Department of Microbiology, CMS college of Commerce and Science, Coimbatore. Tamilnadu. India.
Article Publishing History
Received: 09/10/2019
Accepted After Revision: 01/12/2019
Siderophores are small molecules and important iron chelators produced by microbes like bacteria,algae, and fungi. This study was undertaken to determine the efficiency of rhizobacterial isolates to produce different types of siderophores that enhance the plant growth promoting substances like Indole acetic acid, HCN production, Phosphate solubilisation, Ammonia production etc. The isolates from the rhizosphere soil region are characterized morphologically and biochemically and identified as Pseudomonas sp. The strains were assessed for their siderophore production by Chrome azurol S assay. The results indicates that isolates were able to produce hydroxmate and carboxylate type of siderophore and these isolates have studied for their PGPR activity. The results shows that the isolates have a potential on plant growth promotion and they enhance the plant growth efficiently.
Chrome azurol S assay, Indole acetic acid, Plant growth promoting rhizobacteria, Siderophores.
Parveen S. R, Latha D. Characterization of Siderophore Producing Pseudomonas Sp for its Plant Growth Promoting Properties. Biosc.Biotech.Res.Comm. 2019;12(4).
Parveen S. R, Latha D. Characterization of Siderophore Producing Pseudomonas Sp for its Plant Growth Promoting Properties. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2BKZVcS
Copyright © Parveen and Latha This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Microbes are diverse in nature which is present in all surrounding areas of earth. In terms of sheer number and mass it is estimated that microbes contain 50 % of the biological carbon and 90 % of the biological nitrogen on earth. Soil microbial communities appear to be more diverse than those found in most fresh water and marine environments. The vicinity of plant roots are surrounded by rhizosphere which is an extremely important and active area for root activity and metabolism. (Agrawal et.al., 2015).The Pseudomonas sp encompasses arguably the most diverse and ecologically significant group of bacteria on the planet. Fluorescent Pseudomonas is gram negative, rod shaped, chemoheterotrophic bacteria with polar flagella and are characterized by the yellow green pigments ( Sullivan et.al., 1992). Fluorescent Pseudomonas is well recognized as plant growth promoting rhizobaceria, phosphate solubilizers and also act as biocontrol agents against many soil born plant pathogens, (Battu et.al .,2009). Three different characteristics have been determined in order to classify the strains as plant growth promoting rhizobacteria. These characteristics are 1.ability to produce auxin,2. Sidrophores 3. The solubilisation of phosphates presents in the medium (Cardenas et al., 2019) Plant Growth Promoting Rhizobacteria (PGPR), which enhances plant growth and increase crop yield via secretion of various plant growth promoting substances as well as biofertilizers. PGPR’s exhibit antagonistic effects to soil-borne pathogens or induce the systemic resistance against pathogens in the entire plant lifespan, (Swamy et at ., 2019).
Siderophores are relatively low molecular weight, ferric ion specific chelating agents synthesized by bacteria, actinomycetes, fungi and certain algae growing under low ionic stress(Kannahi et.al ., 2014). The iron ligation groups have been tentatively classified into three main chemical types: hydroxamate, catecholate and hydroxycarboxylates. The type of siderophore synthesized by bacteria depends on the amount and accessibility of nutrients and it may differ in culture rich conditions as compared to natural habitat. (Maheshwari, et al.,(2019).
Pseudomonas fluorescence and Pseudomonas putida produce siderophores of two general types, pyochelin and pyoverdin( Dava et.al.,2000 ). Pyoverdin are water soluble pigments that fluoresce under ultra violet light (Budzikiewicz et.al., 1993). Siderophores produced by rhizosphere inhabitants has been studied well and it has been reported that ability to produce siderophores not only improve rhizosphere colonization of producer strain but also play an important role in iron nutrition of plant.
MATERIALS AND METHODS
Sample collection
Rhizosphere soil sample was collected from Palladam region, Tirupur district, Tamil Nadu, South India. The collected soil sample was brought to the laboratory in a sterile ziplock bag aseptically and maintained at the laboratory for further study.
Isolation of bacteria
0.1 ml of serially diluted sample was taken from 10–4 to 10–7 dilutions, was spreaded on King’s B medium plates and incubated at 28◦C for 48 h. After incubation the plates were exposed to UV light at 365 nm for few seconds and the colonies exhibiting the fluorescence were picked up and purified on King’s B medium plates.
Characterisation and identification
Phenotypic characterisation was carried out by subjecting the bacterial isolates to morphological (colony morphology), microscopical (grams staining), motility, and biochemical tests (utilisation of different carbon sources and enzyme activity) following standard procedure as per Bergey’s manual of systematic bacteriology.
Production and Detection of Siderosphores: CAS Agar Plate Technique
(Agrawal et.al.,2015: Siderophore production by all the isolates were tested by chrome azurol dye method. Freshly grown bacterial cultures were inoculated into CAS agar plate and incubated at 37˚C for 24 -48 hours. After incubation appearance of an orange zone against the dark blue background indicates the siderophore production.
Tetrazolium Salt Test to Detect Hydroxamate Type Of Siderophore (Syamala et al 2017)
To a pinch of tetrazolium salt, 1-2 drops of 2N NaOH and 0.1ml of test culture supernatant were added. Instant appearance of deep red colour indicated hydroxamate nature of siderophores.
Vogel’s Chemical Test to Detect Carboxylate Type Of Siderophore
To 3 drops of 2N NaOH , 1 drop of phenolphthalein and then water was added until light pink colour was developed. Disappearance of pink colour on addition of test sample, indicate carboxylate nature of siderophores.
Detection of Plant Growth Promoting Substances.
Indole Acitic Acid Production
(Agrawal et.al.,2015): Isolates were inoculated in King’s B medium supplemented with 0.1mg/ml tryptophan and incubated at 37◦C for 4 days. The cells were removed by centrifugation at 10,000 rpm for 15 minutes. 1ml of the cell free supernatant was transferred to a fresh tube to which 50µl of 10mm orthophosphoric acid and 2ml of Salkowski’s reagent was added. The tubes were observed for the presence or absence of pink colour.
Phosphate Solubilization
(Agrawal et.al.,2015): The Pikovskaya’s medium was prepared and sterilized at 121oC for 15 minutes at 15lbs pressure. It was poured onto sterile petriplates, inoculated and incubated with the bacterial isolates at 37◦C for 4-5 days. The plates were examined for the presence or absence of clear zone surrounding each test isolates.
HCN Production
(Syamala et al 2017): All the isolates were screened for the production of hydrogen cyanide .King’s B medium was mixed with 4.4g of glycine and the isolates were streaked onto the agar plate. Whatmann filter paper soaked in the solution of 2% sodium carbonate and 0.5% picric acid solution was placed on streak plate’s upper lid and the plates were sealed with paraffin. The plates incubated at 37oC for 4-5 days. The change in the colour from yellow to orange red indicates the HCN production.
Protease Production
(Apastamph et.al 2014):The active bacterial cultures were spot inoculated on caseincontaining sterile media plate and incubated at 30 ◦C for 48 hrs and observed for zone of clearance.
Cellulose Production
(Apastamph et.al 2014): The Czapek mineral salt media was prepared and sterilized at 121◦C for 15 minutes at 15 lbs pressure. It was poured on a sterile petriplates, inoculated and incubated with bacterial isolates at 37oC for 24 hours. The plates were examined for the presence or absence of clear zone surrounding each test isolates on exposure to 1% Congo red and 1M sodium chloride.
Nitrogen Content
(Kaushal et al., 2013): Nitrate broth was inoculated with bacterial cultures and incubated at 30◦C for 48 hrs3 drops of reagent and 1drop of sulphuric acid were added in a petriplate and 1 drop of culture was added to it. Blue colour appeared indicates nitrite is produced. Zinc dust was added, if no blue colour appeared indicates nitrate was reduced to nitrite.
RESULT AND DISCUSSION
The organisms were isolated from rhizosphere soil, after 24 hrs the colonies were appeared as crowded, it was then purified on kings B agar plate and observed under UV light at 365 nm. Strains were identified based on its cultural, morphological, microscopic and motility tests. The colonies that can able to produce yellow green colour pigment have been selected for further analysis (Battu et al 2009 ). The isolates were further maintained on nutrient agar slants and used for furthers studies. (Table 1) (Figure 1) .
With the help of biochemical characteristics, as per bergys manual of determinative bacteriology the colonies were named as Pseudomonas Ps 1 to Ps9 respectively. Results showed that the strains were positive for citrate, catalase positive,Urease positive, liquefy gelatinase, and produce H2S. The strains were able to produce acid butt and alkaline slant.It was also observed that it is positive for oxidase Ps 5 –Ps9 (Holt et al 1994 ). All the isolates are positive for urease production. The stains can able to utilize various type of carbohydrates like glucose,sucrose and mannitol and it shows negative results for lactose utilization.( Table 2).
Siderophore are iron specific compounds which are synthesized and secreted under iron stress condition.(Budizikiewic 1993) Pseudomonas sp have been known for their siderophore production for many years and therefore many reports on the isolation and characterization of their siderophores have been published ( Dava et al.,2000). Siderophores produced by Pseudomonas sp., have been employed efficiently as biocontrol agent against certain soil born plant pathogens ( Fekadualemu 2013). Appearance of orange zone after 48 hrs of incubation indicates that Ps 1 to Ps 9 were able to produce siderophore in Chrome Azural ager medium(Belkar Y.K. et al., 2012). Tetrazolium and neilands spectrophotometric assay is used for hrdroxamate type of siderophore ,Arnows assay is used for catecholate type of siderophore,Vogals assay for carboxylate type of sideophore .(Table 3)
Instant appearance of deep red colour in tetrozolium test indicate the presence of hydroxamate nature of siderophore , disappearance of pink color on the addition of phenolphthalein indicates the carboxylate type of siderophores.( Syamala et al 2017). Strains Ps 1-2 and Ps 5-7 were able to produce hydroxamate and carboxylate type of siderophores is used for further analysis. Siderophores have been classified based on their main chelating groups. Generally they are categorized into two groups.(Payne 1994) . Siderophores in the rhizosphere region of the plant provide iron nutrition,serve us a first defence against root invading parasites and helps in removing toxic metals from the plants. Pseudomonas sp can enhance the plant growth by producing pyoveridine siderophores.(Joseph et al 2007)
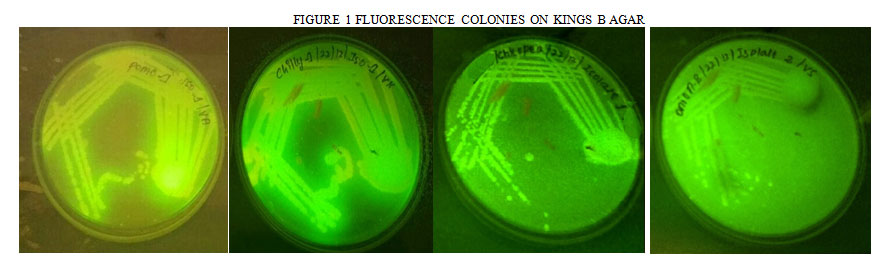 |
Figure 1: Fluorescence Colonies on Kings B Agar |
Table 1: Morphological identification of Pseudomonas strains Ps1 – Ps9
| S.no | Sample | Colony morphology | Grams nature | Motility | Pigment production | Fluorescence under UV |
| 1 | Pomegranate Ps1 | Opaque round colony | Gram Negative, rod | Motile | Yellowish colony | Yellow Fluorescence colony |
| Pomegranate Ps 2 | Round colony | Gram Negative, rod | Motile | Yellowish colony | Yellow Fluorescence colony | |
| 2 | Chilly Ps3 | Transparent colony | Gram Negative, rod | Motile | Yellowish green | Fluorescence colony |
| Chilly Ps4 | Opaque colony | Gram Negative, rod | Motile | Yellowish green | Fluorescence colony | |
| 3 | Chickpea Ps 5 | Dome shaped colony | Gram Negative, rod | Motile | Yellow | Yellow fluorescence colony |
| Chickpea Ps6 | Small pointed colonies | Gram Negative, rod | Motile | Yellowish Green | Yellow Fluorescence | |
| Chickpea Ps7 | Small dome shaped colonies | Gram Negative, rod | Motile | Yellowish green | GreenishFluorescence | |
| 4 | Onion Ps 8 | Opaque colony | Gram Negative, rod | Motile | Yellowish Green | GreenishFluorescence |
| Onion Ps 9 | Transparent colony | Gram Negative, rod | Motile | Yellowish Green | GreenishFluorescence |
Table 2: Physiological and Biochemical properties of the selected bacterial isolates
| TEST | Ps 1 | Ps 2 | Ps 3 | Ps 4 | Ps 5 | Ps 6 | Ps 7 | Ps 8 | Ps 9 | |
| 1 | Indole production test | – | – | – | – | – | – | – | – | – |
| 2 | Methyl red test | – | – | – | – | – | – | – | – | – |
| 3 | Voges proskauer test | – | – | – | – | – | – | – | – | – |
| 4 | Citrate utilization test | + | + | + | + | + | + | + | + | + |
| 5 | Oxidase test | – | – | – | – | + | + | + | + | + |
| 6 | Catalase test | + | + | + | + | + | + | + | + | + |
| 7 | Gelatin hydrolysis | + | + | + | + | + | + | + | + | + |
| 8 | H2S production test | + | + | + | + | + | + | + | + | + |
| 9 | Urease test | + | + | + | + | + | + | + | + | + |
| 10 | Triple sugar iron test | AB/AS | AB/AS | AB/AS | AB/AS | AB/AS | AB/AS | AB/AS | AB/AS | AB/AS |
| Carbohydrate fermentation tes | ||||||||||
| 1 | Glucose | + | + | + | + | + | + | + | + | + |
| 2 | Lactose | – | – | – | – | – | – | – | – | – |
| 3 | Mannitol | + | + | + | + | + | + | + | + | + |
| 4 | Sucrose | + | + | + | + | + | + | + | + | + |
Note : + -positive,- -negative ,AB/AS Acid butt/ Acid slant
Table 3: Detection and types of Siderophores
| s.no | Strain no. | Types of siderophores | ||
| Hydroxemate | Carboxylate | |||
| 1 | Ps 1 | + | + | |
| 2 | Ps 2 | + | + | |
| 3 | Ps 3 | – | – | |
| 4 | Ps 4 | – | – | |
| 5 | Ps 5 | + | + | |
| 6 | Ps 6 | + | + | |
| 7 | Ps 7 | + | + | |
| 8 | Ps8 | – | – | |
| 9 | Ps 9 | – | – | |
Table 4: Plant growth promoting properties of the seleced Pseudomonas strains.
| S.NO | TEST | Ps 1 | Ps 2 | Ps 5 | Ps 6 | Ps7 |
| 1 | IAA production | + | + | + | + | + |
| 2 | HCN production | – | – | + | + | + |
| 3 | Ammonia production | + | + | + | + | + |
| 4 | Phosphate solubilization | + | + | + | + | + |
| 5 | Nitrate reduction | + | + | + | + | + |
| 6 | Cellulose | + | + | + | + | + |
| 7 | Protease | + | + | + | + | + |
Plant growth promoting properties of the selected Pseudomonas strains
The product of microbial metabolism that are released into the soil influence the growth of plant. Interaction between plant and microbes is well known for beneficial effect and such free living bacteria isolated from the rhizosphere of plants are known as plant growth promoting rhizobacteria ( Kloepper et al.,1980). Pseudomonas sp showed the positive reaction towards IAA, (Agrawal et.al 2015). The presence of tryptophan in the medium significantly enhanced the indole acetic acid production. Indole acetic acid is the main auxin having influence on cell enlargement,division,tissue differentiation and response to light and gravity (Josip colo et al.,2014).
Diverse bacterial species has the ability to produce IAA, ( Reetha et al ., 2014). The improved plant growth is due to the ability of the organism to produce phytohormones such as indole acetic acid,gibberilic acid, cytokinins etc. IAA producing bacteria may be a efficient biofertilizer inoculants to promote plant growth and protecting the medicinal plants for future generations (Glick 1995). The rhizosphere microorganism especially fluorescent pseudomonas have exceptional ability to promote the growth of the host plant by various mechanism to suppress plant disease including production of powerful siderophores, ( Sullivan et al., 1992) Furthermore siderophores are able to reduce the oxidative stress by inhibiting the free radicle formation and can inhibit the IAA destruction. (Table 4) The isolates were positive for protease production, cellulose production,nitrate reduction (Apastamph et al., 2014). Qualitative analysis showed that all the bacterial isolates produce IAA,ammonia,siderophore etc (Anitha et al.,2013). The selected isolates were found to be positive for conversion of ammonia into nitrate that plants can absorb and use it, (Ahmed et al.,2008).
Phosphorous is an essential nutrient for plant growth but often limiting due to its low solubility and fixation in the soil (Deshwal et al., 2013). The ability of the microorganisms to convert insoluble phosphorus to soluble form is an important trait in PGPR for increasing plant yield (Rodriquez et al., 2006) .Strains has shown to be positive for tricalcium phosphate solubilizers .Fluorescent pseudomonas have also been reported as phospahate solubilizers due to the excretion of organic acids (Banu et al.,2004). The exact mechanism by which PGPR stimulate the plant growth is not clearly known, although the mechanisms such as activation of phosphate solubilisation and promotion of mineral nutrient uptake are usually believed to be involved in plant growth promotion. The HCN production is found to be a common trait of Pseudomonas sp and Bacillus in the rhizospheric soil and plant root nodules, (Ahmed et al., 2008).Rhizosphere colonizing Fluorescent Pseudomonas significantly increased growth and yield of crops.
CONCLUSION
The results suggested that pigment producing Pseudomons sp present in the rhizosphere soil region. All the strains can able to produce sideophores ,among these the strains Ps 1-2 to Ps 5-7 that can able to produce hydroxmate and carboxylate type of siderophores was selected and analysed for their plant growth promoting properties. These results shows that siderophore producing Pseudomonas sp has been an efficient plant growth promoters and control the plant pathogen by acting as a first defence. However, to ascertain the effectivity of PGP traits on growth and productivity of crops, through phytohormone production and control against harmful microbes, needs to be evaluated in further studies.
REFERENCES
Anitha G. and B.S. Kumudini.(2013) Isolation and characterization of Fluorescent pseudomonads and their effect on Plant growth promotion.Journal of Environmental Biology.vol 35 ,627-634,
Agrawal Pavan Kumar,Kundan Rishi,Agarwal Shruti (2015).Characterization of Pseudomonas sp from rhizosphere of tomato plants (Lycopersicon esculentum) and its efficacy on plant growth promotion. Journal of Biological and Scientific Opinion.Vol 3 (3) 114-121.
Ahmed F.,Ahmed I.,Khan M S.,(2008).Screening of free living rhizospheric bacteria for their multiple plant growth promoting activities. Microbial Research 163 (suppl 2) 173-181.
Apastamph A.R., and Beig M.M.V. (2014) Screening of Fluorescent pseudomonas for plant growth promoting activity.CIB tech Journal of Microbiology,Vol .3 (4), 47-51
Banu N and Musarrat (2004) Charaterization of a novel carbofuran degrading Pseudomonas sp with collateral biocontrol and plant growth promoting potential.FEMS Microbial let.231:13-17.
Battu P.R. and Reddy M.S.(2009) Isolation of secondary metabolites from Pseudomonas fluroscenes and it characterization. Asian Journal of Research Chemistry.2 26-29.
Belkar Y.K.,R.M.Gade.(2012) Biochemical characterization and growth promotion activities of Pseudomonas fluorescens .J.Pl.Dis.Sci.Vol 7 (2) (170-174).
Budzikiewicz,H (1993) Secondary metabolites from Fluorsecent pseudomonas ,FEMS,Microbial rev.104 209-228
Cardenas rodriguez, rodriguez berbal N,Canosa perez fragero I and Camacho M(2019) Biosaia (revista de los másteres de Biotecnología Sanitaria y Biotecnología Ambiental, Industrial y Alimentaria de la UPO) n08 march.
Dava B.P., and Dube H.C. (2000) Chemical characterization of fungal siderophores ,Indian Journal of Experimental Biology 38, 56-62.
Deshwal VK and Punkaj Kumar., (2013) Production of plant growth promoting substance by Pseudomonads.Journal of academia and industrial research. 2 ( 4) 221-225.
Fekadualemu .(2013) Isolation of Pseudomonas fluroscenes species from rhizospheric soil of faba bean and their assessment of their siderophore production.Int.J.of Advanced research Vol 1 8 203-210.
Glick B.R.(1995) The enhancement of Plant growth by free living bacteria .Can.J.Microbiol. 41 (2) 109-117.
Holt J.G.,Krieg N R.,Sneath P.H.A.,Staley J.T.,and Williams S.T.(1994):Bergeys manual of determinative bacteriology.London:9 th Edition –Williams and Willkins co,Baltimore
Joseph B.,Patra R.P., and Lawrence R.,(2007) Characterization of plant growth promoting rhizobacteria associated with chickpea.Cicer arietinum.Int.J.Plant Produc.Vol 1 2 141-152.
Josip Colo.,Timea I Hajnal –Jafari, Simonida Duric,Dragana Stemenov and Saud Hamidovic (2014) Plant growth promotion rhizobacteria in onion, Production.Vol 63,No 1,83-88
Kannahi M., Senbagam N.(2014) Studies on siderophore production by microbial isolates obtained from rhizosphere soil and its antibacterial activity. Journal of Chemical and Pharmaceutical Research 6(4) 1142-1145.
Kaushal Shruti., Karnwal Arun and Rai Yuvneet.(2013) Potential plant growth promoting activity of rhizobacteria Pseudomonas sp in oryza sativa.J.Nat.Prod.Plant Resour.vol 3 4:38-50
Kloepper JW.,Leong J.Teintze M.,Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria.Nature 268 (1980) 885- 886
Payne S.M.(1994),Detection Isolation and characterization of siderophores,Methods in Enzymol,235 329-344
Prashantkumar S. Chakra ,P.G. Vinay Kumar and C.T. Swamy (2019) Isolation and Biochemical Characterization of Plant Growth Promoting Bacteria from a Maize Crop Field. Int.J.Curr.Microbiol.App.Sci (4): 1415-1422
Rajat Maheshwari, Namita Bhutani, Pooja Suneja (2019) Screening and characterization of siderophore producing endophytic bacteria from Cicer arietinum and Pisum sativum plants. Journal of Applied Biology & Biotechnology Vol. 7 (05) 7-14,
Reetha S.Bhuvaneswari G.,Thamizhiniyan P and Ravi mycin T.(2014) Isolation of indole acetic acid producing rhizobacteria of Pseudomonas florescence and Bacillus subtilis and enhance growth of onion.(Allium cepa.L.).International Journal of Current Microbiology and Applied Sciences, vol 3 (2).568-574.
Rodriquez H.,Fraga R.,Gonzalez T and Bashan Y.(2006) Genetics of phosphate solubilisation and its potential application for improving plant growth promoting bacteria, Plant and Soil. Vol 287, 15-21.
Sullivan O DJ and O Gara F(1992) Traits of fluorescent pseudomonads sp.involved in suppression of plant root pathogens, Microbiological Reviews 56 (1992) 662-676.
Schwyn B. and Neilands J.B. (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem.160. 47-56.
Syamala M and Sivaji M. (2017) Isolation and functional characterization of plant growth promoting rhizobacteria against soft rot in Aloe Vera (L).Journal of Entomology and Zoology., 5(5) 187-191.


