Postgraduate Department of Zoology the Assam Royal Global
University Guwhati, Assam 782447 India.
Article Publishing History
Received: 10/10/2024
Accepted After Revision: 24/11/2024
Copper is highly toxic to fish, leading to harmful effects such as cell damage, genetic mutations, and cancer In aquatic environment it affects both human health and the aquatic ecosystem. Accumulation of copper sulphate on blood and tissue causes changes in the liver and kidney of Channa punctatus. Three sub-lethal concentrations of CuSO4 (0 .45 mg/l, 0.72 mg/l, and 1.2 mg/l) were taken, One group with pretreatment of BFE exposed to CuSO4, another group exposed to highest dose of CuSO4 were treated BFE. Changes noted at intervals of 10, 20, and 30 days of blood , and tissues samples in haematology and histological parameters.
In chemically treated groups. A decrease in RBC and Hb counts ,increase in WBC counts , and some nuclear abnormalities were observed Improvement in blood parameters noted in fish treated with BFE. Chronological histopathological damage, including swelling of hepatic cells, hepatocellular necrosis, vacuolization, inflammation, and hepatic cell damage observed in fish exposed to CuSO4. In BFE treatment, improvements of vital organs were noted, with decreased inflammation, necrosis, and vacuolation in hepatic and renal structures. The banana inflorescence extract can be used as antioxidants to counteract the damage induced by copper sulphate in C. punctatus.
Channa puntatus, CuSO4, necrosis, vacuolation, nuclear abnormalities
Arjun J, Debi Y. R. Ameliorative effects of banana inflorescence Musa sp to mitigate the impact of CuSO4 on Channa punctatus.Biosc.Biotech.Res.Comm.2024;17(4).
Arjun J, Debi Y. R. Ameliorative effects of banana inflorescence Musa sp to mitigate the impact of CuSO4 on Channa punctatus.Biosc.Biotech.Res.Comm.2024;17(4). Availablefrom:<ahref=”https://shorturl.at/9YYgl“>https://shorturl.at/9YYgl</a>
INTRODUCTION
Heavy metals are natural environmental components and large quantities of these heavy metals are accumulated as a result of land- based activities in the aquatic ecosystem (Javed et al. 2017 ). Nowadays, heavy metal residues have become a serious concern because of their continuous increase in air and aquatic environment (Abah et al. 2016; Javed and Usmani 2019). Increased human population with their anthropogenic activities, both underground and surface water supplies are now affected with the heavy metals resulting in depletion of the aquatic organisms (Waqar et al. 2013 Vasconselos et al 2024).
The contamination of aquatic ecosystem with heavy metals is regarded to be dangerous not only for aquatic fauna but also for the human as the consumer of fish for food, (Sabullah et al. 2015). At present, the aquatic pollution has increased many times due to the introduction of modern technologies using heavy metals as raw materials for different functions (Wong et al. 2001, Wu et al 2019). The adverse impacts of these pollutants on aquatic ecosystems and human health emphasize the need for effective pollution control measures, proper waste management, and sustainable practices to protect and preserve water quality ( Perkumien et al., 2023).
Among metals, copper is one of the essential trace elements that plays an important role in the growth and development of organisms. At the same time, Cu is also one of the most poisonous metals that affects aquatic species and ecosystem (Singh et al. 2010). Heavy metal in relatively high concentrations and excessive use can result in toxic metal complexes that build in water, creating biological system imbalances and results in oxidative stress, ( Lushchak 2011,Vasconselos et al 2024).
Apparently, the demand for copper continues to increase annually as it is used in water pipelines, intelligent houses and buildings, electrical motors, power lines, electrical appliances, healthcare, environment-related industries, computers and communication devices. This has further increased the use of copper in industry, while increasing copper contamination of the environment (Wani et al., 2020).
Heavy metals accumulate mostly in water bodies, affects a wide variety of aquatic organisms. In recent years, chemical biomonitoring and histopathology have often been combined with the evaluation of biomarkers representing early indicators of biological effects Chana punctatus is one of the most widely distributed freshwater fish and 26 species discovered in Asia, North eastern India gives home to the nine species of Channa (Vishwanath and Geetakumari, 2009). Eight species belonging to C. barcaand and C. bleheri) are found all over India ). Considering Channa punctatus and adaptable, it has It is taken as least concern by the IUCN red list threatened data book (IUCN 2014)
According to El-Moselhy et a (2014), metal bioaccumulation by fish is subsequently accumulated in specific organs and later distributed to different organs such as the liver, kidney, gills, heart, bone, brain and digestive tract. Fish has the ability to easily accumulate toxicants in their organs and cellular functions during exposure (Ajani and Akpoilih 2010). Thus, the effect of accumulated toxicants will cause severe damage to the piscine systems, leading to an overall toxic effect of metabolic reactions as well as physical and behavioural activities.
Haematological studies are useful in assessing the health of fish subjected to changing environmental conditions. Fish blood is being studied as an indicator of pathological changes and environmental monitoring system (Walter et al. 2006). Therefore, it is important to diagnose the functional and systemic state of fish exposed to toxicants.Metals can cause alteration in haematological indices in fish. Increase or decrease in blood parameters is considered to be the symbol of unhealthy state, environmental stress, or tissue injury ( Hassan et al. 2018). The histopathological changes due to exposure of heavy metals are helpful in evaluating their toxic effects in different species of fishes (Clemente et al. 2013 Chakraborty and Sarkar 2023).
The liver of fish exposed to heavy metals showed congestion of the central vein, edema, and nuclear pyknosis and degeneration in hepatic cells of different species (Kaoud and El-Dahshan 2010; The histological alterations due to heavy metals in the intestines of fish include atrophy in the muscularis, necrotic changes in the intestinal mucosa, and sub-mucosa with degenerative cells in the intestinal lumen (Padrilah et al. 2018). Thus, the current study was planned to investigate the effect of induced toxicity of Copper Sulphate on the haematology and the histolopathology of Channa punctatus. .
Many researchers’ study to neutralize the toxicity induced by heavy metals by using naturally found minerals and vitamins. Liu et al. (2023) in their study, the anti- inflammatory and antioxidant activities of the tocotrienol-rich fraction from rice bran oil and its potential mechanism were verified in a zebrafish CuSO4 inflammation model. According to a study conducted by China et al. (2010), banana flower extract is a potential natural source of antioxidants that might help reduce oxidative stress by lowering hepatic cell damage caused by free radicals in the iron-mediated Fenton reaction. The anti-cancer activities and anti-proliferative effects were also assessed.
The current study selected banana inflorescence extract to explore its potential in protecting freshwater fish from copper poisoning. This research focuses on the haematological response to chemical treatment with CuSO4, emphasizing blood analysis. Additionally, the study examined the histological structure of key organs like the liver and kidney, due to their crucial roles in metabolizing various chemicals and eliminating cytotoxic and metabolic byproducts. The liver and kidneys are essential in processing toxic substances, meaning no chemical is entirely safe from potentially harming these organs. As a result, liver and kidney damage often serve as the first indication of toxicity. Thus, research over the past few decades has aimed to identify compounds that can mitigate these harmful effects and facilitate recovery from the damage caused by toxins. However, the protective role of banana inflorescence extract against the degree of toxicity of copper sulphate are not well documented.
MATERIAL AND METHODS
Collection of fish: Channa punctatus, ranging in length from 13 to 15 cm and weighing between 24 and 30 grams, were procured from a nearby market. To eliminate external fungal and algal infections and prevent potential skin issues, the fish underwent thorough washing in tap water followed by treatment with a 0.02% potassium permanganate (KMNO4) solution. They were then placed in aerated dechlorinated water under laboratory conditions, maintaining a natural photoperiod of 12 hours light and 12 hours dark, and a temperature of 26°C for a 15-day acclimation period prior to the commencement of the experiment. Throughout this period, the fish were fed twice daily with “Optimum” artificial fish food from Perfect Companion Group Co., Ltd., Thailand, and daily maintenance included the removal of waste materials and uneaten feed to ensure optimal fish health.
Experimental design and specimen treatment: The selection of healthy fish for copper exposure were divided Karber Method into seven groups The LC50 was determined (Hamilton et al., 1977). Group I served as the control, while groups II, III, and IV were exposed to different sublethal concentrations of copper sulphate (0.45 mg/l, 0.72 mg/l, & 1.20 mg/L) for 10, 20, and 30 days, respectively. Groups V received treatment with banana flower extract (BFE), while group VI were chemical pretreated BFE followed by 1.20 mg/l of CuSO4 exposure and in contrast other groups were first exposed to 1.20 mg/l CuSO4 followed BFE treatment. Each treatment was performed in triplicate ).Copper sulphate, formalin, ethanol, KMnO4, methanol, Giemsa 5% solution, haematoxylin, eosin are used during the experiment from the Zoology Lab, Royal School of life Science. Fresh banana (Musa sp.) inflorescence were purchased at the neighbourhood market, cleaned, sliced, dried at 40 degrees Celsius, powdered, and kept at 4 degrees Celsius.
Diet preparation: A total of 2 kg of banana inflorescence powder was extracted and processed using the decoction method. The resulting extract was then combined with 0.5 kg of salt (NaCl) and 1.0 kg of “Optimum” fish feed powder at a ratio of 10:1. Sterilized water was added as needed to form a dough, which was then used to create pellet feed. These pellets were dried in an oven at a temperature of 40°C and stored in an airtight container. The experimental diets were fed to the fish at a rate of 3% of their live body weight, with the daily ration split into two equal meals at 8:45 am and 2:45 pm over a period of 2 weeks.
Sample Collection: Six samples of blood were taken from every group of fish Whole blood count parameters were determined using EDTA- containing blood. cells. Blood. Smear was fixed with absolute methanol, air dried and room temperature and counterstained with 5 and studied under the light microscope.
TOTAL COUNT (TC) RBC (Erythrocyte): Erythrocytes were counted in the hemocytometry chamber and expressed as 10^6 cells per mm^3, following Wintrobe’s (1967) methodology. Counting was done within five smaller squares: the 1st, 5th, 13th, 21st, and 25th
WBC (Leucocyte): Blood samples were diluted 1:20 with Turk’s diluting solution before being put onto the haemocytomer. The four huge (1 square millimetre) corner squares of the haemocytomer were inspected under a microscope. Cells that touched the boundary . The total number of white blood cells was determined and expressed as cells per cubic millimetre using the method outlined by Wintrobe (1967).
Estimation of haemoglobin: A haemometer tube was filled with 10 drops of N/10 hydrochloric acid (HCl). A pipette was used to extract blood up to 20 cm, which was then discharged into the tube, mixed, and allowed to stand for 30 minutes. The tube was gradually filled with distilled water until it matched the colour of the
Statistical Analysis: Experiments were conducted in triplicates. The data observed in the experiment were statistically analyzed on One way ANOVA for individual group wise comparison was administered for testing the hypothesis. The data shown are the average of three replicates + SE and statistical significance was tested at p<0.001(***), p<0.01(**),& p<0.05(*).
Histopathological studies: The selected fish were euthanized and organs such as the liver and kidney were removed and fixed in 10% formalin for 48 hours and dehydration using various concentrations of ethanol (50%, 70%, 90%, and 100%) and were for kept for 20-30 mins each time. The tissues were then cleaned in Xylene for 30 minutes, immersed in liquid paraffin wax heated to 60°C, and embedded in blocks. A rotatory microtome was utilized to cut the sample blocks into slices that were 5 µm thick. These sections were then , flattened on a hot plate, and stained with hematoxylin for one minute followed by counterstaining with eosin for two minutes. After appropriate preparation, the sections were mounted using DPX. Images were captured using an oil immersion compound microscope with a 100X objective lens magnification.
RESULTS AND DISCUSSION
The behavioural changes and deformities included alterations in swimming patterns, food intake, and excretions from the fish, with severity increasing in correlation with higher CuSO4 concentrations the fish became more lethargic, exhibited reduced activity, and lost their healthy appearance and vigour.
Figure 1.1 Photos taken during experimental period I, control; head lesion (II), &
upward swimming (III); body lesion (IV), & scale loss and discoloration (V)

Table 1.1 Behavioral changes and morphological deformities of Channa punctatus
upon exposure to different concentration of CuSO4.
| Observation | Control | Pretreatment with BFE | Exposing pretreated fishes to CuSO4 | Effects of CuSO4 | Recovery response after
treated with BFE |
| Swimming
Pattern |
R | R | S | S | S |
| Scale loss | – | – | * | *** | ** |
| Head lesion | – | – | + | +++ | ++ |
| Fin and tail
movement |
A | A | A | In | In |
| Discolouration
of fish |
N | N | * | *** | * |
| Excretion | + | + | ++ | ++ | |
| Mucus
secretion |
– | – | + | +++ | ++ |
R, relax; S, at the surface of the water; Iv, Inverted; A, active; In, inactive; N. normal; *, little loss; **, moderate loss; +, little; ++, moderate; +++, high; -. nil.
Table 1.1 shows the observation of the behavioural changes of C. punctatus treated with CuSO4. Upon CuSO4 exposure, C. punctatus was recorded to change its swimming pattern as the fish tend to swim erratically with signs of suffocation at the water surface. Swimming pattern changes is one of the obvious signs of chemical exposure onto fish regardless of the exposure routes either by oral exposure or by a flow-through system. Table 1.1 shows the C. punctatus swimming style, which has changed to become inverted and the fish tend to swim at the surface of the water upon increasing CuSO4 concentrations in the water. The behavioural changes and deformities observed are in accordance with a previous study by Sabullah and Khayat (2015) on Puntius schwanenfeldii. After thorough observation, the fishes in the BFE treated groups were seen improving in their motility such as increased in their fins and tail movement as well as their swimming behaviour.
Excretion and mucus secretion: In response to CuSO4 treatment, the experimental fish exhibited increased excretory activity as a means of expelling toxins from their bodies. Prolonged exposure to CuSO4 resulted in higher excretion levels. Sometimes, body released mucus as mechanism to neutralize the toxic effects from metals. After exposure, the fish produced more excretory waste, while a slight decrease in excretion was observed in the BFE-treated groups.
Haematological analysis::The fish in each of the five groups—control, BFE treated, copper sulphate treated, BFE pretreated before being exposed to CuSO4, and CuSO4 treated before BFE treatment— had a differential count . The experimental data is documented in a table that suggests CuSO4 interferes with the body’s immunoreaction. Even while the fish treated with BFE and exposed to CuSO4 do not exhibit total normalcy, the pretreatment group’s blood parameters show a comparable range, which unquestionably demonstrates the antitoxic action of BFE.
The fish group that received only BFE treatment had blood characteristics that were similar to the control group, with a small increase in WBC count and higher levels of both haemoglobin and RBC counts. After Channa punctatus that had previously been exposed to chemicals received BFE, there was a modest increase in the number of red blood cells and haemoglobin, with the white blood cell counts coming closer to those of the control group ( Table 1.2). The values for RBC and hb count mentioned above showed a significant (P<0.05) decrease when compared to the control group while WBC count showed a significant increase (P<0.01) when compared to control group.
The reduction in haemoglobin content and low RBC count as compared to control appear to be a direct effect of copper sulphate toxicity which is reported to interfering haemoglobin synthesis by different toxicants like other heavy metals and pesticides.The revival effect on haemoglobin content erythrocyte count following BFE treatment have been clearly revealed in present findings which suggested the protective tole of antioxidants from BFE as an effective dietary factor in neutralizing toxic stress due to chemical pollutant exposure.
In response to CuSO4 treatment, the experimental fish exhibited increased excretory activity as a means of expelling toxins from their bodies. Sometimes body releases mucus to neutralized the against the toxic from the heavy metals. Prolonged exposure to CuSO4 resulted in higher excretion and releases more mucus levels. After exposure, the fish produced more excretory waste, while a slight decrease in excretion was observed in the BFE-treated groups.The revival effect on haemoglobin content erythrocyte count following BFE treatment have been clearly revealed in present findings which suggested the protective tole of antioxidants from BFE as an effective dietary factor in neutralizing toxic stress due to chemical pollutant exposure.
Table 1.2 Haematological indices after pretreatment of BFE on C. punctatus
| Control | Days of exposure | |||
| 10 days | 20 days | 30 days | ||
| Hb (g/dl) | 10.73 | 10.73 | 10.79 | 10.8 |
| RBC (106/µL) | 2.86 | 2.86 | 2.88 | 2.89 |
| WBC (103/µL) | 4.3 | 4.35 | 4.5 | 4.6 |
Table 1.3 Effect of copper sulphate on haematological parameters of C. punctatus
| Control | Days of exposure period | |||
| 10 days | 20 days | 30 days | ||
| Hb (g/dL) | 10.73 | 8.91 | 7.62 | 5.7 |
| RBC (106/µL) | 2.86 | 2.46 | 2.08 | 1.8 |
| WBC (103/µL) | 4.3 | 4.78 | 4.92 | 5.1 |
Table 1.4 Recovery response of BFE on copper sulphate toxicity on C. punctatus
| Control | Days of exposure | |||
| 10 days | 20 days | 30 days | ||
| Hb (g/dL) | 10.73 | 7.6 | 8.5 | 9.2 |
| RBC (106/µL) | 2.86 | 1.88 | 2.39 | 2.62 |
| WBC (103/µL) | 4.3 | 4.78 | 4.52 | 4.4 |
Table 1.5 Effects of CuSO4 on haematological parameters of BFE pretreated Channa punctatus
| Control | Days of exposure | |||
| 10 days | 20 days | 30 days | ||
| Hb (g/dL) | 10.73 | 10.8 | 10.67 | 10.60 |
| RBC (106/µL) | 2.86 | 2.88 | 2.70 | 1.99 |
| WBC (103/µL) | 4.3 | 4.48 | 4.52 | 5.4 |
Figure 1.2 Haematological parameters of Channa punctatus with control and
after giving BFE for 30 days 30 days experimental period.
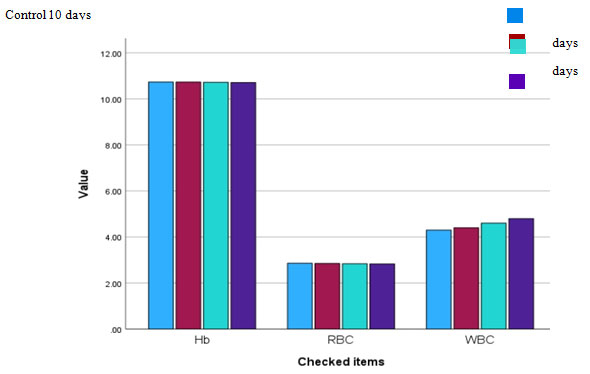
Figure. 1.3 Haematological parameters of Channa punctatus with control and
after exposing to CuSO4 for 30 days experimental period.
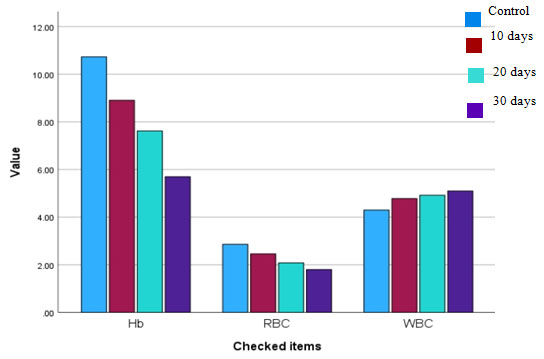
Figure 1.4 Recovery response showed by the group of fishes after
treated with BFE after exposure to the CuSO4.
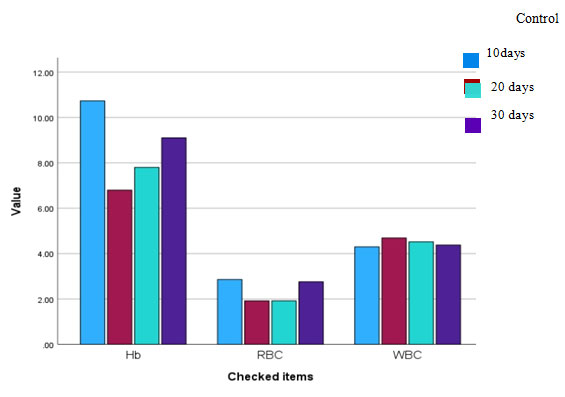
Figure. 1.5 Effects of CuSO4 on haematological parameters of BFE
pretreated Channa punctatus

Figure 1.6 Histogram showing the effects of BFE against Copper- induced toxicity on C. punctatus’s blood parameters. (A) Effect of CuSO4, BFE, BFE then CuSO4, CuSO4 then BFE on RBC count (B) Effect of CuSO4, BFE, BFE then CuSO4, CuSO4 then BFE on WBC count (C) Effect of CuSO4, BFE, BFE then CuSO4, CuSO4 then BFE on haemoglobin percentage. Value is significantly different from the control at p < 0.001 (***), p < 0.01 **), p <0.05(*). (A) In RBC count, similar letters above the bar indicate.
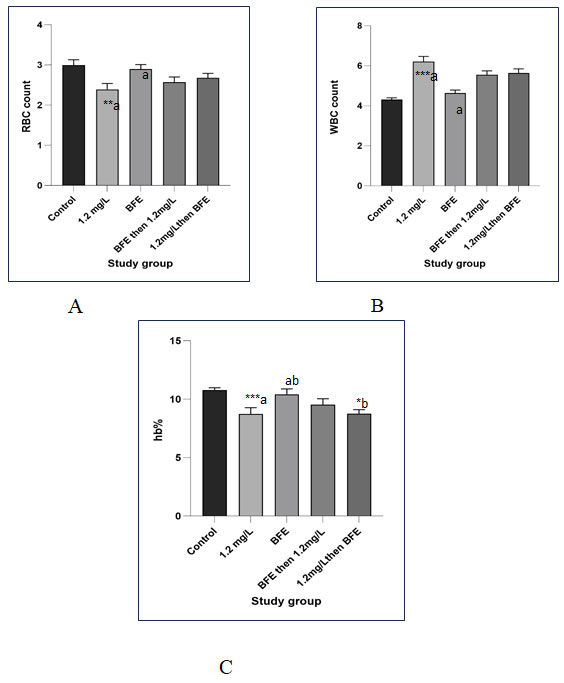
values are significantly different at p < 0.01 (a), and (a). (B) In WBC count, a similar letter above the bar indicates values are significantly different at p < 0.001(a) and (a), and in hb estimation at p < 0.001 (a), (a) (b) and p < 0.05 (b). average and SD (error bars) of the three specimens tested are displayed in each of the columns of the histogram (n = 3).The percentage of different types of leucocytes shows abnormalities The development of neutropenia {high count of neutrophil),lymphopenia (ow levels of lymphocytes) ,eosinophilia (Increase no of eosinophil) and clumping of platelets occurs due to the toxic action of copper sulphate.
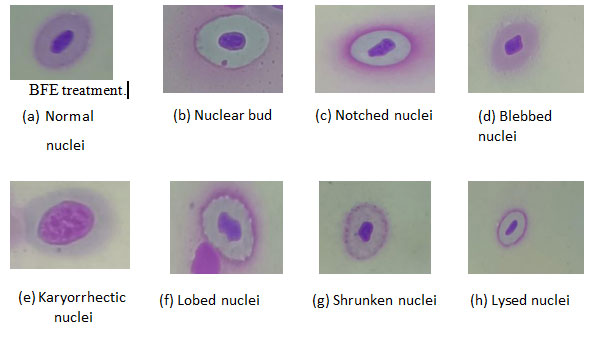
Assessment of nuclear abnormalities:Abnormalities of nuclei (Nas) were observed in the peripheral blood cells, which were identified previously by several authors (Anbumanii and Mohankumar,2011; Shahjahan et al., 2020; Sarangi, 2021). In comparison to the control group (Fig. 1.7a), various nuclear irregularities were observed. These included (Fig. 1.7b) nuclear buds(NB), characterized by micronucleus-like structures attached to the nucleus.
Additionally, (Fig. 1.7c) nuclei displaying vacuoles and significant invagination lacking nuclear material, were designated as notched nuclei (NN). (Fig. 1.7d) Blebbed nuclei (BN) exhibited minor invaginations in the nuclear membrane. (Fig. 1.7e) Karyorrhectic nuclei (KN) were marked by fragmented and disrupted nuclear structures. (Fig. 1.7 f) Lobed nuclei (LN) presented with two or more differentiated lobes. (fig.1.7 g) Shrunken nuclei (SN) indicated reduced nucleus size due to hypoxic conditions. Lastly, (Fig. 1.7h) lysed nuclei (LN) displayed incomplete or ruptured nuclear integrity. These observations contribute collectively to a deeper understanding of nuclear anomalies and their implications in C. punctatus.
In the present work high percentage of shrunken nuclei and lysed nuclei and few blebbed nuclei, nuclear bud, and lobe nuclei in individuals exposed to copper sulphate also suggested that the metal can act as toxicants that affects nuclear membranes of erythrocytes. The erythrocyte from toxic changes were found closer to control after
Figure. 1.7 Micrograph showing nuclear abnormalities (NAs) in blood cells of C.
punctatus in all the Groups at 100x magnification
Histological analysis of liver: After a 30-day exposure, there was increased in necrosis as dose of chemical increased the higher the concentration of CuSO4 the higher the chance of necrosis found in the hepatocytes. Similarly, the damage percentage of vacuolization and pyknotic nuclei are observed, respectively, after the completion of the experimental period. Vacuolization (fig 1.8 c), pyknotic nuclei (fig 1.8d), necrosis (fig 1.8e), and cytoplasmic degeneration (fig 1.8 b) as well as sinusoids (fig 1.8 f) were noted in all the treated Groups with an escalating pattern and dose-dependently; however, the greatest modifications were seen in Group IV, which was given the highest dosage.
Zeng et al. (2020) and Zebral et al. (2019) suggested that fish may have developed mechanisms to cope with copper exposure, potentially reducing liver damage. In their study, continuous exposure to copper sulphate caused vacuolization, necrosis, and cytoplasmic degeneration in fish liver cells, resembling previous observations.In another group, after exposure to CuSO4, BFE treatment started and studied. There were subtle modifications observed in the hepatic cord structure, mild adjustments in cell arrangement, and slight alterations in nuclear structure. These changes suggested a minimal deviation from the effects seen in chemical (CuSO4) treatment. In similar analysis of CuSO4 exposure to the BFE pretreated fishes, the damage were found lesser compare to CuSO4 such as decreasing in vacuolation, didn’t progress necrosis rapidly. These findings suggested the protective role of BFE against metal toxicity.
Figure. 1.8 Microphotographs taken from control and exposed Groups of C. punctatus’s liver segment.
Liver section demonstrating (a) vacuolization, (b) Cytoplasmic degeneration,
(c)Pyknotic nuclei, and (d)Necrosis induced by CuSO4.X100
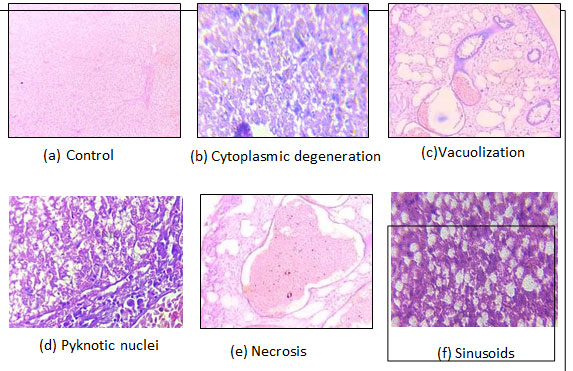
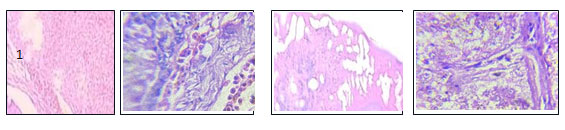
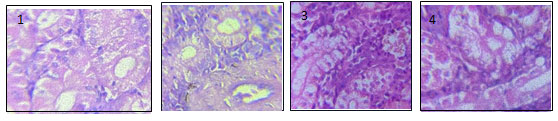
Figure. 1.9 Hepatic tissues from different groups including 1, BFE; 2, CuSO4;
3, BFE then CuSO4; & CuSO4 then BFE. X 100
A regular mass of parenchyma cells makes up the liver of C. punctatus in its natural structural configuration. Hepatocytes, or polygonal cells with rounded nuclei, are seen in the histology of the liver and are grouped in the bile ducts, blood arteries, and liver parenchyma. The histopathological results of fish liver tissue from the 20-day exposed and control groups of C. punctatus are shown in Figure 1.6. The most significant change that began in the liver was vacuolization, pyknotic nuclei (Fig. 1.6 d), necrosis (Fig. 1.6e), cytoplasm degeneration (Fig. 1.6 b), and Sinusoids (Fig. 1.6 f). Whil1e cytoplasmic degeneration exhibits the highest damage percentage of all types of damage, the liver tissue of the control group was in good condition and showed no signs of damage.
After a 30-day exposure, there was increased in necrosis as dose of chemical increased the higher the concentration of CuSO4 the higher the chance of necrosis found in the hepatocytes. Similarly, the damage percentage of vacuolization and pyknotic nuclei are observed, respectively, after the completion of the experimental period. Vacuolization, pyknosis, necrosis, and cytoplasmic degeneration were noted in all the treated Groups with an escalating pattern and dose-dependently; however, the greatest modifications were seen in Group IV, which was given the highest dosage.
Histological analysis of kidney: In the untreated fish group, kidney sections exhibited a typical structure characterized by Bowman’s capsules neatly arranged within renal tubules. The component of kidney tissue is altered by repeated exposure to CuSO4, and the alterations in the test animal’s kidney are expressed as a percentage of damage. Comparatively to the control group
(Fig. 1.10a), the kidneys exposed to sublethal concentrations of CuSO4 after 30 days showed multiple changes. Cavity reduction in renal tubule (CRRT) (Fig. 1.10b) was the most significant alteration seen in the renal tubule, while necrosis (Fig. 1.10c), hypertrophy (Fig. 1.10d) and vacuolization (Fig. 1.10e) were the most frequent modifications in the tubules. Kidney tissue of Group 1 was healthy and did not show any damage. The CRRT damage were observed highest in the animals treated with highest dose, i.e., 1.2 mg/l of CuSO4. However, necrosis, hypertrophy and vacuolization was observed in most part of the tissue. Among all four kinds of damage, the percentage of CRRT was the highest in the kidney tissue.
Figure.1.10 Micrographs of the kidney section taken from the exposed and control Groups of C. punctatus. Section of the kidney displaying the conditions: (a) control; (b) cavity reduction in the renal tubule (CRRT); (c) necrosis; (d) hypertrophy; and (e)vacuolization brought on by CuSO4 exposure shown at 100X
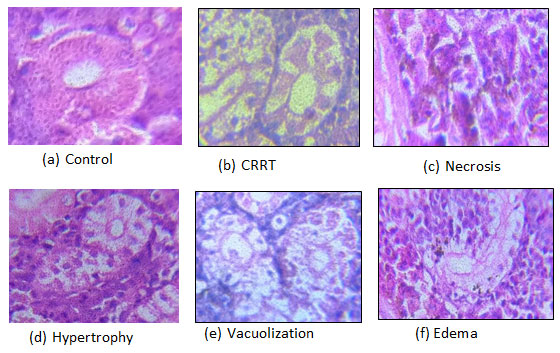
Figure.1.11 Renal tissues from different groups including 1, BFE; 2, CuSO4; 3,
BFE then CUSO4; & 4, BFE then CuSO4. (X 100)
The animals grow increasingly lethargic and exhibit a noticeable inhibition of active movement as the exposure period lengthens. It was shown that feeding habits changed when mucus output and excretion increased. This could be because the metal, copper sulphate, gets absorbed by intestinal cells and interferes with the digestion process. The decrease of scale and discolouration around the lesion on their heads was another obvious alteration. Studying fish behaviour is highly valuable, as behavioural changes are linked to physiological biomarkers in aquatic species, reflecting their responses to biotic and abiotic environmental factors.
Alterations in swimming patterns indicate fish avoidance or attraction in response to xenobiotic exposure. When exposed to foreign substances, gills utilize solitary chemosensory cells, taste, or pain perception to detect and react to the exposure. The connection between swimming patterns and chemical avoidance was demonstrated in Lake Whitefish (Coregonus clupeaformis) exposed to 0.2 µg/L of cadmium, where a dichotomous response was noted (Price 2013). Additionally, an upward swimming pattern towards the water surface is observed in response to oxygen depletion in copper-contaminated water.
The observation revealed that the metal had a general harmful effect on fish physiology, including changes in motility as well as histological damage to key organs like the liver and kidney. The haematological study’s conclusions demonstrated a significant level of physiological stress since exposure to CuSO4 significantly altered the blood picture. Blood is an extremely sensitive marker of both internal and external stress brought on by a variety of circumstances. The current results show that the number of red blood cells decreased in proportion to the number of days of exposure, whereas the number of white blood cells increased from the moment copper sulphate therapy began. An increase in CuSO4 dosage was likewise associated with a decrease in haemoglobin %.
Blood parameters are considered as pathophysiological indicators of the whole body and lawful are important in diagnosing the structural and functional study of fish exposed to toxicants (Adhikari et al. 2004). When assessing the combined impact of zinc and lead on the haematological parameters of carp, a synergistic effect was observed on red blood cell count, leukocyte count, and haemoglobin concentration. Comparable alterations were noted in Channa punctatus upon exposure to copper sulphate.
This study on haematological changes in fish serves as an effective tool in the diagnosis of the extent of environmental pollution and also the abiotic fish diseases. Hypoxia, anaemia, and hyperthermia are related stresses causing an osmotic imbalance and decreased capacity of the RBC to carry sufficient oxygen unless otherwise compensated by erythropoiesis or suitable physiological adjustments. Decreased availability of oxygen generally causes increased synthesis of haemoglobin, release of blood cells from storage sites, and enhanced erythropoiesis.
Adakole (2012) demonstrated that exposure of C. gariepinus to effluents from metal finishing companies initially increased red blood cell (RBC) count, but this count decreased after prolonged exposure. In the current study, the decrease in total RBC count along with reduced haemoglobin content could be attributed to the detrimental effects of pollutants on erythrocytes, which were observed after chronic exposure. This could potentially impact the viability of the cells, a phenomenon also noted by Karuppasamy (2000). In total WBC, increase in total WBC count in the present study is the result of direct stimulation for the defense from decrease due to the preserve of heavy metals. Progressive increased levels of total WBC count have also been repeated.
Chandanshive et al. (2012) also reported decrease in RBC of fish Labeo rohita after exposure to mixture of heavy metals. All these reports are in agreement with the present study of reduction in total RBC count and Hb content of fish from polluted sewage fed pond due to the inhibition of aerobic glycolysis curtailing of iron and haemoglobin via the lowered energy status in fish. Leucocytes is directly proportional to severely to stress condition in maturing fish and as a result of direct stimulation of immunological defense due to the presence of heavy metal.
Hence results of the present investigation have confirmed that stress due to heavy metals present in water create haematological disturbances erythrocyte destruction (haemolysis) and leucocytes in fish population affecting immune system and making the fish vulnerable to diseases. Therefore, the fish is provided as a bioindicator of deteriorating water quality and due should be taken to monitor the environment.In fish, exposure to chemical pollutants can induce either increase or decrease in haematological indices such as haemoglobin (Hb), red blood cells (RBCs) & so on are used to assess the functional status of oxygen carrying capacity of blood stream and used as an indicator of pollution in aquatic environment (Shah & Altindag, 2004).
The hepatocytes show heterogenicity in size and were found to be undissociated in the present investigation. Cell damage were evident at places lesions of various size were present. Hepatocytes of large size were found to form disorganized running in all direction. BFE pretreatment for 30 days followed by copper sulphate revealed that many of these structural defects do not exist and the cell surface and other cellular features were more less similar to the normal hepatocytes.The haematological and biochemical profiles of fish Labeo rohita were examined using the medicinal plant Curcuma amada (Malik et al. 2019). The plant extract was combined with salt and fish feed powder, and after 30 days, the fish had much more red blood cells, white blood cells, serum protein, and globulin. The herbal diet also preserved liver problem enzymes, indicating enhanced health.
Kaur et al. (2018) conducted a study investigating the impact of copper on the liver histology of Labeo rohita. It was discovered that there was a positive correlation between the concentration of copper exposure and the severity of liver damage. This damage included vacuolation, congestion, and necrosis. Similarly, the study by Noureen et al. (2018) on the liver histology of Cyprinus carpio (common carp) exposed to copper showed severe damage, including degeneration, necrosis, and fibrosis. Their findings revealed that exposure to higher concentrations of copper resulted in dose- dependent liver damage in the fish. In contrast, the research done by Sangeetha and Aruljothi (2019) on the liver histology of common carp C. carpio exposed to copper showed mild damage, including vacuolation and lipid accumulation.
The kidney plays a crucial role in excretion, osmoregulation, and maintaining overall bodily equilibrium. Additionally, it facilitates selective reabsorption, aiding in the regulation of blood and body fluid volume and pH balance, as well as erythropoiesis.
In a study conducted by Wu et al. (2019) investigated the impact of copper treatment on the kidney histology of Gobiocypris rarus. They found moderate damage, including tubular necrosis, interstitial edema, and cellular infiltration. The authors suggested that copper accumulation in the kidney could impair renal function and potentially lead to fish mortality. Abdel et al. (2021) reported kidney damage in Oreochromis niloticus exposed to copper. The kidney showed severe tubular necrosis and interstitial fibrosis, leading to decreased renal function. The study by Tavares-Dias (2021) on the kidney histology of fish Labeo rohita exposed to copper showed congestion of blood vessels, tubular necrosis of glomerulus, interstitial oedema, and cellular infiltration.
According to the study’s findings, C. punctatus‘s copper buildup caused nuclear anomalies, histological alterations in fish organs like the liver and kidneys, a decline in RBC and Hb, rise in WBC, and other effects. Under the experimental parameters mentioned above, the BFE therapy demonstrated a recovery and a neutralizing effect in response to the toxic effects of copper sulphate, encompassing both haematological and histopathological criteria. The most significant impact was seen in the recovery of blood parameters (RBC, Hb, and WBC).
As there is currently no treatment for copper intoxication and because the metal’s abundance in the environment poses a threat to future generations as well as fish populations in aquatic bodies, it is strongly suggested by the current experimental findings that using high concentrations of banana flower as a raw ingredient in diet preparation may enhance the body’s immunocompetence and serve as a useful tool in the fight against copper intoxication.Thus, it is evident from the results of the current study that antioxidants have a special role in preventing tissue damage as well as in reviving and shielding pathological damage caused by CuSO4. It is anticipated that these results would pave the way for the use of banana inflorescence as a phytochemical instrument to mitigate the harmful effects of heavy metals on humans and other animals.
CONCLUSION
Adult C. punctatus has been shown to exhibit early behavioural and morphological changes in response to copper contamination in the aquatic environment, even at low concentrations. Prolonged exposure to copper sulphate was found to cause morphological and behavioural alterations. In addition to exhibiting vacuolation, necrosis, and hypertrophy, cytoplasmic degeneration were also observed in the tissues. The study concluded that the accumulation of copper in C. punctatus resulted in the reduction of RBC and Hb, and increase of WBC as well as nuclear abnormalities, and histological changes in organs such as the liver, and kidneys of the fish. In the above experimental parameters, the BFE treatment exhibited a revival and neutralizing action in response to copper sulphate induced toxic impact including haematological and histopathological parameters.
The most important effect of antioxidants was observed in recovery of blood parameters (RBC, Hb, and WBC). This research underscores the potential adverse effects of copper accumulation on fish health, emphasizing the necessity for effective measures to prevent such accumulation in aquatic environments. These findings can inform the development of strategies for the sustainable management of aquatic resources and ensure the long-term well-being of fish populations. To further understand the underlying mechanisms of copper- induced toxicity and create viable mitigation methods, more research is necessary.
Ethical Clearance: All ethical clearances have been obtained vide The Institutional Ethical Committee of the Institute, Department of Zoology, The Assam Royal Global University Guwhati, Assam India
Author declares no Conflict of interest
Data Availability: Data are available with the corresponding author
REFERENCES
Abah, J., Mashebe, P., & Onjefu, S. A. (2016). Preliminary assessment of some heavy metals pollution status of Lisikili River Water In Zambezi Region, Namibia. International Journal of Environment and Pollution Research, 4(2), 13-30.
Abdel-Khalek, A. A., Badran, S. R., & Marie, M. A. S. (2016). Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using haematological, bioaccumulation and histological biomarkers. Fish physiology and biochemistry, 42, 1225-1236.
Ahmad K, I., Kausar, R., Tabassum, T., & Muhammad, A. (2013). Use of Bioremediated Sewage Effluent for Fish Survival. International Journal of Agriculture & Waqar Biology, 15(5).
Ajani, E. K., & Akpoilih, B. U. (2010). Effect of chronic dietary copper exposure on haematology and histology of common carp (Cyprinus carpio ). Journal of Applied Sciences and Environmental Management, 14(4).
Anbumani, S., & Mohankumar, M. N. (2011). Nuclear and cytoplasmic abnormalities in the fish Catla catla (Hamilton) exposed to chemicals and ionizing radiation. Research Journal of Environmental Sciences, 5(12), 867-877. Chicago.
Chandanshive, S. S., Sarwade, P. P., Humbe, A., & Mohekar, A. D. (2012). Effect of heavy metal model mixture on haematological parameters of Labeo rohita from Gharni Dam Nalegaon, Latur. International Multidisciplinary Research Journal, 2(4).
Chakraborty H and Chandan Sarkar (2023)Assessment of acute toxic effects of copper sulphate on Catla catla fry: mortality rates, behavioral changes and opercular movement dynamics CONSCIENTIA ISSN: 2278-6554 Vol. XII December 23
China, R., Dutta, S., Sen, S., Chakrabarti, R. Maia, A. H. N., & Fraceto, L. F. (2013). Fish exposure to nano-TiO2 under different experimental conditions: Methodological aspects for nanoecotoxicology investigations. Science of the Total Environment, 463, 647-656.
El-Moselhy, K. M., Othman, A. I., Abd El-Azem, H., & El-Metwally, M. E. A. (2014). Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian journal of basic and applied sciences, 1(2), 97-105.
El-Moselhy, K. M., Othman, A. I., Abd El-Azem, H., & El-Metwally, M. E. A. (2014). Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian journal of basic and applied sciences, 1(2), 97-105.
Javed, M., & Usmani, N. (2019). An overview of the adverse effects of heavy metal contamination on fish health. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 89, 389-403.
Javed, M., Ahmad, M. I., Usmani, N., & Ahmad, M. (2017). Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Scientific reports, 7(1), 1675.
Kaoud, H. A., & El-Dahshan, A. R. (2010). Bioaccumulation and histopathological alterations of the heavy metals in Oreochromis niloticus fish. Nature and science, 8(4), 147-156.
Liu, N., Zhang, P., Xue, M., Zhang, M., Xiao, Z., Xu, C., & Zhou, Y. (2023). Anti-inflammatory and antioxidant properties of rice bran oil extract in copper sulphate-induced inflammation in zebrafish (Danio rerio). Fish & Shellfish Immunology, 136, 108740.
Lushchak V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquatic toxicology (Amsterdam, Netherlands), 101(1), 13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak, V. I. (2016). Contaminant-induced oxidative stress in fish: a mechanistic approach. Fish physiology and biochemistry, 42, 711-747.
Mallik, A. R., Shammi, Q. J., & Telang, S. (2019). Formulation of fish feed using medicinal herb Curcuma amada and its biochemical and haematological changes in Labeo rohita. Journal of Drug Delivery and Therapeutics, 9(3-s), 96-99.
Naz, S., Hussain, R., Ullah, Q., Chatha, A. M. M., Shaheen, A., & Khan, R. U. (2021). Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environmental science and pollution research, 28, 6533-6539.
Padrilah, S. N., Sabullah, M. K., Shukor, M. Y. A., Yasid, N. A., Shamaan, N. A., & Ahmad, S. A. (2018). Toxicity effects of fish histopathology on copper accumulation. Pertanika Journal of Tropical Agricultural Science.
Perkumien E D., Atalay, A., Safaa, L., and Grigien˙ J. (2023). Sustainable waste management for clean and safe environments in the recreation and tourism sector: A case study of Lithuania. Turkey and Morocco. Recycling, 8 (4), 56. https://doi.org/ 10.3390/recycling8040056
Sabullah, M. K., Ahmad, S. A., Shukor, M. Y., Gansau, A. J., Syed, M. A., Sulaiman, M. R., & Shamaan, N. A. (2015). Heavy metal biomarker: Fish behaviour, cellular alteration, enzymatic reaction and proteomics approaches.
Sabullah, M. K., Sulaiman, M. R., Shukor, M. S., Yusof, M. T., Johari, W. L. W., Shukor, M. Y., & Syahir, A. (2015). Heavy metals biomonitoring via inhibitive assay of acetylcholinesterase from Periophthalmodon schlosseri. Rendiconti Lincei, 26, 151-158.
Sabullah, M. K., Sulaiman, M. R., Shukor, M. Y. A., Syed, M. A., Shamaan, N. A., Khalid, A., & Ahmad, S. A. (2014). The assessment of cholinesterase from the liver of Puntius javanicus as detection of metal ions. The Scientific World Journal, 2014.
Shah SL and Altindag A (2004). Haematological parameters of tench (Tinca tinca L) after acute and chronic exposure to lethal and sublethal mercury treatments. Bull. Environ. Contam. Toxicol. 73:911-918.
Shuhaimi-Othman, M., Nadzifah, Y., & Ahmad, A. K. (2010). Toxicity of copper and cadmium to freshwater fishes. International Journal of Bioengineering and Life Sciences, 4(5), 319-321.
Singh D, Nath K, Trivedi SP, Sharma YK. Impact of copper on haematological profile of freshwater fish, Channa punctatus. J Environ Biol. 2008;29(2):253- 7..
Singh, A., Sharma, R. K., Agrawal, M., & Marshall, F. M. (2010). Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Tropical ecology, 51(2), 375-387.om Periophtha Waqar,
Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 222, 90-99.
Vishwanath, W and Geetakumari, K. (2009). Diagnosis and interrelationships of fishes of the genus Channa scopoli (Teleostei: Channidae) of northeastern India. Journal of Threatened Taxa, 1(2), 97-105.
Wani, A. A., Sikdar-Bar, M., & Khan, H. A. (2013). Acute toxicity of copper sulphate to African catfish, Clarias gariepinus. GERF Bulletin of Biosciences, 4(1), 14-18..
Wani, K. A., Manzoor, J., Dar, A. A., Shuab, R., & Lone, R. (2020). Heavy metal intrusion and accumulation in aquatic ecosystems. Fresh water pollution dynamics and remediation, 83-104.
Wintrobe (1967) Wintrobe’s Clinical Hematology Fifteenth Edition Ref 2023
Wong, J. W. C., Li, K., Fang, M., & Su, D. C. (2001). Toxicity evaluation of sewage sludges in Hong Kong. Environment international, 27(5), 373-380
Wu, L., Yu, Q., Zhang, G., Wu, F., Zhang, Y., Yuan, C., & Wang, Z. (2019) In vitro antioxidant activity of different cultivars of banana flower (Musa paradicicus L.) extracts available in India. Journal of food science, 76(9), 1292-1299.
Vasconcelos, RT CE Copatti, AC Albinat (2024) Waterborne copper sulfate toxicity in Nile tilapia (Oreochromis niloticus) juveniles affect survival, growth, and physiology Journal of Applied Toxicology Vol 21 4 202-214


