for most of the watermelon plant parts, however, this shortage might be explained by their denaturization during analysis or their values are very limited in the plant. To compensate this limitation in watermelon, additional Utilization of animal or plant proteins, for example, milk, egg, lentils, and heartbeats are profoundly prescribed (Silva et al. 2014).
Antimicrobial activity
Antimicrobial peptide (AMPs) is a part of inborn safeguard framework in most multicellular life forms, from people to plants to insects. To date more than 2200 common or synthetic AMPs have been accounted for through the antimicrobial peptide database (APD) (Silva et al. 2014). Antimicrobial peptides (AMPs) are commonly composed of short sequences of 10–100 amino acids deposits and profoundly and membrane active (Reddy et al. 2004). AMPs have a wide range of antibacterial, antifungal, antiviral, and anti-tumor activity at low concentrations. In contrast to traditional antibiotics, antimicrobial peptides can direct the host resistant immune system and kill bacteria directly (Gottler and Ramamoorthy 2009). Moreover, other biological activities of AMPs have also been described as following: neutralization of endotoxins, immune-modulating properties, chemokine-like activities, and induction of both angiogenesis and wound repair (Guaní-Guerra et al. 2010).
It has been reported that Gram-positive bacteria are known to be more susceptible to amino acids complexes than gram-negative bacteria. The weak antibacterial activity against gram negative bacteria was attributed to the existence of an external film which stances hydrophilic polysaccharides chains as a a boundary to the amino acids complexes. It has also been reported that the antibacterial action of a complex is affected by its dependability. The lower strength of the amino acid complex, the more prominent is the antibacterial action. This is likely because they have more free ions in the solution, which can improve the cooperative association between the metal particles and the ligands, (Marcu et al., 2008; Stanila et al., 2007).
The variety in the adequacy of various compounds against various organisms depends either on the impermeability of the cells of the microorganisms or the distinction in ribosomes of microbial cells. It has additionally been suggested that concentration assumes a fundamental role in expanding the level of inhibition; as the concentration increases, the activity increments as it was accounted for likewise in the present cases (El-Wahab et al., 2005). In this context further investigations are expected to understanding the antibacterial or antifungal mechanism Antimicrobial peptides which are composted of a great number of amino acids have a broad spectrum of activity against a wide range of microorganism including Gram negative and Gram positive bacteria, fungi, parasites and viruses (Brown et al., 2007; Hancock (2001); Andrea and Rivas (1998); Bradshaw 2003).
CONCLUSION
There are a total of 17 free amino acids that were found in all organs of watermelon plant, out of which glutamic acid, arginine and aspartic acids were found to be in higher concentration in all organs of the plant. However, the highest contents of most of the essential amino acids were concentrated in the leaves, while the highest contents of most of the non-essential amino acids were concentrated in the roots. The results of antimicrobial activity tests, show that amino acids complexes have inhibitory effect against the bacteria: Staphylococcus aureus, Escherichia coli, and the fungus, Candida albicans, and less efficient against Bacillus subtilis. Studies regarding the mode of action for the amino acids in the microbial bacterial cell should be investigated, since these substances are natural, their hazardous potential is lower when compared with other products.
ACKNOWLEDGEMENTS
The authors would like to acknowledge The Ministry of Higher Education and Scientific Research of the Sudan for their financial support, Medicinal and Aromatic Plants Research Institute, National Centre for Research-Sudan, Biotechnology & Genetic Engineering , National Centre for Research, Khartoum- Sudan, Ministry of Science & Technology Center Laboratory- Sudan, Beijing University of Chemical Technology Life Science Center Microbiology and University of Beijing University of Chemical Technology, China. For providing laboratory facilities to conduct the various experiments.
REFERENCES
Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. (2006). Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrobial agents and chemotherapy 2006: 50:43-8.
Andreu D, Rivas. L. (1998). Animal antimicrobial peptides: an overview. Peptide Science 1998; 47:415-33.
Andini, R.; Yoshida, S.; Yoshida, Y.; Ohsawa, R.O. (2013). Amaranthus genetic resources in Indonesia: Morphological and protein content assessment in comparison with worldwide amaranths. Gen. Resour. Crop Evol. 2013, doi:10.1007/s10722-013-9979-y. Available online: http://link.springer. com/content/pdf/10.1007s10722-013-9979-y.pdf (accessed on 2 May 2013).
Baltzer SA, Brown MH (2011). Antimicrobial peptides–promising alternatives to conventional antibiotics. Journal of molecular microbiology and biotechnology 2011; 20:228-35.
Bradshaw JP (2003). Cationic antimicrobial peptides. BioDrugs 2003; 17:233-40
Becker, E. W. (2007). Micro- algae as a source of protein. Biotechnology Advances, 25, 207–210.
Brown KL, Mookherjee N, Hancock RE (2007). Antimicrobial, host defence peptides and proteins. Encyclopedia of Life Sciences. Chichester, Wiley, 2007; 1–11.
Collins J.K., Wu G., Perkins-Veazie P., spears K., Claypool P.L., Baker R.A., Clevidence B.A. (2013). Watermelon consumption increases plasma arginine concentration in adults, Nutrition (2003), Vol. 23, pp.261-266.
Cutrufello, P.T.; Gadomski, S.J.; Zavorsjy, G.S. (2015). The effect of L-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J. Sport Sci. 2015, 33, 1459–1466. [CrossRef] [PubMed]
El‐Adawy T., Rahma E., El‐Bedawey A., & Gafar A. (2001). Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates. Food Chemistry, 74, 455–462.
El-Wahab ZHA, Mashaly MM, Salman AA, El-Shetary BA, Faheim AA. (2004). Co(II), Ce(III) and UO2VI) bissalicylatothiosemicarbazide complexes: binary and ternary complexes, thermal studies and antimicrobial activity. Spectrochim Acta A 60(12):2861-2873.
FAO. (2018). Protein quality assessment in follow-up formula for young children and ready to use therapeutic foods. Rome. 50 pp. Licence: CC BY-NC-SA 3.0 IGO.
Gottler LM, Ramamoorthy A. (2009) Structure, membrane orientation, mechanism, and function of pexiganan-A highly potent antimicrobial peptide designed from magainin. Biochimica Et Biophysica Acta-Biomembranes 1788(8):1680–1686
Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM (2010) Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol 135(1):1–11
Hancock RE. (2001). Cationic peptides: effectors in innate immunity and novel antimicrobials. The Lancet infectious diseases. 2001; 1:156-64.
Hobohm U., Houthaeve T., and Sander C. (1994) Amino acid analysis and protein database compositional search as a rapid and inexpensive method to identify proteins. Anal. Biochem. 222, 202–209.
Jordan L. Hartman , Todd C. Wehner ,Guoying Ma and Penelope Perkins-Veazie (2019). Citrulline and Arginine Content of Taxa of Cucurbitaceae. Horticulturae 2019, 5, 22; doi:10.3390/horticulturae5010022.
Kingsbury D. T. and Wagner G.E. (1990). Edition, 2, illustrated. Publisher, Wiley, 1990. Original from, the University of California. Digitized, Jun 14, 2011. ISBN-10: 0683062344 https://books.google.com › Science › Life Sciences › Biology
Manchanda V, Sanchaita S, Singh NP (2010). Multidrug resistant Acetobacter. Journal of global infectious diseases 2010; 2:291.
Marcu A, Stanila A, Cozar O, David L (2008). Structural investigations of some metallic complexes with threonine as ligand. J Optoelectron Adv M 10(4):830-833.
Mune M. A., Minka S. R., Mbome I. L., & Etoa F. X. (2011). Nutritional potential of bambara bean protein concentrate. Pakistan Journal of Nutrition, 10, 112–119.
Ogunlade I., Olaifa O., Adeniran O. A., & Ogunlade A. O. (2011). Effect of domestic processing on the amino acid profile of Dioscorea rotundata (White yam). African Journal of Food Science, 5, 36–39.
Reddy KV, Yedery RD, Aranha C. (2004). Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004 Dec;24(6):536-47.
Sánchez‐Vioque R., Clemente A., Vioque J., Bautista J., & Millán F. (1999). Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chemistry, 64, 237–243.
Schegg K. M., Denslow N. D., Andersen T. T., Bao Y.A., Cohen S.A., Mahrenholz A. M., and Mann K. (1997) Quantitation and identification of proteins by amino acid analysis: ABRF-96 collaborative trial, in Techniques in Protein Chemistry VIII (Marshak D., ed.), Academic San Diego, CA, pp. 207–216.
Silva N. H., Artur F.R., Isabel F., Almeida P.C., Costa C. R., Carlos P.N.., Armando J.D., Silvestre. C., S.R. (2014) Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydrate Polymers Volume 106, 15 June 2014, Pages 264-269
Stanila A, Marcu A, Rusu D, Rusu M, David L (2007). Spectroscopic studies of some copper (II) complexes with amino acids. J Mol Struct 834:364-368
Tedesco T.A., Benford S.A., Foster R.C., Barness L.A. (1984). Free amino acids in Citrullus vulgaris (watermelon), Pediatrics (1984), Vol. 73, No. 6, pp. 879
Usman A, Haruna M, Itodo V, Oforghor OA. (2010). Protein and amino acid composition of water melon (Citrullus lanatus) seeds. International Journal of Tropical Agriculture and Food Systems 4:343- 356.
Wang S, Zeng X, Yang Q, Qiao S. (2016). Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. International journal of molecular sciences 2016; 17:603.`
Yu H, Mou SF. (2005) Method development for amino acid analysis. Chinese J Anal Chem 33:398–404


