1Ecology and Range Management Department, Desert Research Center, Egypt
2Chemistry Department, Faculty of Science, Northern Borders University, Arar, Saudi Arabia
Corresponding author email: elsharqawyeman2017@gmail.com
Article Publishing History
Received: 10/10/2019
Accepted After Revision: 20/12/2019
The aim of current research was to evaluate of the allopathic effect of aqueous extracts and alkaloid fraction of Withania somnifera and Hyoscyamus muticus against germination and growth of Cichorium intybus, in a laboratory. Alkaloid fraction and aqueous extract of aerial part of W. somnifera and H. muticus at different concentration, were applied to determine their effect on the seed germination and seedling growth of tested plant under laboratory condition. The germination and growth of Cichorium intybus were assessed using growth parameters. The results revealed that all the aqueous extracts markedly suppressed the germination and seedling growth of Cichorium intybus. Withania extracts showed remarkable effects on germination and the growth of Cichorium intybus in comparison to controls. The aqueous extract and alkaloid fraction of W. somnifera were more pronounced than Hyoscyamus muticus extracts in germination assay. All the aqueous extracts significantly suppressed shoot length, and root length, of Cichorium intybus. The inhibition of germination and suppression of growth parameters could be attributed to the presence of active phytochemicals compounds present in the extracts and alkaloid fraction which, subject to GC-MS analysis to study these compound, GC-MS revealed the presence of many active phytochemical compounds, of W. somnifera and H. muticus extracts ( Ferulic acid, Methyl ferulate, Mandolic acid), Scopolamine and atropine are the major compounds in H. muticus with concentrations of 13.79% and 3.80% in alkaloid fraction, while in W. somnifera, Pterin-6-carboxylic acid are the major compounds with concentration of 2.5 %. The present study concludes that W. somnifera and H.muticus contain bio-herbicidal compounds in the plant extracts and alkaloid fractions which showed an inhibitory allelopathic effect on the development of wild chicory.
Allelopathy, Hyoscyamus muticus L. , Wathenia somnifera, Harm Seeds
Elsharkawy E. R. Allelopathic effects of alkaloid contents of Hyoscyamus muticus and Withania somnifera on the germination of Cichorium intybus seeds. Biosc.Biotech.Res.Comm. 2019;12(4).
Elsharkawy E. R. Allelopathic effects of alkaloid contents of Hyoscyamus muticus and Withania somnifera on the germination of Cichorium intybus seeds. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2MKSFnw
Copyright © Elsharkawy This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Allelopathy is formulated as any process involving secondary metabolites produced by plants, algae, bacteria, and fungi that influence the growth and development of agricultural and biological systems. The allelopathic organism releases chemicals that inhibit the growth of a competing organism and thus indirectly prevents it from using common resources. For a long time, it has been recognized that several compounds that are present in the soil in replant situations, actively or passively alter the prevailing soil conditions, inducing a plant population, therefore, due to their allelopathic characteristics, these substances could somehow act as a pesticide (Awatief et al., 2013, Fang and Cheng 2015).
Withania somnifera is a small shrub to 2 m high, the whole plant is covered with short, fine, silver-grey, branched hairs. The stems are brownish and prostrate to erect, sometimes leafless below. The leaves are alternate simple, margins entire to slightly wavy, broadly ovate, obovate or oblong, 30–80 mm long and 20–50 mm broad, flowering time is mostly from October to June, while the fruiting time is mostly from October to July (Boulos, 1995).
The medicinal importance of W. somnifera is due to presence of diverse secondary metabolites. The main chemical constituent of leave of W. somnifera are alkaloids ( isopelletierine, anaferine, cuseohygrine, anahygrine), steroidal lactones (withanolides withaferins) and saponins (Atta-ur-Rahman 1996).Withania somnifera (L.) Dunal screened to investigate the biological activities i.e. antimicrobial, antioxidant, Anti-inflammatory, Anti-aging, Anti-carcinogenic, Cardioprotective, Hypothyroid, Pharmacological activities. The demonstration of broad spectrum of W. somnifera may help to discover new chemical classes of antibiotic substances that could serve as selective agents for infectious disease chemotherapy and control (Kapoor 2001; Kalpana et al., 2014, Elsharkawy and Shiboop 2017).
Egyptian henbane (Hyoscyamus muticus) is one of the most important medicinal plant of the Solanaceae family. It contains valuable tropane alkaloids, hyoscyamin and traces atropine (Lavania, 2005). Tropane alkaloids, especially hyoscyamin and scopolamine, are widely used in medicine for their mydriatic, antispasmodic, anticholinergic, analgesic and sedative properties. These alkaloids are synthesized in roots and then transported to aerial parts of the plant (Pal Bais et al., 2001). The genus Hyoscyamus L. belongs to the tribe Hyoscyameae Miers of Solanaceae family with 18 species all over the world (Yousaf, 2008) and 3 species in Egyp. Hyoscyamus species are rich sources of tropane alkaloids, mainly hyoscyamine and scopolamine, Tropane alkaloid have pharmacological and toxicological importance, and are spread in solanaceae family (Kartal et al., 2003).
Fatemeh et al., (2012), determined scopolamine and hyoscyamine contents in five Hyoscyamus species including H.niger L., H.reticulatus L., H.pusillus L., H. arachnoideus Pojark., and H. kurdicus Bornm., collected from different geographical origins of North West of Iran by HPLC The range of genetic similarity was obtained between 91.07 and 99.89 within Hyoscyamus accessions based on scopolamine and hyoscyamine alkaloids composition. Allelopathy is the science that studies processes in which secondary metabolites from plants and microorganisms are involved, affecting growth and development of biological systems (Singh et al., 2003) . The use of secondary metabolites implicated in allelopathic interactions as sources for news agrochemical models could satisfy the requirements for crop protection and weeds management (Qiming et al., 2016).
The aim of current research was to study positive or negative the allopathy of the application different concentration of aqueous extracts and alkaloid fraction of Withania somnifera and Hyoscyamus muticus against germination and growth of, Cichorium intybus in laboratory along with to also study the phytochemical compounds present in plant extract, where the alkaloid fraction was analyzed to understand the role of chemical composition of plants and their allophathic effects.
MATERIAL AND METHODS
Collection and Preparation of Samples
Plants for the study (Withania somnifera and Hyoscyamus muticus) were collected in June, 2016 from the wild population in Alamain- Wadi El- Natrun Desert Road, Egypt. Seeds of plant weed (Cichorium intybus) were collected also collected from Farms of onion in near the plant and kept until germination process. The plant was identified in Desert Research center and the sample was deposited in the Herbarium of Desert Research center.
Laboratory Study: Water Extract preparation
500 g of dried aerial part of each plants were boiled with 1000 ml distilled water for 2 hour, vigorously stirred and allowed to stand for 24 hours. These were then vigorously re-stirred and filtered into wash bottles until use, thereafter filtered using muslin cloth to obtain a stock solution of 0. 5 g /ml concentration. The stock solution was then adjusted accordingly to obtain four different levels concentrations i.e. 25%, 50%, 75% 100% . Distilled water was used as a control .
50 fifty Petri dishes double layered with Whatman No. 1 filter paper were taken and divided into two (2) sets, one set for Aqueous extracts and the other set for alkaloid extracts of roots. The same Experiment was done for each plant. Ten (10) seeds were sown in each Petri dish and moistened with 25 mls of an appropriate water extract of the weed. There were three (3) replications of each treatment (an aqueous and alkaloid extract). Twenty-five (25 mls) of distilled water was applied to the control. Filter paper linings of each Petri dish were moistened daily with an appropriate extract to prevent them drying up before final germination counts (10 days after sowing). The petri dishes were kept in a growth chamber at room temperature until the final germination count.Germination inhibition/stimulation Percentages of inhibition/stimulation effect on seed germination over control were calculated using the formula proposed by Singh and Chaudhary (2011).
Inhibition (-) or stimulation (+) = [(Germinated seeds in extracts – Germinated seed in control)/Geminated seeds in control] x 100
Measurement of growth parameters
After the completion of seed germination, shoot length was measured using ruler with different days of developmental period. The root length and inhibition percentage of the root length for each seeds of weed was calculated. Germination data obtained were analyzed using analysis (ANOVA) test, the means of treatment were grouped on the basis of (LSD) at the 0.05 probability level.
Preparation of Alkaloid fraction
100 gm of plant powder of each plant under investigation (W. somnifera and H.mutcus) was separately extracted by refluxing by 70% ethanol three time for 3 hours ,filtered of and concentrated by rotary evaporator then suspended in 200 ml water and separated in separating funnel, the filtered aqueous layer was fractionated by chloroform take chloroform fraction and evaporated to obtain (1.8 and 2.6 gm ) from two plant ((W. somnifera and H. mutcus)) respectively, (Elsharkawy and Shiboop 2017).
Preliminary Phytochemical screening
Preliminary phytochemical screening of water extracts for each plant for plant secondary metabolite (flavonoids, saponins, alkaloid, terpenes and tannins) was carried out using standard methods (Bao et al., 2005).
GC-MS Analysis
Gas chromatography-mass spectroscopy (GC-MS) analysis GC-MS analysis of the plant extracts and fraction were carried out using a Clarus 500 Perkin – elmer (Auto system XL) gas chromatograph equipped and coupled to a mass detector Turbo mass gold – Perkin Elmer Turbomass 5.1 spectrometer with an Elite – 1 (100% dimethyl poly siloxane), 30 m × 0.25 mm ID × 1 μm of capillary column. For GC-MS detection, an electron ionization system was operated in electron impact mode with ionization system operated in electron impact mode with ionization energy of 70 ev.
RESULTS AND DISCUSSION
The allophathic effect of plants (W. somenifera and H. muticus) were carried out by study the effect of aqueous extract and alkaloid fraction of the two plants on the germination of weeds (ciocherum,). The effects of aqueous extract and alkaloid fraction on germination of weeds after 7 days and 15 days in Petri dishes and in pots respectively are given in Table 1 and Table 2.Our findings demonstrated that two plant W. somnifera and Hyoscyamus muticus exhibited significant allelopathic activities in all parameters measured (germination, shoot and root length). As shown in Table 1&2, both alkaloid and aqueous extract significantly inhibited germination and reduced shoot and root length of Cichorium intybus. The result obtained were concentration dependent as increasing extract concentration significantly inhibited germination of Cichorium intybus. The highest inhibitory effect was shown by aqueous extract of W. somnifera than H. muticus (100%) of high extract concentration(100%), while the alkaloid fraction of two plants have similar inhibition effect especially at high concentration while at low concentration the inhibition of germination decrease reach to (90% and 85%) for W. somnifera and H. muticus respectively as illustrated in Table 1.
Table 1: Effect of plant extract and alkaloid fraction on the germination Cichorium intybus
| CONC % |
W. somnifea |
H. mutcus |
||
| Germination %
Plant Extract |
Germination % alkaloid fr action | Germination % Water extract % | Germination % alkaloid fraction | |
| 100 | 0% | 0% | 0% | 0% |
| 75 | 0% | 0% | 0 % | 5% |
| 50 | 0% | 0% | 5% | 10% |
| 25 | 10% | 5% | 10% | 15% |
Table 2: Effect of plant extract and alkaloid fraction on the growth parameters of weed Cichorium intybus
W. somnifea |
H. muticus |
||||
| CONC%
|
Germination% Water extract | Germination % alkaloid fraction | Germination % Water extract | Germination% alkaloid fraction | |
| Shoot length | |||||
| 100 % | 0 | 0 | 0 | 0 | |
| 75% | 0 | 0 | 28% | 0 | |
| 50% | 84% | 0 | 83% | 0 | |
| 25% | 88% | 0 | 94% | 0 | |
| Water extract %Germination % | Germination % alkaloid fraction | Water extract Germination % | Germination % alkaloid fraction % | ||
| Root length | |||||
| 100 % | 0 | 0 | 0 | 0 | |
| 75% | 0 | 0 | 0 | 0 | |
| 50% | 68% | 0 | 76% | 0 | |
| 25% | 84% | 0 | 88% | 0 | |
The effect of aqueous extracts and alkaloid fractions of two plants on root and shoot lengths on the studied weed were, like its effects on the germination indices, depending on concentration Table 2,. The root length of Cichorium intybus, was significantly reduced by aqueous plant extract and alkaloid fraction, which completely inhibited the root growth. The remaining concentrations of the extract completely inhibited the root growth of weed. In application of low concentration 25% of plant extract and 50% of alkaloid fraction showed significantly increased root length, whereas the higher concentrations induced significant gradual reductions in root length. The effect of extract on shoot length of the weed species exhibited the same pattern as that observed for root growth. At all concentrations, the plant extract inhibited the shoot elongation of , the degree of that inhibition increased gradually in parallel with increasing concentrations of the plant extracts and alkaloid fraction.Phytochemical analysis of aqueous extract of plants under study revealed the presence of many phytochemical class in two plant alkaloid, saponin, flavonoid and alkaloid as in Table 3
Table 3: phytochemical analysis of Withania somnifera and Hyoscyamus muticus
| H. muticus | W. somnifera | Class |
| + | ++ | Alkaloids |
| ++ | +++ | Saponins |
| +++ | ++ | Sterols and steroids |
| + | _ | Flavonoïd |
| – | _ | Tanins |
| – | _ | Anthocyanosids |
| + | _ | Terpene |
GC-MS analysis of plants extract showed the presence of many bioactive compounds As in Table 4, with their retention index (RI), structure formula and concentration. The results demonstrated the water extract of two plant contain the same phenolic compounds, 4-Hydroxy-3-methoxycinnamic acid (Ferulic acid), Methyl ferulate (, Hydroxy-6,7,8-trimethoxy-2,3-dimethyl-4H-chromen-4-one and mono benzylidene- glucose and Mandolic acid, also two plant contain some steroidal compounds, Sarreroside and Ergosta 5,22 dien, 3-ol, acetate. There are some compounds are found in W. somnifera extract and not detected in H. muticus, Corynan-17-ol, 18, 19 di-dehydro10-methoxy-acetate and 3,9-Epoxypregnan-14 ol, 20-one,3,11,18-tri-acetoxy.the major compound in H. muticus are methyl ferulate (2.5%) and mono benzylidene- glucose (2.80).
Table 4: GC-MS- analysis of water extract of withania somnifera and Hyoscyamus muticus
| Hyoscyamus muticus | withania somnifera | RI | Compounds |
| 2.5 | 0.08 | 604 | Methyl ferulate |
| 0.08 | 0.06 | 615 | 4-Hydroxy-3-methoxycinnamic acid |
| 0.65 | 0.28 | 604 | 4-hydroxy-mandonlic acid |
| 0.20 | 0.35 | 501 | psi.,.psi.-Carotene |
| 0.66 | 0.10 | 611 | Sarreroside |
| 2.80 | 0.44 | 521 | mono benzylidene- glucose |
| 0.44 | 0.33 | 420 | Hydroxy-6,7,8-trimethoxy-2,3-dimethyl-4H-chromen-4-one |
| 0.26 | 0.45 | 632 | Ergosta 5,22 dien, 3-ol, acetate |
| – | 0.27 | 493 | Cis Vaccenic acid |
| – | 1.32 | 502 | 3,9-Epoxypregnan-14-ol, 20-one,3,11,18-triacetoxy |
| – | 0.31 | 404 | Corynan-17-ol,18,19-didehydro10-methoxy, acetate |
| 0.11 | 0.08 | 586 | α-D-Glucopyranoside |
GC-MS analysis of alkaloid fractions of two plant showed the presence of many bioactive compounds As in Table 5,6 with their retention index (RI), structure formula and concentration. The results demonstrated the alkaloid fraction of two plant contain many same alkaloid compounds Morphinan4,5-epoxy3,6-diol, Strychane, 1-acetyl-20, α-hydroxy-16-methylene, Dasycarpidan1-methanol, Acetate and β-Hydroxyque brachamine with different concentration. Pterin-6-carboxylic acid are the major compound with concentration (2.5) in plant Withenia somnifera where it found in H. muticus with low concentration. Scopolamine and atropine are the major compounds in H. muticus with concentration (13.79% and 3.80%) respectively.
Table 5: phytochemical analysis of Alkaloid fraction of W. somnifera and H. muticus
| Hyoscyamus muticus | Withania somnifera | RI | Compounds |
| 0.60 | 0.26 | 446 | Pregn-4ene-3, 20 dione, 17, 21dihydroxy, |
| 0.70 | 2.3 | 677 | Pterin-6-carboxylic acid |
| 0.61 | 1.28 | 627 | Dasycarpidan1-methanol, Acetate |
| 0.68 | 0.53 | 421 | Morphinan-7,8-didehydro-4,5 epoxy-3,6 –diol-17- methyl (Kadain) |
| – | 0.25 | 547 | Morphinan-6 ol,7,8-didehydro4,5-epoxy-3-methoxy17-methyl, acetate, (Acetylcodeine) |
| 0.11 | – | 607 | Desulphosingrin |
| 0.27 | 0.50 | 440 | β-Hydroxyque brachamine |
| 0.03 | 0.17 | 603 | Curan-17-oic acid, 19,20-dihydroxy-, methyl ester |
| 0.04 | 0.13 | 582 | Strychane, 1-acetyl-20, α-hydroxy-16-methylene |
| 13.79 | – | 2264 | Scopolamine |
| 3.80 | – | 2253 | Atropine |
| – | 0.28 | 671 | Aristolochic acid |
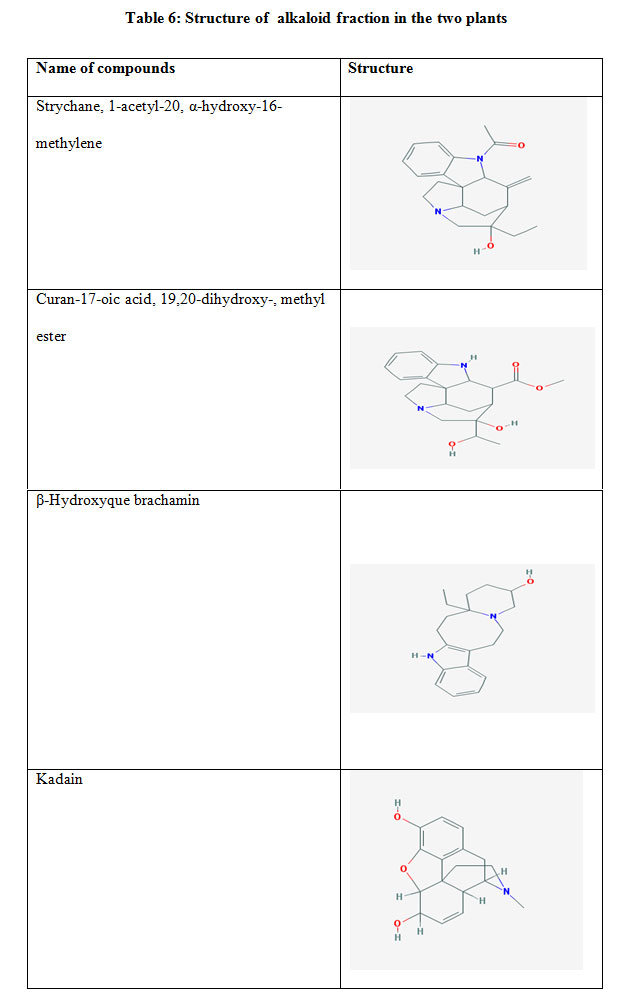 |
Table 6: Structure of alkaloid fraction in the two plants |
somnifera and H. mutcus are considered indicators allelopathic activity due to their ability of releasing allelophatic substance which has an affect on the germination of other plants . The allelophatic phenomena are found in gardening and agricultural fields since ancient times and this term mean biochemically mediated interaction in plants, many plant were noticed have this phenomena ,so in this study we need to use this phenomena to help us as natural pest side by using plant extract of W. somnifera and H. mutcus and their alkaloid fraction in germination inhibition of weed Ciochoruim instead of synthetic pest side and try to determine the chemical compound responsible for this inhibition. Secondary metabolites that exert allelopathy can be released in the form of volatile compounds, root exudates, above-ground plant leachates or plant litter [Duke, 2010]. Released allelochemicals are indeed subject to sorption on soil particles as well as chemical and microbial decomposition, (Kaur et al. 2009; Lankau 2010).
(Macel et al. 2014), studied the role of many metabolite in six plant in allophathy effect the studied showed that plant chemistry is highly Specific-specific and diverse among both exotic and native species. Between class of metabolite have a role of allopathic effect are, phenolic compound, sesquiterpene, substituted alkaloid and flavonde in between phenolic compound have allophathy effect cinnamic acid derivatives (Lankau, 2010.), this are agree with our results which demonstrate the presence of cinnamic acid derivatives and methyl ferulate as constituents of two plant extracts. Also alkaloid compounds present in plant extract have a role in germination inhibition.
CONCLUSION
It is concluded that the aqueous extract and alkaloid fraction of Withania somnifera and H. mutcus showed an inhibitory allelophathic effect on the germination of wild chicory and the inhibitory effect depended on the concentration. Phytochemical analysis showed the plant contain different class of phytochemical compounds GC-Ms analysis of aqueous extract revealed the presence of hydroxyl cinnamic acid and methyl ferulate and other phenolic compound which may have a major role in germination inhibition also alkaloid fraction have many compounds which consider as alellochemical finally we can recommended by use these plant extracts as a pest side after further studies.
ACKNOWLEDGMENT
The authors are grateful to Northern border University and Desert Research Center for support and provision of facility.
CONFLICT of INTEREST
The author declare any conflict of interest
REFERENCES
Atta-ur-Rahman, Jamal, A.S.; Choudary, M.I.; Asif, I. (1991): Two withanolides from Withania somnifera. Phytochemistry, 30, 3824-3825.
Awatief F. Hifney, M.S. Adam, G. Ghareib, A.A. Issa (2013): Allelopathic effects of some weeds on rhizosphere algae at El-Kharga Oasis (New Valley), Egypt, Journal of Biology and Earth Sciences, , Vol 3, Issue 1, B42-B53.
Bao, J. . Cai, Y. Sun, M. Wang G., and Corke, H. (2005), Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability, Journal of Agricultural and Food Chemistry, vol. 53, no. 6, pp. 2327–2332.
Boulos, L.(1995): Flora of Egypt Checklist , Al Hadara Publishing; First edition .
Duke SO. (2010), Allelopathy : Current status of research and future of the discipline : A Commentary Allelopathy Journal 25: 17–30.
Elsharkawy E.R. and Shiboop M.H. (2017), Antioxidant Activity of Phenolic and Alkaloid Fractions Accumulated in Artemisia judaica and Artemisia herba Journal of Natural Remedies, 17(4).
Fang Cheng, Zhihui Cheng (2015): Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy, Front Plant Sci. ; 6: 1020. doi: 10.3389/fpls.
Fatemeh N., Fatemeh R., Reza H., Rashid J. and Farzaneh A. (2012), Study of inheritance and environment on tropane alkaloids within Hyoscyamus species ,AJCS 6(10):1428-1434.
Kato-Noguchi H, Moriyasu M, Ohno O, Suenaga K. (2014), Growth limiting effects on various terrestrial plant species by an allelopathic substance, loliolide, from water hyacinth. Aquatic Botany 117: 56–61.
Kaur H, Kaur R, Kaur S, Baldwin IT, Inderjit. 2009. Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PloS one 4: e4700.
Kartal M, Kurucus A ,Ceyhan T, Sayar E, Cevheroglu S Yetkin Y ( 2003), Quantitative analysis of L- hyoscyamine in Hyoscyamus reticulatus L. by GC-MS. Turk J Chem:,27: 565-569.
Kapoor, L.D. Handbook of Ayurvedic Medicinal Plants; CRC Press: London, UK, 2001; pp. 337-338.
Kalpana Gavande ,Kirti Jain and Rakesh Mehta (2014), Few medicinal activities of Ashwagandha (Withania somnifera), Int. J. of Pharm. Life Sci, 5(6):3603-3606.
Lankau R. (2010), Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biological Invasions 12: 2059–2068.
Lavania, U.C., (2005), Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet. Resour., 3: 170-177.
Macel M, de Vos RCH, Jansen JJ, van der Putten WH, van Dam NM. (2014), Novel chemistry of invasive plants: exotic species have more unique metabolomic profiles than native congeners. Ecology and evolution 4:2777-86.
Pal Bais, H., V.M. Loyola-Vargas, H.E. Flores and J.M. Vivanco (2001), Root-specific metabolism: The biology and biochemistry of underground organs. In Vitro Cell Dev. Biol. Plant, 37: 730-741.
Qiming X, Haidong C, Huixian Z, Daqiang Y (2006), Allelopathic activity of volatile substance from submerged macrophytes on Microcystin aeruginosa. Acta Ecol. Sin. 26:3549-3554.
Singh HP, Batish DR, Kohli RK (2003), Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management Crit. Rev. Plant Sci. 22:239-311.
Singh, A.P. and Chaudhary, B.R. (2011), Allelopathic of algae weed Pithophora aedogonia (Mont.) ittrock on the germination and seedling growth of Oryza sativa Botany Research International, 4(2): 36-40.
Yousaf Z, Masood S, Shinwari ZK, Khan MA, Rabani A (2008), Evaluation of taxonomic status of medicinal species of the genus Hyoscyamus, Withania, Atropa and Datura based on polyacrylamide gel electrophoresis. Pak J Bot: 2289-2297.


