1Ecotoxicology, Fisheries and Aquaculture Extension Laboratory, Department of Zoology, University of Kalyani, Kalyani, India.
2The University of Burdwan, Burdwan, India.
3Retired Professor, Department of Zoology, Berhampur University, Bhanjabihar, Berhampur, Odisha India.
Corresponding author email: panigrahi.ashis@gmail.com
Article Publishing History
Received: 08/12/2020
Accepted After Revision: 21/03/2021
An alarming threat for environment, pollution is undoubtedly posing different adverse impacts on the living organisms. Aquatic environment is not the expectation. Among all the sources of aquatic pollution, heavy metal induced hazards better have been found to be most important for both freshwater and marine ecosystems. Heavy metals can prove to be deleterious for aquatic organism when exposed for short term (acute) as well as long term (chronic) period. Fishes are best known model for determining the degree of aquatic pollution. Thus, it is very essential to find a consolidated research article describing the accumulation pathway of heavy metals in freshwater fishes. In this review, attempts have been made to compile all the available scientific data related to the uptake and accumulation of different heavy metals (As, Hg, Cd, Cu, Cr and Pb) and the general histopathological changes due to chronic exposure to sublethal concentrations. Data obtained from the previous researches are meticulously chosen in order to avoid ambiguous presentation here. The focal objective of the scientific review is to offer an imminent guideline for the students, scientific community, and public officials involved in environmental health risk assessment and management ensuring a better future environmental condition. During the review process, we have found out that entry routes of different heavy metal is mainly GI tract, gill and/or skin. Most of the heavy metal may be present in more than one form and have specific way of accumulation in tissues. This review also provides the accumulated data of heavy metal contamination in Indian rivers, factors related to metal uptake in fishes and scientific information about the source and bioaccumulation of selected heavy metals.
Heavy Metals, Freshwater Fish, Uptake, Bioaccumulation, Histopathology
Panigrahi A. K, Bakshi A, Pattanayak S. A Comprehensive Review on the Uptake by and Accumulation of Some Heavy Metals in Fresh Water Fishes. Biosc.Biotech.Res.Comm. 2021;14(1).
Panigrahi A. K, Bakshi A, Pattanayak S. A Comprehensive Review on the Uptake by and Accumulation of Some Heavy Metals in Fresh Water Fishes. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3enems3″>https://bit.ly/3enems3</a>
Copyright © Panigrahi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nowadays, pollution, especially in aquatic ecosystem, due to the contamination of heavy metal becomes a significant issue of concern to the researchers of environmental sciences. It is evident that wide spreading of industries, rapid urbanization and population explosion impose deleterious impact on the hydro-biological quality of both lentic and lotic ecosystems viz., ponds, lakes, and rivers. The consequences become more critical because the small and large-scale industries frequently discharge their wastes containing different heavy metallic contaminants directly into the environment which often go beyond the permissible limit of the environment (Velma et al., 2009; Praveena et al., 2013). In spite of the development in waste management technologies, the difficulties due to heavy metal release are continuously putting immense adverse effect on the biolife of aquatic ecosystems (Bakshi and Panigrahi, 2018). Especially class- B or lithophilic metals are considered to be more deleterious to the ecosystem and fundamental group of aquatic pollutants because of the long persisting nature (or longer half-life), mechanism of bioaccumulation, process of biomagnification and non-biodegradability. Another important reason behind the consideration is their potency to destroy the framework of species diversity in any ecosystem (Vutukuru et al., 2007; Lodhi et al., 2007; Saha and Zamman, 2011; Ahmed et al., 2014).
Thus, entering into the food chain heavy metals often show high toxicity even in minimum concentration providing cumulative injurious effects in an aquatic system (Velma et al., 2009; Velma and Tchounwou, 2009). Heavy metals are thus considered to put ecological, evolutionary, nutritional and environmental impact on the ecosystem (Jaishankar et al., 2014). In the recent days, fresh water ecosystems are mostly polluted by waste waters released from different industries and municipalities. The most frequently available heavy metals in the waste water are Lead, Arsenic, Cadmium, Chromium and Mercury (Bakshi, 2016; Mehana et al., 2020). The main objective of the review is to provide insight of the uptake and accumulation of some heavy metals like lead, cadmium, arsenic, chromium and mercury in fresh water fishes. Accumulation rate of heavy metals is very specific in different fishes (Khan et al, 2020). In this review we have only tried to consolidate the data related to heavy metal accumulation in fresh water fishes though further studies can be done on the heavy metal accumulation in estuarine or marine fishes (Khan et al, 2020).
MATERIAL AND METHODS
An attempt is made to produce an utmost consolidated manuscript on this topic. In order to make the manuscript more comprehensive and relevant for the future study, extensive review has been done compiling and consolidating the maximum number of available scientific data. All data has been collected, from science journals of repute, published reports (particularly from international agencies) and doctoral or postdoctoral theses. Priority has been paid to the reproducible articles which are indexed in science journal database like Copernicus, Scopus, PubMed etc. The scientific articles highlighting ambiguous working methodologies are avoided carefully. Key words have been meticulously selected and searched based on systematic scientific approaches. Our own experimental findings (both laboratory and field) have been encompassed at various parts of the manuscript to improve the essence of the article.
Heavy metal concentration in fresh water: Most of the heavy metals are available in natural water in the form of soluble and/or in particulate form. Water soluble forms of heavy metals are found in labile or non-labile fractions (Jezierska and Witeska, 2001). Labile metallic forms are most detrimental to the aquatic organisms, especially fishes. Aquatic ecosystem contains not only the heavy metals but also the essential metals (both major and trace metals). In aquatic environment, trace metals are present in very low amount affecting fresh water fishes detectable in minimum. Amount of metal concentration in fresh water ecosystem is continuously increasing directly through atmospheric deposition and waste water contamination or indirectly through rising solubilisation followed by mobilization from sediments. EPA and BIS recommended permissible limit of various metals in water are often crossed in some rivers in India (EPA,1972; BIS 10500,2012). Central Water Commission conducts surveys to estimate the concentration of the heavy metals in the Indian river waters (Table 1) (CWC, 2014; CWC, 2018).
Table 1. Status of heavy metals concentration in Indian Rivers (CWC, 2014; CWC, 2018; BIS 10500,2012; EPA, 1972)
| Metal | Chemical symbol | Sources | Maximum permissible concentration (µg/ l) | Number of the rivers in India with metal concentration beyond permissible limit | ||||
| 2014 | 2018 | |||||||
| EPA | BIS | Rivers | Maximum Observed Conc. (µg/ l) | Rivers | Maximum Observed Conc. (µg/ l) | |||
| Arsenic | As | Pesticide industries, mining, Chemical industries | 50 | 10 | 0 | 9.47 | 0 | 9.53 |
| Cadmium | Cd | Cd-NI batteries, Nuclear reactors, Television phosphor | 10 | 3 | 4 | 4.0 | 25 | 70.51 |
| Chromium | Cr | Dyeing, Mines, Electroplating | 50 | 50 | 11 | 366.91 | 21 | 450.26 |
| Copper | Cu | Electroplating, Pesticide industries | 1000 | 50 | 68 | 180.70 | 10 | 314.93 |
| Lead | Pb | Paint, Pesticide, Batteries, Crystal glass industries | 5 | 10 | 30 | 48.92 | 69 | 374.58 |
| Mercury | Hg | Mining, Pesticide industries. | 2 (1.44) | 1 | 0 | <1 | – | – |
Central Water Commission report, 2018 shows that forty-two rivers of India have been found to be polluted due to receive of neurotoxic heavy metals. The report also describes that river Ganga is highly contaminated by chromium, copper, lead, iron and nickel due to receive of run-off mostly from milling, plating, mining and surface finishing industries. Report shows that Cadmium contamination in Indian rivers has increased during the last four years. In the rivers of Godavari basin, most of the rivers contain cadmium, chromium, arsenic, nickel and zinc within the acceptable or permissible limit of Bureau of Indian Standards (BIS 10500, 2012) (CWC, 2018).
Environmental factors affecting metal uptake and accumulation in fish: Fishes in the heavy metal contaminated aquatic system must accumulate the metals in their tissues. The rate of accumulation solely depends on the concentration of the metal, method of uptake and time of exposure. Some extrinsic factors and some intrinsic factors are also important parameters which determine the rate of accumulation (Jezierska and Witeska, 2001).
The environmental factors like water temperature, hydrogen ion concentration, hardness etc. influence the uptake, accumulation and depuration of metals in fish. Water temperature is a key environmental factor that influence metal uptake, accumulation and depuration (Jezierska and Witeska, 2001). Metal accumulation in fishes is also related with some biological or intrinsic factors like age, size, feeding habits. Except mercury, other heavy metals have shown an inverse relation with age and size (Jezierska and Witeska, 2006). Accumulated metals show different tissue affinity but most of them accumulate especially in gill, liver and kidney. Very small number of metals is found to be accumulated in muscles in most of the fishes. Except mercury, other heavy metals have shown an inverse relation with age and size (Jezierska and Witeska, 2001; Jezierska and Witeska, 2006).
Uptake and accumulation dynamics of different metals in fish: In recent days, pollution (especially water pollution) due to heavy metal contamination has undoubtedly grow into a great issue of concern to the environmental scientists. Extensive industrialization, exploitation and rapid increase of urban communities have measurably forced adversative impact on the hydrobiological quality of lakes, ponds and rivers all over the world (Praveena et al., 2013). Heavy metals especially cadmium (Cd), copper (Cu), Chromium (Cr) and Lead (Pb) are found to be highly available in Indian rivers. Though, contamination of detectable arsenic (As) and mercury (Hg) is not found to be reported in any river of India. In this review, an attempt has been made to focus on the various environmental forms of the heavy metals (As, Hg, Cd, Cu, Cr and Pb), method of uptake, accumulation and dynamics in the fish body (Praveena et al., 2013).
Arsenic: Arsenic is one of the harmful heavy metals (metalloid) in the aquatic bioloife. It has a metalloid property and is predominantly available in the form of oxides (arsenate and arsenite) or sulfides or as a salt of sodium, iron, copper, calcium, etc. (Singh et al., 2007). Excessive use of arsenical pesticides, industrial activities, mining operations and chemical laboratory exhaustion has led to the global occurrence of water-soluble arsenic concentration above the permissible limit (Table 1). Water soluble inorganic arsenic (iAS) are converted to methylated arsenical forms i.e., monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) through enzymatic activities in organism body. These arsenical forms are main end metabolites and biomarker of the long-term arsenic exposure (Jaishankar et al., 2014; Kumari et al., 2016). Arsenic exposure may be waterborne and diet-borne. So, main routes of entry are gill and GI tract. Waterborne arsenic after taking entry through gill significantly accumulated in gill, liver and intestine and manipulates growth of the fish (Tsai and Liao, 2006; Han et al., 2019).
Inorganic arsenic (iAs) may be present in two forms i.e., iAs(III) and iAs(V). According to Kumari et al., (2016), after entry of iAs (V), it converts into iAs(III). Then iAs (III) changes into MMA(V) coupled with SAM –SAH conversion (SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine). After that MMA(V) transforms into most toxic and accumulating form MMA (III) through reduction reaction by the action of MMA(V) reductase or GSTO 1 (glutathione S-transferase omega 1). MMA (III) can also be converted into DMA coupled with a SAM –SAH conversion (SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine). Most of the bio-transformation reactions are taken place in liver (Fig 1) (Han et al., 2019).
Figure 1: Biotransformation of arsenic compound (Source: Modified from Kumari et al., 2016).
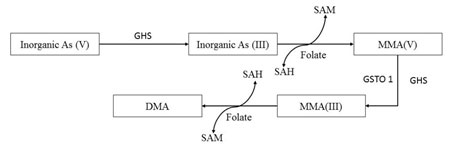
When arsenical compounds enter through the dietary route, it basically accumulates into digestive tract. From GI tract it goes to Liver where most of the biotransformation takes place. Then it deposited into the other organs or tissues of the body viz., brain gonads, muscles either directly or via gill circulation and get accumulated (Tsai et al., 2012). Elimination of little amount of metal is also observed through feces during depuration experiments (Kumari et al., 2016). Water dissolved arsenical compounds can also enter through gills and can be deposited directly into the brain, kidney, gonads and other tissue through speciation. Dietary uptake shows accumulation in the digestive tract till the end of exposure but the concentration gradually decreases during depuration. Though several researches are there but, in most cases, liver is said to be the highest accumulator organ of the metal (Kumari et al., 2016; Han et al., 2019).
Cadmium: Being completely non-essential to all the organisms, cadmium, is considered as a highly toxic heavy metal. With the increase of industrialization, deposition of cadmium in fresh water bodies (lakes, rivers etc.) becomes a major issue of concern to the environmentalists. Cadmium related contamination in the aquatic organism has been reported to be increased in last decade with a high degree of its accumulating property (Okocha and Adedeji, 2011). According to ATSDR (1999) report, the main sources of cadmium in the environment is anthropogenic (90%) and very low amount of cadmium is contributed by natural activities (viz., volcanic eruption, decaying of vegetables, forest fire etc.). Anthropogenic activities like agricultural uses, electroplating, mining, industrialization etc.
are main contributors of the cadmium into the environment (Table 1). Aquatic organism like fish can readily uptake cadmium in its ionic form (Cd II) through the gills (AMAP, 1998; AMAP, 2002). The ions are usually absorbed through carrier mediated transport or passive diffusion over the chloride cells of the gills. It has been reported by many researchers that cadmium enters into the cell through calcium ion channels and interacts with the cytoplasmic components like metabolic enzymes and metallothioneine (Rodriguez et al., 2015). It is believed that high affinity of Cd2+ ion for Ca2+ binding site in the gill facilitates its entry through the apical side of the chloride cells (Okocha and Adedeji, 2011).
Cadmium can also bind with the active site of Ca2+– ATPases present on the basolateral side of the chloride cell facilitating the translocation of ionic cadmium into the blood circulation (Okocha and Adedeji, 2011). Another route of cadmium entry is through dietary ingestion when cadmium is associated with organic material. Then the ions are absorbed by endocytosis through intestine Cadmium is highly accumulated in liver and kidney causing various deletorious pathological changes (Sumet and Blust, 2001). Cadmium is found to be present in maximum concentration in the kidney of the fishes posing various degree of renal damage (Kumar et al., 2009; Vesey, 2010). A very little amount of cadmium is found to be liberated out through feces (from intestine) and bile (from liver) secretion at the time of depuration (Okocha and Adedeji, 2011).
After renal damage cadmium can also be liberated from kidney of the fishes (Kumar et al., 2009; Vesey, 2010). Gills are said to be the storehouse of the cadmium showing high degree of morphological and biochemical changes after chronic exposure. The prime target of cadmium ion is chloride cells of the gill where it competes with calcium ion for the entry into the cell resulting hypocalcemia in fish (Wong and Wong, 2000). Several workers have reported that cadmium is highly toxic in both acute and chronic exposure causing nephrotoxicity, hepatotoxicity, lamellar degeneration and hypocalcemia in fish which also put some deleterious impact on the human life though food chain (Okocha and Adedeji, 2011; Khan et al., 2020).
Chromium: Chromium is present in three oxidation states viz., Cr2+, Cr3+, Cr6+, among which divalent Chromium is most unsteady. Only, the Cr3+ and the Cr6+ are the stable chemical state of Chromium available in the environment. Being one of the most common ubiquitous pollutants in the aquatic medium, Chromium and its particulates get contaminated into the aquatic medium through effluents discharged from various industries like electroplating workshops, printing-photographic, tanneries, textiles, ore mining, dyeing, and medical industries (Bakshi and Panigrahi, 2018). Among all the oxidation state, hexavalent chromium can be considered as the most toxic form because it can readily pass the cellular bio-membranes and then reduced to trivalent form.
Then, trivalent chromium reacts with different cellular molecules, and ultimately exposes the mutagenic and toxic properties of chromium industries (Bakshi and Panigrahi, 2018). Chromium enters into fish body either through gastro-intestinal tract and/or respiratory tract (Bakshi, 2016). The amount of the metal inside the fish varies with the form of available chromium time of exposure and its concentration (Mallesh et al., 2015). Bakshi and Panigrahi (2018) reported that chromium (VI) gets associated with the plasma protein and encompasses in transportation after penetrating the plasma membrane through sulphate ion channel. After that, the metal biologically gets accumulated in various internal organs of fish. The general pattern of distribution of Cr6+ in fishes is as follows: Gills> Liver> Skin> Muscles (Jaishankar et., al., 2014; Bakshi, 2016).
After getting entry through passages for isoelectric and isostructural anions (such as SO42- and HPO42-) of cell membrane, the hexavalent chromium undergoes metabolic reduction within the cell. During these metabolic reactions, different reactive intermediates are released which are reported to be detrimental to ensuring the stability of DNA helix, causing fatal effects in the affected individual (Wang et al., 1997; Jaishankar et., al., 2014). The same authors have also reported that migration of various intermediate chromium metabolites to nuclei and interaction with DNA are evident during this process causing the final negative effect (Vutukuru, 2005; Velma et al., 2009).
The primary storage and detoxification site for chromium is said to be liver in experimental condition. Higher concentration of metals is evident in bile of the experimental organism (Clarias batrachus) being exposed to metal contaminated food and environment (Bakshi, 2016; Bakshi and Panigrahi, 2018). It is reported that this storage is stabilized mainly by protein linkage or small peptide linkage such as glutathione linkage. In case of fishes the main elimination route of chromium or its compounds is through feces (Bakshi, 2016; Bakshi and Panigrahi, 2018).
Lead: Lead is considered to be a toxic metal which do not have any importance in the physiological processes of any living organisms. The metal is highly toxic in aquatic environment as it easily accumulates in fish tissues like gill, liver, kidney, bones and scales. It also can cross the blood-brain barrier causing neurotoxicity in fish (Rabitto et al., 2005; Ju-Wook et. al., 2019). Lead has now becoming a ubiquitous metal with various source in the environment. The sources are mainly of industrial, agricultural and domestic origin.
Gasoline and house paints also contribute lead in the environment, furthermore, lead bullets, plumbing pipes, storage batteries, pewter pitchers, faucets and toys are also helping in lead contamination (Sharma and Agarwal, 2005; Jaishankar et al., 2014). Automobile exhaust and smoking also contaminate lead into the air. Lead can enter through the gill, altering the morphological character of gill when gets attached to the mucus (Mobarak and Sharaf, 2011). Then it enters into the blood stream and accumulates in liver. Liver is the main organ for detoxification in fish. The metal can enter also through gastrointestinal pathway if lead contaminated diet is consumed or through skin (Łuszczek-Trojnar et al., 2013; Ju-Wook et. al., 2019).
The divalent lead can compete with the divalent calcium ion for entry through the gills (Ju-Wook et al., 2019). After getting entry through the metal traverses the basal membrane and enters into the blood flow from where it readily accumulates in liver. Dietary entry of lead also leads to the accumulation in liver though very little amount of the metal is defecated out. Then the metal accumulated into the kidney, it makes a huge damage to the organ. As the metal can cross blood brain barrier, it shows high degree of neurological damage. Several researchers reported about the acculamation of this metal into bones and scales also (Rabitto et al., 2005; Ju-Wook et. al., 2019). Several researchers have confirmed that lead can be bioacumulated in different tissues of the fish and can also be biomagnified with the food chain (Shaukat et al., 2018; Ju-Wook et al., 2019; Khan et al., 2020). Apart from the defecation very small amount of lead have been found to be eliminated out through bones and scales during depuration (Łuszczek-Trojnar et al., 2013; Ju-Wook et al., 2019).
Mercury: Mercury, a highly toxic and non-essential metal, also termed as quicksilver, is prevalent in the environment as a result of natural and anthropogenic activities. Exposure of mercury is considered to be the second highest cause of toxic metal poisoning. Best known accident related to mercury pollution is Minamata disaster of Japan (Vasanthi et al., 2019).
Mercury is present in three states with different metabolic fate: mercury vapour or metallic mercury or elemental mercury (Hg0), mercury salts or inorganic mercury (including mercurous chloride or Hg2cl2, mercuric chloride or HgCl2 and mercuric sulfide or HgS) and organic mercury (methyl mercury, ethyl mercury, phenyl mercury and alkyl mercury). There are some natural sources of mercury pollution like elemental mercury vapour from volcanoes and forest-fire, inorganic mercury by rock weathering etc. (Martinez-Finley and Aschner, 2014; Raihan et al., 2020). But after industrial revolution, source of mercury in the environment is mainly anthropogenic (Rice et. al., 2014). viz., gold mining, fossil fuel combustion, paper and pulp industries, electronic wastes, medical wastes, electroplating, metal industries, pharmaceutical industries etc.
In fishes, mercury can be taken up through gills, skin or digestive tract (Sweet and Zelikoff, 2001; Morcillo et al., 2017). Metallic mercury or mercury vapour (Hg0) can be oxidized into water soluble inorganic mercury (Hg2+) which is basically taken up by the fishes or can be reduced back to metallic mercury (Hg0) (Tokar et al., 2015). Metallic mercury often can be converted into organic mercury (Methyl mercury or phenyl mercury) by microorganisms (Rodriguez et al., 2015). Toxicity of mercury depends upon the state of mercury, environmental media, conditions, age and life history of the specimen and sensitivity of the organism. Organic form of mercury is most toxic to the aquatic organisms as it can be biomagnified through food chain (Fig 2) (Vasanthi et al., 2019).
Figure 2: Forms of mercury in environment.

Mercury uptake can be energy-dependent or passive depending on state of mercury (Aschner et al., 2010). Water soluble inorganic mercury (Hg2+) or mercury in mercuric or mercurus salt can be absorbed (15%) through digestive tract whereas, methyl mercury absorption is 90-95% in food. Most of the methyl mercury is found in the muscles (80-100%). Mercury can be absorbed through gills, gastrointestinal tract and very little amount through skin. The inorganic Hg can cross the epithelia and bound with plasma proteins and transported to different organs via systemic circulation (Aschner et al., 2010; Rodriguez et al., 2015).
In Rainbow trout (Oreochromis mykiss) major part of whole blood methyl mercury (90%) efficiently binds with beta chain of haemoglobin of RBC (Jasim et al., 2016). Due to the lipophilic property of methylmercury, it can easily pass the gut cell membrane and enters into the cell. Then methyl mercury can bind reversibly to sulphur containing amino acid (Cysteine). Therefore, cellular molecules like glutathione (GSH) can easily bind with methyl mercury (Morcillo et al., 2017). The cysteine bound form facilitates its transport to sensitive tissues like brain by an L-neutral amino acid transport system.
In the digestive tract, methyl mercury is absorbed and transported to blood plasma and started to distributed in different tissue. Jasim et al., (2016) reported that liver accumulate more amount of mercury than gill and muscles (liver> gills> muscles) in Oreochromis niloticus. Methyl mercury can readily bind to metalloproteins and metallothioneines. About 10% of total ingested methyl mercury entrapped into central Nervous system (CNS) as it can pass blood brain barrier and the rest is transported to liver and kidney, from where it is excreted through urine or bile (Rodriguez et al., 2015). It is also proved that not only methyl mercury but also total mercury amount also put some deleterious impact on freshwater fishes (Subhavana et al., 2020).
Copper: Copper is an essential trace element for the living organisms. This is very much necessary for completing the metabolic reaction for growth of any organism. Copper is particularly important in activating cuproenzymes that catalyses many important metabolic reactions of living organisms. However, this element may be converted to hazardous substance if exposed beyond its permissible limit (50 ppm.) for long time. Extensive use of copper in agriculture, and industries like textile, tanneries, paints, battery, laundry, photographic studio, copper ware manufacturer, pipe making industries introduce the copper in high amount in the environment, becoming the principal source of contamination (Table 1).
Copper is essential element for living organisms for its involvement in many biological processes like oxidative phosphorylation, gene regulation and also acts as cofactor for enzymes but the metal becomes toxic when it exceeds its tolerance level in the surrounding aquatic medium. The main route of entry is through gill and dietary uptake whereas a very little amount of the metal can be taken up through skin (Padrilah et al., 2018). After entry copper bind to the plasma proteins and carried to different organs of the fish. Particularly, copper becomes toxic when an excessive amount of copper entered into the cell and binds to the cellular proteins and nucleic acid altering the natural metabolic reactions and gene expression. During chronic exposure at high concentration, Copper first accumulates in gill at higher concentration at which it may be toxic (Padrilah et al., 2018).
Then the metal gets absorbed into the plasma. Similarly, plasma takes the metal from the gut cells as well (Annabi et al., 2013). Then the metal is distributed in other organs like liver, spleen and kidney through the blood and bioaccumulated. Several researchers showed that liver is the main depot for the copper accumulation (Rajkowska and Protasowicki, 2013). Das and Gupta (2013) reported the accumulation of copper in the selected fish (Esomus danricus) organs as follows: liver>gill>kidney>flesh>bones>brain. Uptake and accumulation of copper in fish body is highly regulated by physico-chemical parameters of water such as pH, hardness, alkalinity, presence of inorganic and organic matter etc. (Malhotra et al., 2020).
Major histopathological alterations in gill, liver and kidney due to heavy metal accumulation in fresh water fishes: Accumulation of heavy metals leads to cellular level, tissue level or organ level toxicity. Chronic exposure to different heavy metals causes various deleterious impact on fish organs. Histopathological study proves the degree of metal infestation though impacts are dose/concentration and time of exposure dependent, organ sensitive and organism specific. In ecotoxicological studies, histopathology of the sensitive organs has been highly recommended as a biomarker of evaluation of stress due to metal contamination.
Gill, liver and kidney are most sensitive to metal pollution thus histopathological studies of these organs become an unavoidable tool for evaluation of metal stress in the environment (Table 2). Several researchers have reported different types of heavy metal induced tissue degradation in different piscian models (Bakshi, 2016; Morcillo et al., 2017; Bakshi and Panigrahi, 2018). Long-term exposure to heavy metals even in very low amounts generally leads to leakage of cellular pathology marker enzymes in diferent tissues of fsh (Islam, 2019; Mustafa, 2020).
Table 2. Histopathological alteration of gill, liver and kidney due to chronic exposure to sub-lethal concentrations of selected heavy metals.
| Selected Heavy Metals | Major histopathological alterations of different organs due to chronic metal exposure to sub-lethal concentrations | References | ||
| Gill | Liver | Kidney | ||
| Arsenic | Epithelial hyperplasia, lifting and oedema, lamellar fusion, desquamation aneumerism, and necrosis | Focal lymphatic and macrophage infiltration, congestion, sinusoid dilation a
& swelling, vacuolization and shrinkage of hepatocytes, necrosis |
Pycnotic nuclei, vacuolization of tubular cells, glomerular shrinkage, lumen enlargement, necrosis. | Ahmed et al. (2013b); Morcillo et al. (2017) |
| Cadmium | Hyperplasia, increase in chloride cells, reduced and shortened length of secondary gill lamellae | Dissociation of hepatocytes, Necrosis, blood congestion in liver sinusoids, vacuolization | Disorganization and degeneration of renal epithelial cells, reduction in glomerulus, hypertrophy, dialation of bowman’s capsule, focal necrosis | Ahmed et al. (2014); Kaur et al. (2018) |
| Chromium | Lamellar disorganization, Necrosis in epithelial cells, atrophied central axis. | Hyperplasia, Necrosis of hepatocytes, Reduced N-C ratio. | Highly fenestrated Bowman’s capsule, Constricted lumen of renal tubes, glomerular disorganization, | Velma et al. (2009); Velma and Tchounwou, (2009); Bakshi, (2016); Bakshi and Panigrahi, (2018) |
| Lead | Hyperplasia, hypertrophy and destruction or disintegration of lamellar architecture, lamellar clubbing and fusion of lamellae. | Disarrangements of hepatic cords, shrinkage of hepatocytes, dialation of sinusoids, exudation of blood, loss of cell adherence of hepatocytes. | Degeneration of renal epithelium, vacuolization, nuclear pycnosis. Renal tube atrophy, oedema, necrosis | Mobarak and Sharaf, (2011); Ahmed et al. (2014); Mustafa (2020) |
| Mercury | Mild congestion and oedema in primary lamellae, hyperplasia, desquamation in epithelial lining secondary lamellae, hyperactivity of chloride and mucous cells, Increase in RBC, macrophages | Vacuolization, Hypertrophy of hepatocytes, intravascular hemolysis, nuclear pycnosis, congestion in central vein, necrosis | Hydropic swelling of tubules, pycnotic nuclei, swelling of proximal convoluted tubule with necrotic nuclei. | Kaviraj, (1983); Kaoud and El-Dahshan, (2010); Selvanathan et al. (2013) |
| Copper | Lifting of Lamellar epithelium, RBC exudes, Necrosis, fusion of adjacent lamella, hyperplasia, oedema | Necrosis, vascular hemorrhage, dilated
sinusoids and vacuolar degeneration |
Damage and degeneration of renal tubules, glomerular oedema, Necrosis, | Nandan and Kumar, (2014); Atabati et al. (2015); Al-Tamimi et al. (2015) |
CONCLUSION
In this review we have compiled the uptake and accumulation process of some heavy metals (viz., Arsenic, Cadmium, Copper, Chromium, Lead and Mercury) of fishes. The bioaccumulation process of these metals poses serious impact on the aquatic food chain also. The magnified concentration of different heavy metals leads to higher mortality rate in fish eating organisms especially aquatic birds. Fish is consumed as a primary source of protein thus contamination of heavy metals can be very dreadful to human being also. To cope up with the serious environmental threat effective legislation guidelines and regular monitoring are highly required. Failure to control the contamination will lead to severe complication in near future because of the imposed adverse impact of the heavy metals. Monitoring the exposure, release of the heavy metals and probable intervention for reducing additional exposure in environment can become a momentous step towards control measures. State, National and international co-operation is very important for framing ideal tactics to avoid the consequences of heavy metal toxicity.
Conflict of Interest :Authors solemnly declare that there is no conflict of interest to disclose.
Contribution of Authors: Avijit Bakshi: Conceptualization, Methodology, Software, Investigation, Data curation, Writing Original Draft; Ashis Kumar Panigrahi: Supervision, resources, visualization and editing; S. Pattanaik: Supervision, Editing.
ACKNOWLEDGEMENTS
Authors are very much thankful to the authorities of Department of Zoology, University of Kalyani, India for their cordial supports for carrying out the research.
REFERENCES
Ahmed MK, Parvin E, Islam MM, Akter MS, and Al-Mamun MH (2014). Lead and cadmium-induced histopathological changes in gill, kidney and liver tissue of freshwater climbing perch Anabas testudineus (Bloch, 1972). Chemistry and Ecology, Vol 30,2014, issue 6, pp 532-540. http://doi.org/10.1080/02757540.2014.889123.
AMAP (1998). Assessment report: Arctic pollution issues. Arctic Monitoring and Assessment Programme, Oslo.
AMAP (2002). Arctic Pollution 2002. Arctic Monitoring and Assessment Programme, Oslo.
Annabi A, Said K and Messaoudi I (2013). Cadmium: Bioaccumulation, histopathology and detoxifying mechanisms in fish. American Journal of Research Communication, 4(1), 60– 79.
Aschner M, Onishchenko N and Ceccatelli S, (2010). Toxicology of alkylmercury compounds, In: Sigel A, Sigel H, Sigel RKO, Organometallics in Environment and Toxicology, Cambridge, UK: RSC Publishing, 403-434.
Atabati A, Keykhosravi A, Askari-Hesni M, Vatandoost J and Motamedi M (2015). Effects of copper sulfate on gill histopathology of grass carp (Ctenopharyngodon idella). Iranian Journal of Ichthyology, 2(1), 35–42.
Athikesavan S, Vincent S, Ambrose T and Velmurugan B (2006). Nickel induced histopathological changes in the different tissues of freshwater fish, Hypophthalmichthys molitrix (Valenciennes). J. environ. Biol., 27: 391-395.
ATSDR (1999). Toxicol. Profile of Cadmium. Agency for Toxic Substances and Drug Registry, Atlanta, GA. US Deptt. of Health and Human Services.
Bakshi A and Panigrahi AK (2018). A Comprehensive Review on Chromium induced Alterations in Fresh Water Fishes, Toxicology Reports 5 (2018) 440-447. http://doi.org/10.1016/j.toxrep.2018.03.007.
Bakshi A, (2016). Analysis of anthropogenic disturbances and impact of pollution on fish fauna of River Churni with special reference to Chromium pollution. (Doctoral Dissertation). Kalyani University, Kalyani, India. pp-188.
Bureau of Indian Standards (BIS) 10500 (2012). Specification for drinking water. Indian Standards Institution, New Delhi, pp1-5.
Central Water Commission Report (2014). Status of Trace and Toxic metals in Indian Rivers, Ministry of Water Resources, New Delhi, Government of India. pp 1-185.
Central Water Commission Report (2018). Status of Trace and Toxic metals in Indian Rivers, Ministry of Water Resources, New Delhi, Government of India. pp 1-225.
Das S and Gupta A (2013). Accumulation of copper in different tissues and changes in oxygen consumption rate in Indian Flying Barb, Esomus danricus (Hamilton Buchanan) exposed to sublethal concentrations of copper. Jordan Journal of Biological Sciences, 6(1), 21–24.
Haines TA and Brumbaugh WG (1994). Metal concentration in the gill, gastrointestinal tract, and carcass of white suckers (Catostomus commersoni) in relation to lake acidity. Water, Air, and Soil Pollution, Vol 73(1) pp 265-274. http://doi.org/10.1007/BF00477991.
Han J-M, Park H-J, Kim J-H, Jeong D-S and Kang J-C (2019). Toxic effects of arsenic on growth, hematological parameters, and plasma components of starry flounder, Platichthys stellatus, at two water temperature conditions. Fisheries and Aquatic Sciences, Vol 22, issue 3, pp 1-8. https://doi.org/10.1186/s41240-019-0116-5.
Islam A (2019). Assessment of heavy metals concentration in water and Tengra fsh (Mystus vittatus) of Surma River in Sylhet region of Bangladesh. Arch. Agric’. Environ. Sci., Vol 4, pp 151–156.
Jaishankar M, Mathew BB, Shah MS and Gowda KRS (2014). Biosorption of Few Heavy Metal Ions Using Agricultural Wastes. Journal of Environment Pollution and Human Health 2(1): 1–6.
Jasim MA, Sofian-Azirun M, Yusoff I and Rahman M (2016). Bioaccumulation and Histopathological Changes induced by Toxicity of Mercury (HgCl2) to Tilapia Fish Oreochromis niloticus. Sains Malaysiana 45(1)(2016): 119–127.
Jezierska B and Witeska M (2001). Metal Toxicity to Fish, Wydawnictwo Akademii Podlaskiej, Siedlce 318 pp.
Jezierska B and Witeska M (2006). The metal uptake and accumulation in fish living in polluted waters. In. Twardowska et al. (eds.). Soil and Water Pollution Monitoring, Protection and Remediation. NATO Science Series, Vol 69. Springer, Dordrecht. ISBN- 978-1-4020-4726-8, ISBN (online) 978-1-4020-4726-2. doi: http://doi.org/10.1007/978-1-4020-4728-2_6.
Ju-Wook L, Choi H, Hwang, Kang UK, Kang YJ, Kim KI and Kim JH (2019). Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ Toxicol Pharmacol.2019; 68:101-108. doi: 10.1016/j.etap.2019.03.010.
Kaoud HA and El-Dahshan AR (2010). Bioaccumulation and histopathological alterations of the heavy metals in Oreochromis niloticus fish. Nature and Science, 2010;8(4).
Kaur S, Khera KS and Kondal JK, (2018). Heavy metal induced histopathological alterationin liver, muscles and kidney of freshwater cyprinid, Labeo rohita (Hamilton). Journal of Entomology and Zoological Studies 2018; 6(2): pp 2137-2144.
Khan MS, Javed M, Rehman MT, Urooj M and Ahmad MI (2020). Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci Rep, vol 10, https://doi.org/10.1038/s41598-020-73468-4.
Kumar P, Prasad Y, Ranjan R, Swarup D, Pattanaik AK and Patra RC (2009). Accumulation Pattern of Cadmium in Tissues of Indian Catfish Clarias batrachus. Animal Nutrition. and Feed Technol., 8(1): 115-119.
Kumari B, Kumar V, Sinha AK, Ahsan J, Ghosh AK, Wang H and DeBoeck G (2016), Toxicology of arsenic in fish and aquatic systems. Environ Chem Lett. DOI 10.1007/s10311-016-0588-9.
Lodhi HS, Khan MA, Verma RS and Sharma UD (2006). Acute toxicity of copper sulphate to fresh water prawns. J. Environ. Biol. 27, 585-588.
Łuszczek-Trojnar E, Drąg-Kozak E and Popek W (2013) Lead accumulation and elimination in tissues of Prussian carp, Carassius gibelio (Bloch, 1782), after long-term dietary exposure, and depuration periods. Environ Sci Pollut Res (2013) 20:3122–3132. DOI 10.1007/s11356-012-1210-8.
Malhotra N, Ger T-R, Uapipatanakul B, Huang J-C, Chen KH-C and Hsiao C-D (2020). Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanoparticles, vol 10, 1126. doi:10.3390/nano10061126.
Mallesh B, Pandey PK, Kumar K, Vennila A and Kumar S (2015) Bioconcentration of hexavalent chromium in Cirrhinus mrigala (Ham 1822): effect on haematological parameters. J. of Bio. & E. Sci.; 5 (1): 59-67. ISSN-2084-3577.
Martinez-Finley EJ and Aschner M (2014) Recent Advances in Mercury Research, Curr Envir Health Rpt (2014) 1:163–171. DOI 10.1007/s40572-014-0014-z.
Mathew BB, Tiwari A and Jatawa SK (2011). Free radicals and antioxidants: A review. Journal of Pharmacy Research 4(12): 4340–4343.
Mehana E-SE, Khafaga AF, Elblehi SS, Abd El-Hack ME, Naiel MAE, Bin-Jumah M, Othman SI and Allam AA (2020). Biomonitoring of Heavy Metal Pollution Using Acanthocephalans Parasite in Ecosystem: An Updated Overview. Animals. [Online]. Vol 10, issue 5. pp 811. Available from: http://dx.doi.org/10.3390/ani10050811.
Mendil D, Uluozlu OD, Hasdemir E, Tuzen M, Sari H and Suic-mez M (2005). Determination of trace metal levels in seven fish species in lakes in Tokat, Turkey. Food Chem.90,175–179.
Mobarak YMS and Sharaf MM (2011). Lead acetate –induced histopathological changes in in the gills and digestive system of Silver Sailfin Molly (Poecilia latipinna) Int. J. Zool. Res. Vol 7, issue 1, pp 1-18. DOI 10.3923/ijzr.2011.1.18.
Morcillo P, Esteban EA and Cuesta A (2017) Mercury and its toxic effects on fish. AIMS Environmental Science, 4(3): 386-402. DOI: 10.3934/environsci.2017.3.386.
Mustafa SA (2020). Histopathology and heavy metal bioaccumulation in some tissues of Luciobarbus xanthopterus collected from Tigris River of Baghdad, Iraq. Egyptian Journal of Aquatic Research 46 (2020) pp- 123–129. https://doi.org/10.1016/j.ejar.2020.01.004.
Okocha RC and Adedeji OB (2011) Overview of Cadmium Toxicity in Fish, Journal of Applied Sciences Research, 7(7): 1195-1207, 2011. ISSN 1819-544X.
Padrilah SN, Sabullah MK, Shukor MYA, Yasid NA, Shamaan NA and Ahmad SA (2018). Toxicity effects of Fish histopathology on Copper accumulation. Pertanika J. Trop. Agric. Sci. 41 (2): 519 – 540 (2018).
Praveena M, Sandeep V, Kavitha N and Jayantha Rao K (2013). Impact of tannery effluent, chromium on Hematological parameters in a fresh water Fish, Labeo Rohita (Hamilton). Res. J. Animal, Veternary and Fishery Sci. Vol. 1(6), 1-5, July (2013). ISSN-23206535.
Rabitto IS, Alves Costa JRM, Silva de Assis HC, Pelletier E, Akaishi FM, Anjos A, Randi MAF and Ribeiro O (2005). Effects of dietary Pb(II) and tributylin an neotroptical fish Hoplias malabarius: histopathological and biochemical findings. Ecotoxicol Environ Saf 60:147–156.
Raihan SM, Moniruzzaman M, Park Y, Lee S and Bai, SC (2020) Evaluation of Dietary Organic and Inorganic Mercury Threshold Levels on Induced Mercury Toxicity in a Marine Fish Model. Animals 2020, 10, 405; doi:10.3390/ani10030405.
Rajkowska M and Protasowicki M (2013). Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in Northwestern Poland. Environmental Monitoring and Assessment, 185(4), 3493–3502.
Rice KM, Walker Jr EM, Wu M, Gillette C and Blough ER (2014) Environmental Mercury and Its Toxic Effects. J Prev Med Public Health 2014; 47:74-83• http://dx.doi.org/10.3961/jpmph.2014.47.2.74.
Rodríguez JZ, Gallegoríos SE and Ramírez Botero M (2015). Content of Hg, Cd, Pb and As in fish species: A review. Vitae, Revista de la facultad de ciencias farmaceuticus alimentarias. ISSN 0121-4004 / ISSNe 2145-2660. Volumen 22 número 2, año 2015. Universidad de Antioquia, Medellín, Colombia. págs. 148-159. DOI: http://dx.doi.org/10.17533/udea.vitae.v22n2a09.
Saha N and Zaman MR (2011). Concentration of selected toxic metals in groundwater and some cereals grown in Shibganj area of Chapai nawabganj, Rajsahi, Bangladesh. Curr. Sci. 101, 427-431.
Selvanathan J, Vincent S and Nirmala A (2013). Histopathology changes in fresh water fish Clarias batrachus(linn.) exposed to mercury and cadmium. International Journal of Life Science and Pharma Research, (3):2.
Sharma RK and Agarwal M (2005). Biological effects of heavy metals, an overview. J. Environ. Biol. 26, 301-313.
Shaukat N, Javed M, Ambreen F and Latif F (2018) Oxidative Stress Biomarker in Assessing the Lead Induced Toxicity in Commercially Important Fish, Labeo rohita, Pak. J. Zool. (2018). DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.2.735.741.
Singh N, Kumar D and Sahu A (2007). Arsenic in the environment: effects on human health and possible prevention. J Environ Biol 28(2 Suppl): 359–365.
Subhavana KL, Keerthana RT and Qureshi A (2020). Mercury in Marine, Freshwater and Aquaculture Species from South India and Human Exposure Risk Assessment. Expo Health, vol-12, pp 897–903. https://doi.org/10.1007/s12403-020-00352-x.
Sumet HD and Blust R (2001). Stress responses and changes in protein metabolism in carp Cyprinus carpio during cadmium exposure. Ecotoxicol. Environm. Saf., 48(30): 255-262.
Sweet LI and Zelikoff JT (2001) Toxicology and immunotoxicology of mercury: a comparative review in fish and humans. J Toxicol Environ Heatlth B 4: 161-205.
Tokar EJ, Boyd WA, Freedman JH and Waalkes MP (2015) Toxic effects of metals. Casarett and Doull’s Toxicology. 8th ed. McGraw-HilL; 2015. p. 933–980.
Tsai JW and Liao CM (2006) Mode of action and growth toxicity of arsenic to tilapia Oreochromis mossambicus can be determined bioenergetically. Arch Environl Contam Toxicol 50:144–152
Tsai JW, Huang YH, Chen WY and Liao CM (2012) Detoxification and bioregulation are critical for long-term waterborne arsenic exposure risk assessment for tilapia. Environ Monit Assess 184(1):61–572
Vasanthi, N., Muthukumaravel, K., Sathick, O. and Sugumaran, J., (2019) Toxic effect of mercury on the freshwater fish Oreochromis mossambicus. Res J. Lsc, Bioin. Pharma. & chem. Sci. ISSN 2454-6348. DOI: 10.26479/2019.0503.30.
Velma V and Tchounwou PB (2009). Hexavalent Chromium-induced multiple biomarker responses in liver and kidney of Gold fish, Carassius auratus, Environ. Toxicol. 26, pp. 649-656.
Velma V, Vutukuru SS and Tchounwou PB (2009). Ecotoxicology of Hexavalent Chromium in fresh water fish: A critical review. Rev. Environ. Health: 24(2): pp.129-145.
Vesey DA (2010). Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Letters., 198(1): 13-19.
Vutukuru SS (2005). Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian Major Carp, Labeo rohita. Int. J. Environ. Res. Public Health 2:456-462.
Vutukuru SS, Prabhat NA, Raghavender M and Yerramilli A (2007). Effect of arsenic and chromium on the serum amino-transferases activity in Indian major carp, Labeo rohita. Int. J. Enviromen Res Public Health 4(3): pp 224-227 [PubMed: 17911661].
Wang JF, Bashir M, Engelsberg BN, Witmer C, Rozmiarek C and Billings PC (1997). High mobility group proteins 1 and 2 recognize chromium damaged DNA. Carcinogenesis, 18:371-375.
Wong CK and Wong MH (2000). Morphological and biochemical changes in the gills of Tilapia (Oreochromis mossambicus) to ambient cadmium exposure. Aquatic Toxicol., 48(4): 517-527.
Wright DA, Metyer MJ and Martin FD (1985). Effect of calcium on cadmium uptake and toxicity in larvae and juveniles of striped bass (Morone saxatilis) Bull. Environ. Contam. Toxicol., 34: 196-204.


