Centre for Drug Discovery and Development, Sathyabama Institute of Science and
Technology (Deemed to be University), Chennai – 600 119, Tamil Nadu, India.
Corresponding author email: mrkactinos@gmail.com
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 15/09/2020
Tuberculosis (TB) is a contagious airborne disease caused by a bacterium, Mycobacterium tuberculosis (MTB). Emergence of multi drug resistant (MDR) and extensively drug resistance (XDR) among M. tuberculosis strains urged the situation to discovery novel anti TB antibiotics. Many assays were developed based on the whole cell and target, but each having some limitations. Being a slow grower as well as air borne pathogen, adopting suitable assay for anti TB screening is challenging. Here, we discussed about the employment of Luciferase Reporter Phage (LRP) assay for the screening of wide range of compounds against M. tuberculosis strains including drug sensitive and drug resistant strains. Literature articles published between 2006 to March 2020 in reputed journal were collected through searching web of science, pubmed and other sites. This review focus on the articles published on screening of extracts and compounds from natural products, synthetic compounds and nanoparticles from the year 2006 – March 2020 by using LRP assay.
Among the whole cell assays, LRP assay provide the results in 72 hours and this assay can be used as preliminary identification of potential anti-TB compound. Hence, LRP assay is a rapid, simple and sensitive assay to screen natural molecules and synthetic compounds to determine their anti TB activity. However, limitations associated with mycobacteriophage entry into the mycobacterial cell need to be optimized to improve its sensitivity. Understanding the importance and advantages of employing LRP assay as an effective high throughput screening method helps in the significant screening of wide range of antimycobacterial agents in a relatively short time of incubation. This assay can effectively help in the development of new potential drug candidates against tuberculosis.
Tuberculosis, Luciferase reporter phage assay, Screening, anti TB, drug Discovery
Anbarasu S, Revathy K, Radhakrishnan M, Krupakar P, Joseph J, Kumar V. Luciferase Reporter Phage (LRP) Assay for Anti Tuberculosis Screening: Current Status and Challenges. Biosc.Biotech.Res.Comm. 2020;13(3).
Anbarasu S, Revathy K, Radhakrishnan M, Krupakar P, Joseph J, Kumar V. Luciferase Reporter Phage (LRP) Assay for Anti Tuberculosis Screening: Current Status and Challenges. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2XlnQuL
Copyright © Anbarasu et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Tuberculosis (TB) is a communicable disease that is a major cause of ill health, one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent. Globally, an estimated 10.0 million people fell ill with TB in 2018, a number that has been relatively stable in recent years. The burden of disease varies enormously among countries, from fewer than five to more than 500 new cases per 100 000 population per year, with the global average being around 130. There were an estimated 1.2 million TB deaths among HIV-negative people in 2018, and an additional 251 000 deaths among HIV positive people (Global Tuberculosis Report, 2019).
Tuberculosis pose serious problem around the world by the way of increase in the rate of HIV-related TB, pediatric TB, latent TB, MDR- TB and XDR-TB. Drug-resistant TB continues to be a public health threat. In 2018, there were about half a million new cases5 of rifampicin-resistant TB (of which 78% had multidrug resistant TB). The three countries with the largest share of the global burden were India (27%), China (14%) and the Russian Federation (9%). Globally, 3.4% of new TB cases and 18% of previously treated cases had multidrug resistant TB or rifampicin-resistant TB (MDR/RR-TB), with the highest proportions (>50% in previously treated cases) in countries of the former Soviet Union. The treatment for tuberculosis requires 6-8 months for new cases and 18-24 months for MDR TB with more toxic drugs and the treatment options for XDR-TB are seriously limited.
The risk of serious adverse events such as hepatotoxicity, discourage both patients and providers (Menzies et al., 2011). Hence, there is always an urgent need for the development of potential candidate to fight against drug resistant strains with improved activity, novel mechanism, with a short duration for treatment by fast acting mechanism. This necessarily leads to the interest in screening the new compounds for their antimycobacterial activity. Both target based and whole cell screening approaches are in practice for anti TB drug discovery however both of them has its own merits and limitations. Conventional whole-cell screening assays were found to have a higher success rate in identifying a series of hits possessing beneficial properties (Kumar et al., 2017).
It is well known that, Mycobacterium tuberculosis is a slow-growing pathogenic organism and its complex cell wall with mycolic acids and other lipid contents, poses numerous restrictions for anti-TB drug research and development (Favrot et al., 2012). Due to the slow growing nature of M. tuberculosis, a screening technique based on growth is difficult and new assays for antimycobacterial screening of natural products and synthetic compounds are required (Forbes et al., 2015).
Background and Purpose : Native compounds acquired from microbial resources and medicinal cultivars have played an essential part as the origin of TB medications (Sari et al., 2019). Slow growth rate of the Mycobacterium species and their long incubation period remains a major obstacle in the antimycobacterial drug discovery process. Drug susceptibility method using egg or agar is the standard method being used for the evaluation of antimycobacterial agents. However, the assay is labor intensive and the incubation time requires upto 2 months (Rakhmawatie et al., 2019). LRP assay is a high throughput screening method and has been used to evaluate the natural, synthetic and nanoparticles for their antimycobacterial activity (Sivaraj et al., 2020). This assay reveals the potential of native compounds in 3-4 days to behave as an antimycobacterial compound. Understanding the importance and efficiency of LRP assay and application of this assay for the screening process against M. tuberculosis strains can help the effective drug discovery process against tuberculosis.
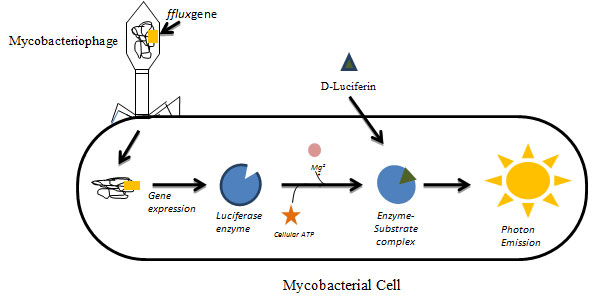
Luciferase Reporter Phage (Lrp) Assay: Luciferase reporter phage assay (LRP) assay utilizes genetically modified mycobacteriophages expressing luciferase gene fflux. Mycobacteriophage have been used as diagnostic tools for tuberculosis and also have various applications in mycobacteriology including gene replacement, development of integration proficient vectors, systems for mycobacterial gene expression, mycobacterial cell wall analysis, delivery systems for reporter phages & transposons, etc,(Parikh et al., 2013; Hatfull, 2014; Fu et al., 2015). In principle, when the constructed phage infects the viable mycobacterial cell, the luciferase gene gets expressed upon addition of luciferin substrate which results in emission of light in the presence of cellular ATP and Mg2+. The emitted light is measured using luminometer which displays proton as Relative Light Unit (RLU) (Fig.1).
Figure 1: Luciferase Reporter Phage (LRP) Assay: Genetically modified mycobacteriophages expressing luciferase gene (fflux gene) infecting viable mycobacterial cells. The gene gets expressed and upon the addition of substrate luciferin results in emission of measurable light in the presence of cellular ATP and Mg2+. The emitted light is measured in the luminometer.
The first evaluation of the diagnostic LRP assay in sputum samples using phAE142 (mycobacteriophage) was reported by Banaiee et al (2001). The authors found the assay comparable with MGIT 960 in sensitivity, specificity and speed. Similar results were reported by Bardarov et al., (2003) as National Institute for Research in Tuberculosis (formerly Tuberculosis Research Centre) at Chennai, India, facilitated an ideal, simple and reliable diagnostic LRP assay using phage Che12 and it was found to be the first ever temperate phage capable of infecting and lysogenising M. tuberculosis. The specificity of the mycobacteriophages to infect mycobacteria has been effectively put in to use for rapid detection of M. tuberculosis in sputum (Kumar et al., 2008). Luciferase phages or M. tuberculosis strains expressing luciferase genes may permit rapid screening of drugs for antituberculosis activity.
In this context, LRP assay has been successfully employed for rapid screening of various natural and synthetic and semi-synthetic compounds against M. tuberculosis including drug resistant strains and dormant TB bacilli. Limitations associated with mycobacteriophage entry into the mycobacterial cell need to be optimized to improve its sensitivity.The authors have also revealed that this might beachieved by engineering of better characterized mycobacteriophages to allow higher expression of luciferase. The constructed mycobacteriophages such as L5 and D29 have various defects remain in LRP assay especially the mild L5 mycobacteriophage is unable to infect the M. tuberculosis complex, which limits its application in the drug resistance M. tuberculosis strain detection in the sample (William et al., 1993). In LRP assay, the relative light units can be detected within a few minutes following LRP infection of the live mycobacterial when the sample contains at least 104/milliliter of M. tuberculosis. The lytic characteristics of D29 and TM4 result in the loss of light output and reduced sensitivity (Fu et al., 2015).
Kumar et al., (2008) have constructed new LRPs using the mild Che12 bacteriophage to increase light output and improved the sensitivity of the assessment. Temperate phage integrate into the host genome at specific sites and replicate along with cells. Che12, first reported temperate mycobacteriophage capable of infecting and lysogenising M. tuberculosis isolated from soil. LRPs developed from temperate mycobacteriophages could be ideal since it does not cells do not lyse, increased light output at a give point could facilitate designing assay format and there is continuous expression of luciferase. Solvents like DMSO make cell membrane more permeable and using it in substrate preparation could ensure its better entry into cells. Setting up the primary liquid culture in dilutions could establish ideal multiplicity of infection and ensure cells are in continuous log phase.
Protocol for Lrp Assay: Briefly, about 350 µl of Middle brook 7H9 broth, 100 µl of M. tuberculosis cell suspension (#2 McFarland) and50 µl of test compound (example: 50 µg/ml; 100 µg/ml; 500µg/ml) was transferred to a 1.8 ml sterile cryovial. The negative control was a cryovial containing 400µl of Middle brook 7H9 broth and 100 µl of M. tuberculosisH37Rv cell suspension (#2 McFarland). After 72 hours of incubation at 37oC, about 50 µl of high titre mycobacteriophage (titre-6.5×109pfu/ml, the phage was kindly gifted by Dr. Vanaja Kumar, NIRT, Chennai)in addition 40µl of 0.1M CaCl2 solution was added into both test and control cryovials (called cell-phage mixture) and incubated at 37°C. After 4 hours of incubation, 100µl of cell-phage mixture waspipette out and transferred to luminometer cuvette.
Then the D-Luciferin (100 µl) substrate was added to luminometer cuvette and relative light unit (RLU) were measured at 10 seconds integration in luminometer (Model: LB9508 Lumat3; make: Berthold, Germany). Compounds/extracts showing RLU reduction by 50% or more when compared to control will be considered as having antimycobacterial activity. Percentage RLU reduction = Control RLU– Test RLU / Control RLU X 100 (Radhakrishnan et al., 2014). The above mentioned procedure could be applied for drug resistant M. tuberculosis.
Anti-Tb Activity Screening By Lrp Assay:
Anti TB natural products: Although different types of anti-TB agents are available in world market, there is a growing interest in natural products for novel anti-TB drug discovery, due to non-specific side effects associated with synthetic therapeutics agents and unusual chemical diversity present in natural products. Natural products have been recognized as the source of most active ingredients of medicine. More than 80% of drugs available in world market were derived from natural products or inspired by them. Natural products derived scaffolds are therapeutic templates for the design of new therapeutic drugs using medicinal chemistry and computer-assisted design techniques. Thus, they have a remarkable impact on the treatment of TB in comparison with classical FDA-approved drugs such as rifampicin, kanamycin and cycloserine. Anti-TB compounds isolated from natural sources such as plants, microbes and marine organisms have been found with different skeleton chemical forms and conformations (Conti et al., 2016; Lei et al., 2016).
Medicinal plants: Plants have been used worldwide in traditional medicines for the treatment of various diseases and it is estimated that even today approximately 80% of the world’s population rely on medicinal plants as the primary source of medicines (Ekor, 2014). The phytochemical study of some of these plants has yielded a number of active natural products. Next to microorganisms, plants are an important source for anti-TB compounds (Singh et al., 2015).
Few studies used LRP assay to screen the plant extracts/compounds for their antimycobacterial properties. Ignacimuthu and Shanmugam (2010) have studied the antimycobacterial activity of two compounds viz.vasicine acetate and 2-acetyl benzylamine obtained from Adhatoda vasica. The results showed that vasicine acetate recorded 99.96%, 97.68 % and 98.93% of RLU reduction against M. tuberculosis, drug resistant M. tuberculosis and drug sensitive M. tuberculosis respectively. Whereas 2- Acetyl benzylamine showed a reduction of RLU by 98.93%, 95.55% and 98.81% in M. tuberculosis, drug resistant M. tuberculosis and 98.81% drug sensitive M. tuberculosis. Antony et al. (2012) have tested the solvent extract of different parts of the plant Alstonia scholaris against three different strains of M.tuberculosis by using LRP assay in which the butanol extract of bark showed potential activity against resistant strains at 500µg/ml concentration. Anti-TB activity of ethyl acetate and ethanol extracts of Sidarhom bifolia L was tested against M.tuberculosis H37Rv and SHRE resistant M.tuberculosis at 100µg/ml and 500µg/ml concentration by using LRP assay. Results showed that ethyl acetate extracts have potent antimycobacterial activity whereas ethanolic extract has no activity (Papitha et al., 2013).
Muthuswamy et al., (2013) have tested 32 plants for antimycobacterial activity against M. tuberculosis H37Rv, MDR M. tuberculosis and sensitive M. tuberculosis. Out of 32 plants, 7 plants were shown to have potent activity against three strains of M. tuberculosis when tested at 500 µg/ml. Authors concluded that Ruta graveolens extract exhibited good antimycobacterial activity against M. tuberculosis H37Rv (76.60%), MDR M. tuberculosis, (87.25%) and sensitive M. tuberculosis (94.32 %) at 100µg/ml concentration. Prabu et al., (2014) have tested the antimycobacterial activity of seven mangrove plants; Ceriops decandra, Aegiceras corniculatum, Excoecaria agollacha, Avicennia officinalis, Rhizophora mucronata, Suaeda monoica and Sesuvium portulacastrum.
They tested both methanol and hexane extracts of all the mangrove plants using LRP assay and the results showedthat methanol extracts have good inhibition at 500 µg/ml against the mycobacterial strains tested whereas hexane extract showed less or no inhibitory activity. The result of the study concluded that the E. agollacha possess a significant inhibitory activity among the seven mangrove plants tested against M. tuberculosis H37Rv, drug sensitive M. tuberculosis and drug resistant M. tuberculosis.The various solvent extracts of Euphorbia hirta leaves viz. methanol extract, n-Hexane extract and Ethyl acetate extract were screened against M.tuberculosis H37Rv at 250 µg/ml and 500µg/ml concentration by LRP assy. The ethyl acetate extract showed 64% RLU reduction at 500µg/ml concentration. In another study, the methanol, ethyl acetate and hexane extract of leaves from T. procumbens were tested by LRP assay against M.tuberculosis H37Rv at 500 and 250µg/ml concentration. This study revealed that the methanol and hexane extracts have both shown antimycobacterial activity at 500 µg/ml (Rajasekar et al., 2015;2016).
Prabu et al., (2015) have tested the hexane and methanol extract of Andrographis paniculata leaves at two different concentrations against M.tuberculosis H37Rv, SHRE sensitive M.tuberculosis and SHRE resistant M. tuberculosis. Among the extracts tested by LRP assay, the methanol extract possess antimycobacterial activity against all the three strains tested. In a study by Suriyamurthy et al., (2016), the antitubercular activity of Tabubeia rosea leaves were tested. LRP assay results showed that methanol extract of the leaves inhibited the standard and clinical isolate of M.tuberculosis at 500µg/ml concentration. Muthuselvi et al., (2017) have screened the ethyl acetate extract obtained from the bark of Cassia marginata against M.tuberculosis H37Rv and SHRE resistant M.tuberculosis at 100 µg/ml and 500µg/ml concentration.
The extracts demonstrated inhibitory activity at whereas against the SHRE resistant strain the extract showed inhibitory activity at 100 µg/ml and against all strains tested at 500 µg/ml. Plumbagin is an organic compound obtained from Plumbago zeylanica. Three compounds have been derived from Plumbagin and tested for antimycobacterial activity against two clinical isolates of drug sensitive M. tuberculosis and M. tuberculosis H37Rv. Of these three compounds, two showed activity at 50 and 100 µg/ml (Nayak et al., 2014).
MI et al., (2018) investigated the methanolic extract of Dendropthoe falcata leaves for anti TB activity against M.tuberculosis H37Rv, all sensitive MTB and MDR-MTB by LRP assay was done at concentrations 100 and 500 μg/ml concentration. Test drug showed inhibition of H37Rv and all sensitive M.tuberculosis and no inhibition with MDR M. tuberculosis. The Minimal Inhibitory Concentration (MIC) of Nigella sativa seeds were determined against all three mycobacterial strains using Luciferase Reporter Phage (LRP) assay. In this study, the methanolic and water extract of N. sativa seeds showed inhibition against M. tuberculosis H37Rv, all drug sensitive M. tuberculosis, MDRM. tuberculosis respectively. The methanol extract showed least inhibition at concentration of 50 µg/ml, 250 µg/ml and 100 µg/ml against M. tuberculosis H37Rv, all drug sensitive M. tuberculosis and MDR M. tuberculosis respectively (Anbarasu et al., 2018).
Quercetin and rutin, two flavonoids were examined for antimycobacterial activities against M. tuberculosis H37Rv (ATCC 27294). The quercetin exhibited (99.30 ± 0.268%) in (LRP) assay at 200 µg/ml and 56.21 ± 0.97% inhibition in (BMD) at 50 µg/ml, whereas rutin exhibited (90.40 ± 0.68%) in LRP assay at 200 µg/ml and 56.10 ± 0.67% inhibition in BMD at 50 µg/ml. The minimum inhibitory concentration (MIC) was found to be 6.25 µg ml−1 and 25 µg ml−1 respectively. The current investigation suggests that quercetin has better inhibitory activity than rutin (Sasikumar et al., 2018).
The methanol extract of Solanum torvum and ethyl acetate extract of Vitex negundo and Zingiber mauritiana were also screened for anti-tubercular activity against M. tuberculosis H37Rv using Luciferase Reporter Phage (LRP) assay. All the three extracts were exhibited anti TB activity at 500 μg/ml concentration. In particular, the S. torvum extract showed 98.46% inhibition (Vaishnavi et al., 2020).
Marine organisms: The potential of marine organisms is well documented in the recent past. Yet, their utility for anti-TB drug discovery is still in its infancy. Until 2000, there are only two reports of in-vitro anti-TB activity from marine origin. There are very few anti-TB compounds isolated from marine macro organisms such as molluscs (kahalalides A and F), sponges (heteronemin), corals (litosterol) (De Souza et al., 2006). Bioactive substances from natural sources are available in extremely low quantities leading to limitations in using the reservoir of marine organisms for bioassay and therapy.
To overcome these problems, few methodologies such as mariculture, bioreactors, sponge cell culture, genetic modification and most importantly chemical and semi-synthetic approach can be pursued (Lindequist, 2016). Certain anti-TB compounds produced by marine sponges (agelasine) and corals (litosterol) have been synthesized by chemical methods (Mancini et al., 2007). Unfortunately, none of the several hundreds of non-microbial natural products with antimycobacterial activity have moved forward in drug development.
Amudha et al.,(2015) tested hexane and ethanol extracts of T. conoides against M. tuberculosis at 100µg/ml and 500µg/ml concentration by LRP assay. Both solvent extracts showed significant inhibitory activity at 500µg/ml concentration. Mayakrishnan et al. (2017) have screened the red algae, Kappaphycus alvarezii for antimycobacterial activity. They tested the extracts of acetone, chloroform and ethanol against M. tuberculosis H37Rv and clinical isolate of Mycobacterium tuberculosis by LRP assay. The acetone and chloroform extracts showed antimycobacterial activity against M. tuberculosis H37Rv at 500µg/ml concentration whereas all the three extracts showed antimycobacterial activity against clinical isolate of M. tuberculosis. Sundar et al., (2018) reported the antimycobacterial activity of Sargassum swartzii against the whole cell M. tuberculosis H37Rv by LRP assay. Among the extracts tested, the methanol extract, ethyl acetate, chloroform extract and aqueous extract showed inhibition at 500 μg/mL concentration against M. tuberculosis H37Rv whereas, n -hexane showed no inhibition against the strain tested.
Microorganisms: Microorganisms that live together in the environment develop long-lasting methods to keep each other at bay. As a result, many of our most effective bactericidal agents have come from environmental organisms. Microbes are the most exploited sources for bioactive natural products including anti-TB compounds. To date, more than 1000 antimycobacterial compounds have been reported from microbial sources among which actinomycetes are the best reported microbial source. More compounds from actinobacteria of terrestrial and marine origin are still in different stages of investigation to be developed as potential anti-TB drugs. Some of the reports on anti TB activity of actinobacteria by LRP assay are described below. Radhakrishnan et al., (2010) screened bioactive extracts from 15 actinobacterial strains isolated from rare marine and forest ecosystems by LRP assay, for the first time. Culture supernatant and mycelia were extracted with ethyl acetate and methanol, respectively.
Culture filtrates and crude extracts were tested against standard strain Mycobacterium tuberculosis H37Rv and drug sensitive and drug resistant clinical isolates of M. tuberculosis by luciferase reporter phage (LRP) assay. Considerable variation was observed in antimycobacterial activity between actinobacterial culture filtrates and solvent extracts. Actinobacterial strains viz., D10, D5 (desert), CSA14 (forest), CA33 (alkaline soil), NEK5 (Neem plant), MSU, ANS2, R2 and M104 (marine) screened in the present study were found to be highly potent showing good antibacterial and antimycobacterial activity. Five of them; A3, CSA1, EE9, ANS5 and R9 were exclusively active against M. tuberculosis. Secretary products of actinobacteria of rare ecosystems are meant to antagonize organisms in their respective environments. These are likely to be novel antimycobacterial compounds as they unknown to human pathogens.
Bioactive potential of actinobacteria isolated from certain less explored Indian ecosystems was tested against Mycobacterium tuberculosis and other non mycobacterial pathogens. Actinobacteria were isolated from the soil samples collected from desert, coffee plantation, rubber forest, and hill area from Western Ghats and Eastern Ghats Ecosystems in India and their cultural and micromorphological characteristics were studied. Crude extracts were prepared by agar surface fermentation and tested against M. tuberculosis isolates by luciferase reporter phage (LRP) assay at 100g/mL. Activity against nonmycobacterial pathogens was studied by agar plug method. A total of 54 purified cultures of actinobacteria including 43 Streptomyces and 11 non Streptomyces were isolated.
While screening for antitubercular activity, extracts of 39 actinobacteria showed activity against one or more M. tuberculosis isolates whereas 27 isolates exhibited antagonistic activity against nonmycobacterial pathogens. In particular crude extracts from sixteen actinobacterial isolates inhibited all the three M. tuberculosis isolates tested. Findings of the present study concluded that less explored ecosystems investigated in this study are the potential resource for bioactive actinobacteria (Radhakrishnan et al., 2011; Manikkam et al., 2014).
Extracellular pigment extracted from the forest soil Streptomyces sp SFA5 using ethyl acetate was tested for antitubercular activity against M. tuberculosis H37Rv by LRP assay. The crude pigment showed activity against M. tuberculosis H37Rv at 250 µg/mL concentration (Manikkam et al., 2016). Gopikrishnan et al., (2017) studied the MIC of quercetin molecule purified from Streptomyces fradiae PE7 at 100 to <1 µg/ml against M. tuberculosis H37Rv, clinical drug sensitive M. tuberculosis and multi drug resistant (MDR) M. tuberculosis strains by adopting LRP assay. Quercetin showed more than 65% reduction against all the three M. tuberculosis strains at 3.1 µg/mL concentrations.
The crude extracts from 15 marine actinobacterial strains isolated from Andaman and Nicobar Islands were tested against the standard strain M. tuberculosis H37Rv, clinical drug sensitive M. tuberculosis, and MDR M. tuberculosis strains by luciferase reporter phage (LRP) assay at 500 µg/ml concentration. Among the 15 extracts that were tested for anti-tubercular activity, the crude ethyl acetate extract of the 14 actinobacterial strains showed anti-tubercular activity against at least one of the three M. tuberculosis strains. Exceptionally, the ethyl acetate extract of strain SACC 168 inhibited all three M. tuberculosis strains tested (Manigundan et al., 2019).
A study by Ameer et al., (2020) have extracted antitubercular protein from Staphylococcus hominis which significantly inhibited the growth of M. tuberculosis with a RLU reduction of more than 90% against M. tuberculosis H37Rv.
Synthetic Compounds: Sivakumar et al., (2010) have synthesized 25 chalcone derivatives based on the Claisen-Schimdt scheme and tested their antimycobacterial activity at two different concentrations. Among them, a compound, designated as C24, has shown 99% reduction against M. tuberculosis H37Rv. In a study, seven 2r,4c-diaryl-3-azabicyclo[3.3.1]nonan-9-one N-isonicotinoylhydrazone derivatives have tested their antimycobacterial activity against M. tuberculosis. All seven derivatives were found to possess antimycobacterial activity with the range of 62-85% of inhibition at both 1 and 2µg/ml concentration (Sankar et al., 2010). Twelve derivatives of 2-methyl-1H-benzimidazole hydrazide have screened against M. tuberculosis H37Rv.
Out of 12 derivatives, 7 derivatives showed significant reduction in RLU at both 50 and 100µg/ml concentration (Uma et al., 2009). In another study, Kanagarajan et al., (2011) have synthesized 6 novel 1,1’-(5,5’-(1,4-phenylene)bis(3-aryl-1H-pyrazole- 5,1-(4H,5H)-diyl))diethanones from bis chalcones and assessed their antimycobacterial activity against M. tuberculosis H37Rv and INH resistant M. tuberculosis. They found all the six compounds possess inhibitory activity against both the strains at 1 and 2µg/ml concentration. Kumar et al., (2011) have synthesized a series of quinolone coupled 1,2,3-triazoles compounds and tested their antimycobacterial activity by LRP assay. They reported that one compound has shown Inhibitory activity against M. tuberculosis at 5 and 25µg/ml concentration.
Durgad et al., (2012) have synthesized 9 benzimidazole derivatives with the intermediate chalcones and results found that 4 compounds possess potent antimycobacterial activity against M. tuberculosis H37Rv strain. Mohan et al., (2012) synthesized the various derivatives of 1,2,3,4-tetrahydropyrimidine-5-carbonitrile based on the bioisosteric similarities of Isoniazid. They selected compounds based on molecular docking and were tested for their antimycobacterial activity against M. tuberculosis H37Rv and drug resistant M. tuberculosis using LRP assay. Among the five test derivatives, three of them showed potential antimycobacterial activity at 500µg/ml concentration. Derivatives of 2-(4-methylpiperazin-1-yl)-N-(4,6-diarylpyrimidin-2-yl)acetamides have tested their antimycobacterial activity against M. tuberculosis H37Rv and INH resistant M. tuberculosis. Results of the study found all 9 derivatives showed inhibitory activity against the strains tested at 1 and 2µg/ml concentration (Kanagarajan and Gopalakrishnan, 2012).
Similarly, Narender et al. (2016) have synthesized 22 derivatives of 3-substituted-7-benzyl-5,6,7,8-tetrahydropyrido[4′,3′:4,5]thieno[2,3-d]pyrimidin-4(3H)-one and 3-substituted-7-benzyl-2-methyl-5,6,7,8-tetrahydropyrido[4′,3′:4,5]thieno[2,3-d]pyrimidin-4(3H)-one and screened their antibacterial activity. Based on their inhibitory effect, five of them were tested for their antimycobacterial activity against a standard and clinical isolate of M.tuberculosis strains. Among them, two of the derivatives have showed potential antimycobacterial activity at less concentration. Two hydrazones,
Benzoic acid (4-allyloxybenzylidene)-hydrazide and 4-chloro-benzoic acid (4-allyloxybenzylidene)-hydrazide have been synthesized and tested against M.tuberculosis H37Rv in which the latter found to possess significant antimycobacterial activity at both 100 and 200µg/ml concentration (Therese and Geethamalika, 2017). Brindha et al. (2017) have demonstrated the repurposing of drug for tuberculosis treatment. They screened 1554 known drugs by docking studies. Based on their potential in docking studies, they have selected five potential drugs and screened them by LRP assay. They found that two drugs lymecycline and cefpodoxime has potential inhibition at 20µg/ml concentration against drug sensitive and drug resistant M. tuberculosis strains.
Nanoparticles: In vitro antitubercular activity of isoniazid (INZ) loaded solid lipid nanoparticles (SLNs) and free isoniazid (INZ) was evaluated by LRP assay against M. tuberculosis H37Rv and MDR MTB. Results of LRP assay in H37Rv strain showed that percentage reduction in relative light unit (RLU) for INZ-SLNs and free INZ were 99.75 and 99.898% respectively, whereas in case of INZ resistant strain they were found to be 90.27 and 90.52% respectively, confirming notable antitubercular activity (Mohanta et al., 2018).
There are few studied which have reported the anti TB activity of nanoparticles and drug loaded nanoparticles by adopting LRP assays. Green synthesis of silver chloride nanoparticles (AgCl NPs) using commercial yeast extract has been carried out. The physico–chemical characterizations of AgCl NPs were carried out by UV–visible spectroscopy, XRD, FTIR, HR-SEM equipped with EDAX and TEM. In vitro efficacy of anti-mycobacterial properties of AgCl NPs were determined by agar well diffusion and Luciferase Reporter Phage (LRP) assay were used against M.smegmatis (MC²155) and M.tuberculosis H37Rv respectively. This shows that the AgCl NPs have potential anti-myobacterial activity against M. tuberculosis H37Rv (Sivaraj et al., 2020). A recent study by Govindaraju et al. (2020) synthesized nanoparticles using seaweed Turbinaria ornata and found that it has the ability to inhibit the growth of M. tuberculosis strain by 73% of RLU reduction. The characterization of these nanoparticles have also been studied with spectral and microscopic analysis.
CONCLUSION
The review of published literature on anti TB screening assays revealed that more actinobacterial extracts were screened for anti TB activity than other sources by adopting LRP assay. Furthermore, the mycobacteriophage constructs used in all the studies had narrow host range and mainly infect M. tuberculosis, limiting utility. Developing new mycobacteriophage constructs with wide host range may widen the utility of LRP assay for drug discovery against non tubercular mycobacterial pathogens.LRP may help to reduce the time required for anti-TB screening compounds against various sub-population of M. tuberculosis and helps to screen large number of compounds as it involves less quantity of compounds as well the turnaround time to detection reduced from weeks to days which enables to accelerate the screening for new antituberculosis natural/synthetic compounds.
ACKNOWLEDGEMENTS
Authors thank the National Institute for Research in Tuberculosis (ICMR) and the management of Sathyabama Institute of Science and Technology for the research facilities provided. Authors thank the financial support of ICMR (Ref. No: 5/8/5/19/2014-ECD-I) and DBT (Ref:BT/5426/AAQ/3/599/2012) in the form of research grant.
REFERENCES
Ameer, K., Chirom, A. and Paul, A., (2020). Production and purification of anti-tubercular and anticancer protein from Staphylococcus hominis under mild stress condition of Mentha piperita L. Journal of Pharmaceutical and Biomedical Analysis, 182, p.113136.
Amudha, P., Harika, N. and Kumar, V., (2015). Bioactivity of selected seaweeds from gulf of mannar, South-East Cost of India. Journal of Coastal Life Medicine, 3(1), pp.52-55.
Anbarasu, S., Sundar, R., Manigundan, K., Rajasekar, T., Shamya, M. and Joseph, J.,(2018) Evaluating the Anti-mycobacterial Activity of Nigella sativa Seed Extracts. Journal of Applied Pharmaceutical Science, Vol 12 (3) 45-56
Antony, M., James, J., Misra, C.S., Sagadevan, L.D.M., Veettil, A.T. and Thankamani, V., (2012). Anti mycobacterial activity of the plant extracts of Alstonia scholaris. International Journal of Current Pharmaceutical Research, 4(1), pp.40-42.
Banaiee, N., Bobadilla-Del-Valle, M., Bardarov, S., Riska, P.F., Small, P.M., Ponce-De-Leon, A., Jacobs, W.R., Hatfull, G.F. and Sifuentes-Osornio, J., (2001). Luciferase reporter mycobacteriophages for detection, identification, and antibiotic susceptibility testing of Mycobacterium tuberculosis in mexico. Journal of Clinical Microbiology, 39(11), pp.3883-3888.
Bardarov Jr, S., Dou, H., Eisenach, K., Banaiee, N., Ya, S.U., Chan, J., Jacobs Jr, W.R. and Riska, P.F., (2003). Detection and drug-susceptibility testing of M. tuberculosis from sputum samples using luciferase reporter phage: comparison with the Mycobacteria Growth Indicator Tube (MGIT) system. Diagnostic microbiology and infectious disease, 45(1), pp.53-61.
Brindha, S., Sundaramurthi, J.C., Vincent, S., Velmurugan, D. and Gnanadoss, J.J., (2017). In silico and in vitro screening of FDA-approved drugs for potential repurposing against tuberculosis. bioRxiv, p.228171.
Conti, R., Chagas, F.O., Caraballo‐Rodriguez, A.M., Melo, W.G.D.P., do Nascimento, A.M., Cavalcanti, B.C., de Moraes, M.O., Pessoa, C., Costa‐Lotufo, L.V., Krogh, R. and Andricopulo, A.D., (2016). Endophytic actinobacteria from the Brazilian medicinal plant Lychnophora ericoides Mart. and the biological potential of their secondary metabolites. Chemistry & Biodiversity, 13(6), pp.727-736.
Durgad, S.A., Singh, K.C. and Bhinge, S.D., (2012). Synthesis and antimycobacterial activity of benzimidazole derivatives. Indian Drugs, 49(02), p.17.
De Souza, M.V.N., (2006) Marine natural products against tuberculosis. The Scientific World Journal, 6, p.847.
Ekor, M., (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology, 4, p.177.
Favrot, L. and Ronning, D.R., (2012). Targeting the mycobacterial envelope for tuberculosis drug development. Expert review of anti-infective therapy, 10(9), pp.1023-1036..
Forbes, L., Ebsworth-Mojica, K., DiDone, L., Li, S.G., Freundlich, J.S., Connell, N., Dunman, P.M. and Krysan, D.J., (2015). A high throughput screening assay for anti-mycobacterial small molecules based on adenylate kinase release as a reporter of cell lysis. PLoS One, 10(6), p.e0129234.
Fu, X., Ding, M., Zhang, N. and Li, J.,(2015). Mycobacteriophages: an important tool for the diagnosis of Mycobacterium tuberculosis. Molecular medicine reports, 12(1), pp.13-19.
Gopikrishnan, V., Radhakrishnan, M., Pazhanimurugan, R., Shanmugasundaram, T. and Balagurunathan, R., (2017). Antimicrobial, antitubercular and antiproliferative activities of quercetin isolated from the marine Streptomyces fradiae. Bangladesh Journal of Pharmacology, 12(3), pp.333-334.
Govindaraju, K., Anand, K.V., Anbarasu, S., Theerthagiri, J., Revathy, S., Krupakar, P., Durai, G., Kannan, M. and Subramanian, K.S., (2020) Seaweed (Turbinaria ornata)-assisted green synthesis of magnesium hydroxide [Mg (OH) 2] nanomaterials and their anti-mycobacterial activity. Materials Chemistry and Physics, 239, p.122007.
Hatfull, G.F., (2014). Mycobacteriophages: windows into tuberculosis. PLoS Pathog, 10(3), p.e1003953.
Ignacimuthu, S. and Shanmugam, N., (2010). Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica Ness. leaves. Journal of biosciences, 35(4), pp.565-570.
Jacobs, W.R., Barletta, R.G., Udani, R., Chan, J., Kalkut, G., Sosne, G., Kieser, T., Sarkis, G.J., Hatfull, G.F. and Bloom, B.R., (1993). Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science, 260(5109), pp.819-822.
Kanagarajan, V. and Gopalakrishnan, M., (2012). Pyrimidino piperazinyl acetamides: innovative class of hybrid acetamide drugs as potent antimicrobial and antimycobacterial agents. Pharmaceutical Chemistry Journal, 46(1), pp.26-34.
Kanagarajan, V., Ezhilarasi, M.R. and Gopalakrishnan, M., (2011). In vitro microbiological evaluation of 1, 1′-(5, 5′-(1, 4-phenylene) bis (3-aryl-1H-pyrazole-5, 1-(4H, 5H)-diyl)) diethanones, novel bis acetylated pyrazoles. Organic and medicinal chemistry letters, 1(1), p.8.
Kumar, A., Chettiar, S. and Parish, T., (2017). Current challenges in drug discovery for tuberculosis.
Kumar, K.K., Seenivasan, S.P., Kumar, V. and Das, T.M., (2011). Synthesis of quinoline coupled [1, 2, 3]-triazoles as a promising class of anti-tuberculosis agents. Carbohydrate research, 346(14), pp.2084-2090.
Kumar, V., Loganathan, P., Sivaramakrishnan, G., Kriakov, J., Dusthakeer, A., Subramanyam, B., Chan, J., Jacobs Jr, W.R. and Rama, N.P., (2008). Characterization of temperate phage Che12 and construction of a new tool for diagnosis of tuberculosis. Tuberculosis, 88(6), pp.616-623.
Lei, W., Peng, Q., Xiu-Feng, Long., Zhang, S., Zhi-Gang, Z. and Yong-Qiang, T., (2016). Comparative analysis of chemical constituents, antimicrobial and antioxidant activities of ethylacetate extracts of Polygonum cuspidatum and its endophytic actinomycete, Streptomyces sp. A0916. Chinese journal of natural medicines, 14(2), pp.117-123.
Lindequist, U., (2016). Marine-derived pharmaceuticals–challenges and opportunities. Biomolecules & therapeutics, 24(6), p.561.
Mancini, I., Defant, A. and Guella, G., (2007). Recent synthesis of marine natural products with antibacterial activities. Anti-Infective Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Infective Agents), 6(1), pp.17-48.
Manigundan, K., Revathy, S., Sivarajan, A., Anbarasu, S., Jerrine, J., Radhakrishnan, M. and Balagurunathan, R., (2019). Bioactive potential of selected actinobacterial strains against Mycobacterium tuberculosis and other clinical pathogens.
Manikkam, R., Ponnuswamy, S., Joseph, J. and Kumar, V., (2016). Antitubercular activity of the pigment from forest soil Streptomyces sp SFA5. Bangladesh Journal of Pharmacology, 11(1), pp.138-140.
Manikkam, R., Venugopal, G., Ramasamy, B. and Kumar, V., (2015). Effect of critical medium components and culture conditions on antitubercular pigment production from novel Streptomyces sp D25 isolated from Thar desert, Rajasthan. Journal of Applied Pharmaceutical Science, 5(06), pp.015-019.
Manikkam, R., Venugopal, G., Subramaniam, B., Ramasamy, B. and Kumar, V., (2014). Bioactive potential of actinomycetes from less explored ecosystems against Mycobacterium tuberculosis and other nonmycobacterial pathogens. International scholarly research notices.
Mayakrishnan, A., Sivakumari, K., Srinivasan, P. and Kumari, V., (2017). Antimycobacterial activity of Kappaphycus alvarezii against Mycobacterium tuberculosis and in silico molecular docking of kappa-carrageenan against InhA enzyme. Int J Drug Res Tech, 5(11).
Menzies, D., Al Jahdali, H. and Al Otaibi, B., (2011). Recent developments in treatment of latent tuberculosis infection. The Indian journal of medical research, 133(3), p.257.
MI, G.M., Anti-oxidant, anti-bacterial and anti-mycobacterial activity of the methanolic extract of Dendrophthoe falcata leaves.
Mohan, S.B., Kumar, B.R., Dinda, S.C., Naik, D., Seenivasan, S.P., Kumar, V., Rana, D.N. and Brahmkshatriya, P.S., 2012. Microwave-assisted synthesis, molecular docking and antitubercular activity of 1, 2, 3, 4-tetrahydropyrimidine-5-carbonitrile derivatives. Bioorganic & medicinal chemistry letters, 22(24), pp.7539-7542.
Mohanta, B.C., Dinda, S.C., Mishra, G., Palei, N.N. and Dusthackeer, V.N.A., (2018). Formulation, characterization, in vitro anti-tubercular activity and cytotoxicity study of solid lipid nanoparticles of Isoniazid. Nano Biomed Eng, 10(4), pp.379-91.
Muthuselvi R, AshokKumar D, Senniappan P, Vijaya BR, Jayshree N. (2017) Anti-tubercular activity on stem bark of Cassia marginata. World J Pharm and Pharm Sci ;6:1464-70.
Muthuswamy, M., Asan, P. and Kumar, V., (2013) Screening of antitubercular activity of some medicinal plants from Western Ghats, India. Int J Pharm Bio Sci, 4(4), pp.328-334.
Narender, M., Umasankar, K., Malathi, J., Reddy, A.R., Umadevi, K.R., Dusthackeer, A.V.N. and Rao, K.V., (2016) Synthesis, in vitro antimycobacterial evaluation and docking studies of some new 5, 6, 7, 8-tetrahydropyrido [4′, 3′: 4, 5] thieno [2, 3-d] pyrimidin-4 (3H)-one schiff bases. Bioorganic & Medicinal Chemistry Letters, 26(3), pp.836-840.
Nayak, N., Bajpai, M. and Razdan, B., (2014). Plumbagin analogs-synthesis, characterization, and antitubercular activity. Journal of Advanced Pharmaceutical Technology & Research, 5(1), p.28.
Papitha, N., Jayshree, N., Sreenivasan, S. and Kumar, V., 2013. Anti-tubercular activity on leaves and roots of Sida rhombifolia L. Int J. Pharm. Sci. Rev. Res, 20(2), pp.135-137.
Parikh, A., Kumar, D., Chawla, Y., Kurthkoti, K., Khan, S., Varshney, U. and Nandicoori, V.K., (2013). Development of a new generation of vectors for gene expression, gene replacement, and protein-protein interaction studies in mycobacteria. Applied and environmental microbiology, 79(5), pp.1718-1729.
Prabu, A., Hassan, S., Shainaba, A.S., Hanna, L.E. and Kumar, V., (2015). Andrographolide: A potent antituberculosis compound that targets Aminoglycoside 2′-N-acetyltransferase in Mycobacterium tuberculosis. Journal of Molecular Graphics and Modelling, 61, pp.133-140.
Prabu, A., Seenivasan, P. and Kumar, V., 2014. Antimycobacterial activity of certain mangrove plants against multi-drug resistant Mycobacterium tuberculosis. Asian Journal of Medical Sciences, 5(3), pp.54-57.
Radhakrishnan, M., Balagurunathan, R., Selvakumar, N., Doble, M. and Kumar, V., (2011). Bioprospecting of marine derived actinomycetes with special reference to antimycobacterial activity.
Radhakrishnan, M., Suganya, S., Balagurunathan, R. and Kumar, V., (2010) Preliminary screening for antibacterial and antimycobacterial activity of actinomycetes from less explored ecosystems. World Journal of Microbiology and Biotechnology, 26(3), pp.561-566.
Rajasekar, T., Anbarasu, S., Manikkam, R., Joseph, J. and Kumar, V., (2015) Inhibitory activity of Euphorbia hirta (Tawa-tawa) extracts against Mycobacterium tuberculosis and other non mycobacterial pathogens. Der Pharma Chemica, 7(8), pp.213-216.
Rajasekar, T., Anbarasu, S., Radhakrishnan, M., Jerrine, J. and Vanaja, K., (2016). In vitro antitubercular activity of Tridax procumbens extracts against whole cell Mycobacterium tuberculosis and its lysine aminotransferase. Bangladesh Journal of Pharmacology, 11(1), pp.192-193.
Rakhmawatie, M.D., Wibawa, T., Lisdiyanti, P. and Pratiwi, W.R., (2019). Evaluation of crystal violet decolorization assay and resazurin microplate assay for antimycobacterial screening. Heliyon, 5(8), p.e02263.
Sankar, C. and Pandiarajan, K., 2010. Synthesis and anti-tubercular and antimicrobial activities of some 2r, 4c-diaryl-3-azabicyclo [3.3. 1] nonan-9-one N-isonicotinoylhydrazone derivatives. European journal of medicinal chemistry, 45(11), pp.5480-5485.
Sari, M., Syahputra, G. and Kusharyoto, W., 2019. The Application of Multiplate Resazurin Reduction Assay in The Screening for Anti-Mycobacterial Activity from Indonesian Medicinal Plants. Indonesian Journal of Pharmacy, 30(3), p.199.
Sasikumar, K., Ghosh, A.R. and Dusthackeer, A., 2018. Antimycobacterial potentials of quercetin and rutin against Mycobacterium tuberculosis H37Rv. 3 Biotech, 8(10), p.427.
Singh, B., Jain, M., Singh, S.V., Dhama, K., Aseri, G.K., Jain, N., Datta, M., Kumar, N., Yadav, P., Jayaraman, S. and Gupta, S., 2015. Plants as future source of anti-mycobacterial molecules and armour for fighting drug resistance. Asian J Anim Vet Adv, 10, pp.443-60.
Sivakumarl, P. M., V. Kumar, S. P. Seenivasan, and M. Mohanapriya Doble.(2010) Antitubercular activity of chalcones–experimental and QSAR studies. Advances in biomedical research, In Proceedings of the 7th WSEAS International Conference on Mathematical Biology and Ecology.(MABE ‘10), Proceedings of the International Conference on Medical Physiology (PHYSIOLOGY ‘10), Proceedings of the International Conference on Biochemistry and Medical Chemistry (BIOMEDCH ‘10). Cambridge, UK, pp. 168-172.
Sivaraj, A., Kumar, V., Sunder, R., Parthasarathy, K. and Kasivelu, G., (2020). Commercial yeast extracts mediated green synthesis of silver chloride nanoparticles and their anti-mycobacterial activity. Journal of Cluster Science, 31(1), pp.287-291.
Sundar, R., Mathayan, M. and Anbarasu, S., 2018. Antimycobacterial activity of marine Sargassum swartzii extracts against Mycobacterium tuberculosis. Bangladesh Journal of Pharmacology, 13(2), pp.130-131.
Suriyamurthy A, Rajamani A, Kannan E, Kandasamy M. (2016) Screening Of Tabubeia rosea Dc: For antituberculosis, antibacterial and antioxidant studies; an in vitro approach. Indo American J Pharm Res 6: 5297-306.
Therese, S.K. and Geethamalika, G., 2017. Synthesis, Characterization and Anti Mycobacterial Activity of Novel Hydrazones. Oriental Journal of Chemistry, 33(1), pp.335-345.
Uma K, Kannan K, Krishnakumar K.(2009) Synthesis and comparative antimycobacterial evaluation of certain substituted benzimidazoles. Int J Chem. Sci 7:80-86.
Vaishnavi C, Anusha R, Radhakrishnan M, Anbarasu S, Wilson Aruni, Jerrine J.(2020) In vitro activity of selected medicinal plant extracts against Mycobacterium tuberculosis and other non mycobacterial pathogen. Biosci Biotech Res Comm 13(1).
World Health Organization. Global Tuberculosis Report 2019.