1Cell and Molecular Biology Laboratory, Department of Zoology, Rammohan College,
Raja Rammohan Sarani, Kolkata, India.
2Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute, Kolkata, India.
3Molecular Biology and Tissue Culture Laboratory, Post Graduate Department of Vidyasagar
College, Vidyasagar College, Kolkata, India.
4Department of Infertility, Institute of Reproductive Medicine, Kolkata, India.
Corresponding author email: kaustavduttachowdhury@gmail.com
Article Publishing History
Received: 15/10/2024
Accepted After Revision: 25/12/2024
Pulmonary disorders are established consequence of air pollution. Emissions from vehicles, factories and combustion of biomass are major contributors to air pollution. Accumulation of pollutants in lungs develops several respiratory diseases like asthma, chronic obstructive pulmonary disease (COPD). Persistent inflammation due to long term exposure to toxicants causes permanent pulmonary tissue damage and often arises serious medical emergency. Calcium carbide (CaC2) and its byproduct acetylene have been recognized as potential environmental toxicant. It is widely used in various commercial fields including fruit ripening and welding, cutting industries.
In the present study we have investigated the inflammatory status and its consequences on lungs during inhalation of CaC2 generated toxicants by mimicking similar situation of people who occupationally get exposed with the same. After 40 days of exposure by calcium carbide generated gas, real-time qPCR has been performed to investigate the level of Cox2 and PGES mRNA. Apoptosis and TH17 as well as Treg percentage have been estimated flow cytometrically. Levels of Cox2 and PGES have been found to increase with the increase in CaC2 exposure. Apoptotic cell percentage has been declined with increasing dose of toxicant. TH17 and Treg cell percentage reduced as the dose of toxicant increased, indicating a balance between the two at 7gm CaC2 exposure. Balance between inflammatory and anti-inflammatory condition of lung tissue has been attempted after chronic CaC2 exposure, but it has been disrupted at the highest level likely due to accumulation of damaged cells as reflected by the spike of Cox2 mRNA level in 7gm CaC2 exposure.
Calcium Carbide, Lungs, Toxicant, Inflammation, Apoptosis
Ghosh P, Banerjee S, Ghosh R, Patra D, Chakraborty P, Saha P, Chowdhury K.D.Alterations in Regulatory Matrix Related to Inflammation in Lungs of Calcium Carbide Exposed Mice. Biosc.Biotech.Res.Comm. 2024;17(4)
Ghosh P, Banerjee S, Ghosh R, Patra D, Chakraborty P, Saha P, Chowdhury K.D.Alterations in Regulatory Matrix Related to Inflammation in Lungs of Calcium Carbide Exposed Mice. Biosc.Biotech.Res.Comm. 2024;17(4). Available from: <a href=”https://shorturl.at/8ewku“>https://shorturl.at/8ewku</a>
INTRODUCTION
Considering today’s environmental situation, the quality of air we breathe every day is coming up as the predominant cause of several health issues. Especially, in industrial areas and large metropolitan cities the ambient air, mainly the emissions from vehicles and factories act as major conduits for airborne toxicant exposure (Kunovac et al., 2020; Lee et al., 2021). The fuels used in cooking, burning of wood and other materials used in fireplaces act as source of indoor air pollutants (Lee et al., 2021). Combustion of biomass fuel by traffic worldwide as well as domestic fire have been recognized as significant contributors to air pollution in developing countries (Laumbach and Kipen, 2012; Lee et al., 2021; Jiang et al., 2024).
The accumulation of pollutants in the lungs is responsible for triggering the airway inflammation, fibrosis and pulmonary dysfunction. These ultimately lead to exacerbation of the symptoms of chronic respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD) (Lee et al., 2021).
Inflammation is a natural physiological process by which body responds to any injury or infection (Medzhitov, 2010; Chen et al., 2018). Though inflammation is a part of natural healing process, but upon long term exposure to toxicants causes persistent inflammation which may lead to permanent damage of lung tissues and alveolar structure (Wong, Magun and Wood, 2016). Severe lung inflammation may cause hypoxemia or hypoxia giving rise to symptoms like bradycardia, restlessness, cyanosis, dizziness etc. Chronic inflammation over long time may result in a progressive condition called bronchiectasis which ultimately may lead to hyercapnia causing medical emergency (Sarkar, Niranjan and Banyal, 2017; Hill et al., 2019; Defnet, Hasday and Shapiro, 2020).
Although calcium carbide (CaC2) and its byproduct acetylene have been recognized as potential environmental toxicant according to related reports, it is widely used in various commercial fields. Calcium carbide decomposes in water forming highly flammable acetylene gas, a reaction discovered by Friedrich Wöhler in 1862. This property of calcium carbide is utilised in fruit ripening industry to hasten the fruit ripening process (Asif, 2012; Bini, Rajesh and Babu, 2022; Okeke et al., 2022). These chemically forced ripening not only minimizes the nutritive values of fruits but also makes the fruits toxic and increases the risk of health complications (Andrew et al., 2018; Cissé et al., 2020; Okeke et al., 2022).
Apart from the immediate symptoms like stomach discomfort, dizziness and vomiting, prolonged hypoxia may also affect the neurological system on consumption of these chemically ripened fruits in large quantities (Per et al., 2007; Okeke et al., 2022). Moreover, this toxicant gain access to body physiology through inhalation of left over powders, aerosols, and gaseous products of CaC2 residues present on the fruits. Besides, this toxicant is also used by some food vendors to boil eggs and hard-to-cook beans (Okeke et al., 2022).
Apart from using in fruit ripening and food processing, calcium carbide is also utilised for the production of nitrolime (a fertilizer), desulphurization of iron in the process of steel making (Güthner and Mertschenk, 2000; Okeke et al., 2022). Calcium carbide generated acetylene gas is used as a raw material in chemical industry, especially for PVC production, also in carbide lamps as well as welding and cutting industries (Tajudeen, Akanfe and Adebayo, 2019; Okeke et al., 2022).
The workers in these industries are the most susceptible to the hazards caused by the toxicants, as they get exposed every day, especially through inhalation, to an environment saturated by the toxicants for a long period for their occupational purposes. Inspite of having such extensive applications of calcium carbide in industrial field the experimental studies on its adverse effects are actually found to be negligible. In the light of this fact, our study is targeted to investigate the probable consequence of the exposure via inhalation calcium carbide by mimicking the situation of people who occupationally get exposed to this toxicant on regular basis.
MATERIALS AND METHODS
Materials: Calcium carbide and other fine chemicals were acquired from Sigma Aldrich, fluorescent tagged antibodies were obtained from Thermo Fisher Scientific whereas the primers were obtained from BioRad.
Animal maintenance: Male 4-6 weeks aged Swiss-albino mice (Mus musculus) with body weight 20-25g were maintained in a cross ventilated room with 12 hours cycle of light and darkness. Temperature of the room was maintained at 27℃ (± 2℃) with relative humidity of 44%-56% and the animals were provided with adequate supply of food and water. All of experimental procedures were executed following institutional animal ethical guidelines implemented by Institutional Animal Ethics Committee (IAEC), Rammohan College, Committee for Control and Supervision of Experiments on Animals (CCSEA), MoFAHD DAHD, Government of India. Minimum necessary number of animals were used for experimentation in order to minimize animal suffering and valid statistical evaluation.
Toxicant (CaC2) exposure: Designated animals were grouped into two categories- control and toxicant exposed group. Exposed group of animals were further divided into four groups. Respective group of animals were exposed to 3gm, 5gm, 7gm and 9gm calcium carbide per day with requisite volume of water (w:v::1:10) for 15 minutes in a leak proof container of 24 litre volume. After 40 days of exposure the animals were sacrificed to perform the experimental procedures.
Real-time qPCR for Cox2 and PGES mRNA level: After obtaining total RNA from lungs of experimental animals, it was preserved in Trizol (ThermoFisher Scientific, Waltham, MA). First cDNA was acquired using iScript reaction mixture prepared with iScript Reverse Transcriptase, Nuclease free water and RNA template and then was incubated with reaction mixture in thermal cycler for 26 min (BIO-RAD, iScript cDNA Synthesis Kit). Next, the cDNA was amplified using the Sso Advanced Universal SYBR Green Supermix (BIO-RAD). Relative level of Cox2 and PGES were calculated with reference to GAPDH using the comparative CT (2-∆∆CT) method (Godfrey, 2009; Rao et al., 2013).
Flow cytometric analysis: Following the standard procedure of collagenase perfusion technique, the lung cells were isolated from the lungs of experimental animals.
To determine the percentage of apoptotic cells, the isolated pulmonary cells of control and exposed animals were suspended in 500μl 1X Binding Buffer and incubated with 5μl AnnexinV-FITC and PI for 15min at room temperature in dark. AnnexinV-FITC staining was analyzed by flow cytometry (Ex = 488 nm; Em = 530 nm) using FITC signal detector (usually FL1) and PI staining by the phycoerythrin emission signal detector (usually FL2) (Chatterjee et al., 2019).
To evaluate the percentage of the T-cell subsets, first trypan blue exclusion assay was performed with the isolated pulmonary cells to determine cell viability. The process was followed by washing and were treated with proteinase inhibitor. In T-cell subpopulation analysis, cells were incubated with fluorescence tagged CD3, CD4, IL17A (for TH17 analysis) and CD25 and FOXP3 (for Treg analysis) antibodies for 15 min at room temperature in dark atmosphere. Flowcytometric analysis and cell sorting were performed by using FACSAria™ (BD BioScience, Franlin Lakes, NJ). Acquisition was carried out against unstained cells by using CELL Quest software (BD Biosciences, Franlin Lakes, NJ) (Dong, 2009).
Statistical analysis: Conventional method was used for the calculation of means and SEM. Statistical differences among experimental groups were evaluated by the student’s t-test. Data analysis was carried out using the GraphPad Instant software (GraphPad, La Jolla, CA, USA).
RESULTS AND DISCUSSION
In case of chronic inflammatory response, a crucial role is played by PGE2 which acts as an important mediator of chronic inflammation associated diseases (Wautier and Wautier, 2023). Biosynthesis of PGE2 is dependent upon two enzymes cyclooxygenase2 (COX2) and prostaglandin E synthase (PGES) (Gosset, Berenbaum and Levy, 2006). Therefore, in order to investigate the inflammatory conditions of lung tissue after scheduled toxicant exposure through inhalation, experimental animals were sacrificed and the levels of Cox2 and PGES mRNA had been observed through RT-PCR technique.
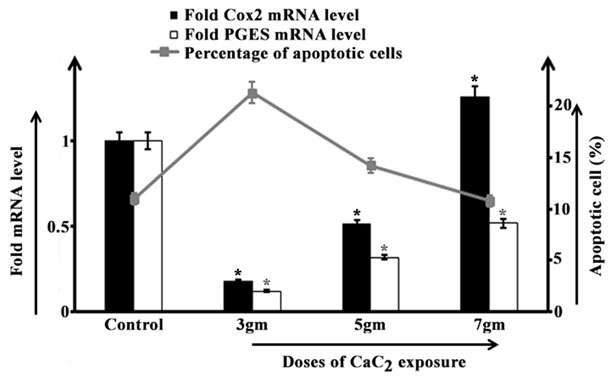
Data demonstrated a sharp decline in both Cox2 and PGES mRNA level after 3gm of CaC2 exposure as compared to control group of animals. Parallelly, a significant increase in percentage of apoptotic cells than control group had been found in the afore-said group. In 5gm CaC2 exposed group a moderate increase in Cox2 level than that in 3gm exposed group had been observed, though the value remained significantly lower than the control group. The mRNA level of Cox2 had been found to increase steeply after 7gm CaC2 exposure with even higher value than the unexposed animals. The PGES mRNA level in 5gm and 7gm CaC2 exposed group had also elevated significantly than 3gm exposed group, though the value remained lower than the control group. But, both of 5gm and 7gm CaC2 exposed group had exhibited a gradual declining pattern in percentage of apoptotic cells (Figure 1) as reported in our previous study (Banerjee et al., 2023).
Figure 1: Cox2 mRNA and PGES mRNA expression level (fold change) in lungs (bar diagram) and representation of apoptotic lung cells (line curve) isolated from control and 3gm, 5gm and 7gm calcium carbide exposed male mice respectively after 40 days of exposure. Data were expressed as mean+SD and were obtained from four independent experiments (n=5). *p<0.05 vs Control.
Inflammatory and anti-inflammatory conditions in our body are balanced by two antagonistic subpopulations of T-cells, i.e., TH17 cells and regulatory T-cells (Treg cells) (Noack and Miossec, 2014).
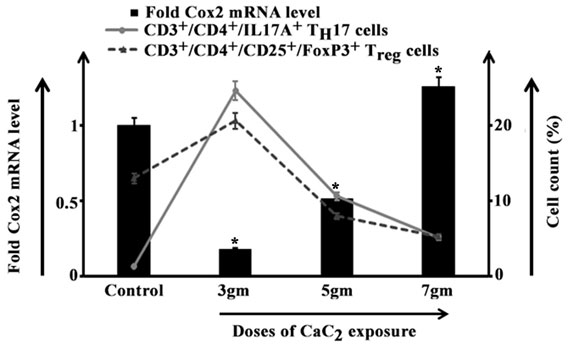
In 3gm CaC2 exposed group Cox2 mRNA level significantly declined than control while both of TH17 and Treg cell percentage escalated with greater percentage of Treg cells as observed in our experiment. In 5gm and 7gm exposed groups the Cox2 mRNA level demonstrated a surging pattern with steep increase in 7gm exposed group in parallel to gradual reduction in TH17 and Treg cell percentages (Figure 2).
Figure 2: Representation of CD3+/CD4+/IL17A+ TH17 and CD3+/CD4+/CD25+/FoxP3+ Treg cells (line curve) isolated from lungs of control and 3gm, 5gm and 7gm calcium carbide (CaC2) exposed male mice after 40 days of exposure along with respective Cox2 mRNA level (bar diagram). Data were expressed as mean+SD and were obtained from four independent experiments (n=5). *p<0.05 vs Control.
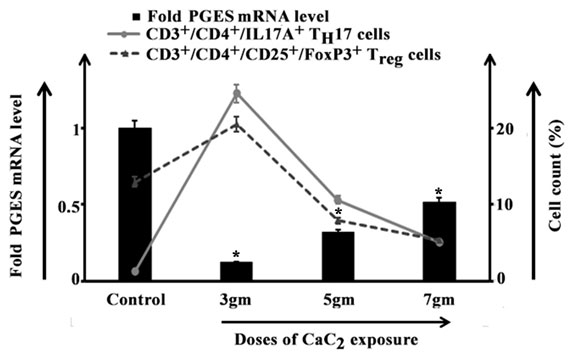
After 3gm toxicant exposure though a marked reduction in the level of PGES mRNA had been observed, but TH17 and Treg cell percentages increased. An increasing pattern of PGES mRNA level had been in both of 5gm and 7gm exposed groups while approximate equivalent percentage of TH17 and Treg cells had been noted after 7gm CaC2 exposure (Figure 3).
Figure 3: Relationship between CD3+/CD4+/IL17A+ TH17 and CD3+/CD4+/CD25+/FoxP3+ Treg cells (line curve) isolated from lungs of control and 3gm, 5gm and 7gm calcium carbide (CaC2) exposed male mice after 40 days of exposure and respective PGES mRNA level (bar diagram). Data were expressed as mean+SD and were obtained from four independent experiments (n=5). *p<0.05 vs Control.
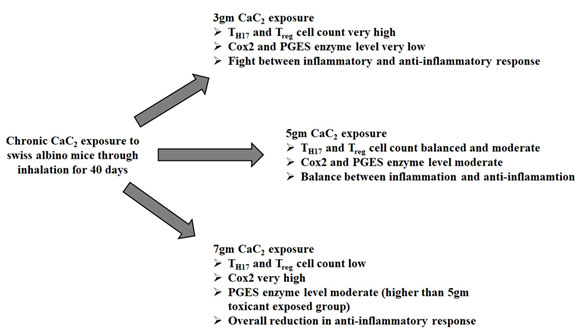
Upon lower grade of stress exerted by the exposure to toxicant (3gm CaC2), the anti-inflammatory pathway probably got activated to balance the inflammatory situation of tissue. In order to accomplish this, anti-inflammatory Treg cell population had been augmented to suppress the inflammatory TH17 cell population which had been increased in response to exerted stress. The higher apoptotic cell percentage as an effect of fight between Treg and TH17 probably suppressed inflammation as demonstrated by low level of both Cox2 and PGES mRNA.
In 5gm toxicant exposed group, TH17 cell population still had been found higher than Treg cells. But all-over inflammatory condition had been reduced as demonstrated by the observed data. Although level of apoptosis had been decreased and therefore moderate inflammatory condition had been portrayed by somehow increased Cox2 and PGES mRNA level. Moreover, the reduced apoptotic cell percentage suggested a pro- to anti-apoptotic environmental shifting due to sustained inflammatory condition.
After 7gm CaC2 exposure, TH17 cell population had been balanced by Treg cell population but percentage of apoptosis had been reduced further. As a result, towering augmentation of Cox2 with a significant increase in PGES mRNA level had been observed. This might lead to possible consequence of extensive cell damage due to overall reduction in anti-inflammatory response (Figure 4).
Figure 4: Diagrammatic status of inflammatory microenvironment of pulmonary tissue of male swiss albino mice after for 40 days of 3gm, 5gm and 7gm calcium carbide exposure through inhalation.
CONCLUSION
3gm CaC2 exposure augmented cell death probably to combat the challenge imposed by stress, while cell death was reduced in 5gm and 7gm CaC2 exposure indicating accumulation of altered and damaged cells. In order to recruit prostaglandin in site of damaged cell, Cox2 and PGES mRNA level augmented with increase in dose of exposure. Anti-inflammatory response was boosted up in 3gm and reduced in 5gm and 7gm CaC2 exposure as reflected by the pattern of Treg and TH17 cell percentage. This disrupted inflammatory response likely due to the accumulation of damaged cells which reflected by the spike of Cox2 mRNA level after chronic exposure.
Author’s contribution: KDC and PC were responsible for conceptualization and designing of the study. PG, SB and RG were responsible for model development, planning of experiments and data collection. KDC, PS, PG, SB and DP were responsible for result analysis and interpretation. All authors equally contributed in literature research, manuscript preparation, editing and review.
ACKNOWLEDGEMENTS
Authors are thankful to Dr. Tuli Biswas, Retired Scientist, CSIR-Indian Institute Chemical Biology, Kolkata, West Bengal; Dr. Gargi Sen, University of Kalyani, Kalyani, Nadia, West Bengal; Dr. Samarendra Nath Banerjee, Department of Zoology, Rammohan College, Kolkata, West Bengal; Dr. Subhadip Hajra, Senior Scientific Officer (Grade II), Chittaranjan National Cancer Institute; Mr. Mriganka Biswas from Chota Jagulia High School (H.S), Chhota Jagulia, North 24 Parganas, West Bengal; Dr. Sujan Chatterjee, University of Nevada, Las Vegus, USA; Dr. Arijit Bhowmik, Research Associate, Chittaranjan National Cancer Institute for critical comment, scientific discussion and helpful suggestions.
Statement of ethics: This study was approved by the Institutional Animal Ethics Committee (IAEC), Rammohan College, affiliated under University of Calcutta, Kolkata, India (1795/PO/Re/S/14/CPCSEA, 28.02.2018 – 27.02.2023) vide approval number RMC/IAEC-2/1 dated 27.11.2021.
Declaration of conflicting interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/ or publication of this article.
Funding: The project was funded by SCIENCE & TECHNOLOGY AND BIOTECHNOLOGY, Government of West Bengal (WB-DST), MEMO NO. 198(Sanc.)/ ST/P/S&T/9G-45/2017 DATED 21/03/2018.
REFERENCES
Andrew, G.S., Simon, U.T., John, A.U., Godwin, O.O., Alexander, N.I. and Ikagu, Y.M., (2018). Studies on changes in some haematological and plasma biochemical parameters in wistar rats fed on diets containing calcium carbide ripened mango fruits. Int J Food Sci Nutr Eng, 8(2), pp.27-36.
Asif, M., (2012). Physico-chemical properties and toxic effect of fruit-ripening agent calcium carbide. Annals of Tropical Medicine & Public Health, 5(3).
Banerjee, S., Ghosh, P., Patra, D., Chakraborty, P., Chowdhury, K.D., (2023). Effect of Calcium Carbide Exposure Through Inhalation in Lungs of Mus musculus. Bioscience Biotechnology Research Communications, 16(4), pp.219-224.
Bini, M., Rajesh, B. and Babu, T.D., (2022). Chronic exposure of industrial grade calcium carbide and ethylene glycol alter histological architecture of systemic organs by disrupting redox balance in rat. Journal of Basic and Clinical Physiology and Pharmacology, 33(3), pp.265-271.
Chatterjee, S., Patra, D., Chakraborti, U., Sengupta, D., Ghosh, P., Basu, A., Sadhukhan, G.C. and Chowdhury, K.D., (2019). Association of p38MAPK‐p53‐Fas aggregation in S‐allyl cysteine mediated regulation of hepatocarcinoma. Environmental toxicology, 34(8), pp.928-940.
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X. and Zhao, L., (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), p.7204.
Cissé, M., Silue, Y., Cissé, M., Kouadio, A.D.S. and Nindjin, C., (2020). Effect of Calcium Carbide Treatment on Ripening Time and Physicochemical Properties of Mango (Mangifera indica L.) Variety “Kent”, Côte d’Ivoire. Current Journal of Applied Science and Technology, 39(38), pp.24-30.
Defnet, A.E., Hasday, J.D. and Shapiro, P., (2020). Kinase inhibitors in the treatment of obstructive pulmonary diseases. Current opinion in pharmacology, 51, pp.11-18.
Dong, C., (2009). Mouse Th17 cells: current understanding of their generation and regulation. European journal of immunology, 39(3), pp.640-644.
Godfrey, T.E., (2009) Intraoperative Molecular Staging Using Real-Time Polymerase Chain Reaction. In Cell and Tissue Based Molecular Pathology (pp. 153-163). Churchill Livingstone.
Gosset, M., Berenbaum, F., Levy, A., Pigenet, A., Thirion, S., Saffar, J.L. and Jacques, C., (2006) Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis research & therapy, 8, pp.1-14.
Güthner, T. and Mertschenk, B., (2000). Cyanamides. Ullmann’s encyclopedia of industrial chemistry.
Hill, A.T., Sullivan, A.L., Chalmers, J.D., De Soyza, A., Elborn, J.S., Floto, R.A., Grillo, L., Gruffydd-Jones, K., Harvey, A., Haworth, C.S. and Hiscocks, E., (2019). British Thoracic Society Guideline for bronchiectasis in adults. Thorax, 74(Suppl 1), pp.1-69.
Jiang, K., Xing, R., Luo, Z., Huang, W., Yi, F., Men, Y., Zhao, N., Chang, Z., Zhao, J., Pan, B. and Shen, G., (2024). Pollutant emissions from biomass burning: A review on emission characteristics, environmental impacts, and research perspectives. Particuology, 85, pp.296-309.
Kunovac, A., Hathaway, Q.A., Pinti, M.V., Taylor, A.D. and Hollander, J.M., (2020). Cardiovascular adaptations to particle inhalation exposure: molecular mechanisms of the toxicology. American Journal of Physiology-Heart and Circulatory Physiology, 319(2), pp.H282-H305.
Laumbach, R.J. and Kipen, H.M., (2012) Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. Journal of allergy and clinical immunology, 129(1), pp.3-11.
Lee, Y.G., Lee, P.H., Choi, S.M., An, M.H. and Jang, A.S., (2021). Effects of air pollutants on airway diseases. International journal of environmental research and public health, 18(18), p.9905.
Medzhitov, R., (2010). Inflammation 2010: new adventures of an old flame. Cell, 140(6), pp.771-776.
Noack, M. and Miossec, P., (2014) Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmunity reviews, 13(6), pp.668-677.
Okeke, E.S., Okagu, I.U., Okoye, C.O. and Ezeorba, T.P.C., (2022). The use of calcium carbide in food and fruit ripening: Potential mechanisms of toxicity to humans and future prospects. Toxicology, 468, p.153112.
Per, H., Kurtoğlu, S., Yağmur, F., Gümüş, H., Kumandaş, S. and Poyrazoğlu, M.H., (2007) Calcium carbide poisoning via food in childhood. The Journal of emergency medicine, 32(2), pp.179-180.
Rao, X., Huang, X., Zhou, Z. and Lin, X., (2013). An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatistics, bioinformatics and biomathematics, 3(3), p.71.
Sarkar, M., Niranjan, N. and Banyal, P.K., (2017). Mechanisms of hypoxemia. Lung India, 34(1), pp.47-60.
Tajudeen, A., Akanfe, F.A. and Adebayo Masaudat, A., (2019). Waste To Wealth: A Case Study Of Chemical Conversion Of Carbide Waste To Laboratory Chemicals. Technology (ICONSEET), 4(25), pp.191-196.
Wautier, J.L. and Wautier, M.P., (2023). Pro-and anti-inflammatory prostaglandins and cytokines in humans: a mini review. International Journal of Molecular Sciences, 24(11), p.9647.
Wong, J., Magun, B.E. and Wood, L.J., (2016). Lung inflammation caused by inhaled toxicants: a review. International journal of chronic obstructive pulmonary disease, pp.1391-1401.


