Biology Department, Faculty of Science, King Abdulaziz University Jeddah, Saudi Arabia,
Corresponding author email: meshafi@kau.edu.sa
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 17/09/2020
Over the past half century, killing of agricultural pests or insect by synthetic chemical pesticides in the field or post-harvest the crops cause insect resistance and health hazards to man and pollute the environments. There is a great need for developing alternative approaches to control harmful insect pests. Essential oils (EOs) and their nanoemulsions (NEs) have broad insecticidal activity against some bests. This study was conducted to investigate toxicological effects of some EOs and their nanoemulsions on the early life stage (24 hr post-hatch nauplii) of brine shrimp Artemia salina. Oil of four plants (Neem, Eucalyptus, Clove and Basil) were collected and their NEs either alone or mixed with Neem were prepared and characterized. The diameter of the nano- particles were 200.1, 211.9, 218,7 and 288.7 nm for Neem, Clove, Basil and Eucalyptus, respectively. All the particles had negative charge. The average diameters were 266.2, 425.1, and 316.1 for Neem+Clove, Neem +Basil and Neem +Eucalyptus, respectively and all the prepared nanoemulsion particles have negative charge. It was found that increasing concentrations of essential oil increased mortality percentage up to 100 %. Moreover, increasing time increased percentage of mortality. The mortality levels were 100 at concentrations of 24, 20 and 16 % after 24, 48 and 72 hr, respectively. The lethal concentration 50 (LC50) of these oils and NEs on Artemia salina nauplii was determined after 24 hours of exposure The LD50 of the tested neem oil was calculated at 50% mortality level.
Similarly, effect of different concentrations of neem oil nanoemulsion on percentage of mortality of Artemia salina nauplii after 24, 48 and 72 hr were determined and compared and increasing concentration of neem oil nanoemulsion increased percentage of mortality and increasing time increased also the percentage of mortality. From the previous results, LD50 doses for neem oil nanoemulsion were 12, 10 and 8 % after 24, 48 and 72 hr. LD50 was calculated after 24 hr for each oil or nanoemulsion prepared from single or mixed oils. LD50 values were ranged from 16-46 % for the tested oil and from 12-41% for nanoemulsion of essential oils. Neem oil showed the greatest activities against the tested larva. In conclusion, essential oils of Neem, Eucalyptus, Clove and Basil, applied singly or mixed with neem and their prepared nanoemulsions have broad insecticidal activity and can be used safely against some insect bests
Oil, insecticidal, Neem, Eucalyptus, Clove, Basil, nanoemulsions, LD50
Shafi M. Chronic and Acute Toxicity of Crude Oils and their Nanoemulsions on the Viability of Larvae of Brine Shrimp, Artimia salina Leach. Biosc.Biotech.Res.Comm. 2020;13(3).
Shafi M. Chronic and Acute Toxicity of Crude Oils and their Nanoemulsions on the Viability of Larvae of Brine Shrimp, Artimia salina Leach. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3jCAkYi
INTRODUCTION
Synthetic chemical pesticides are mainly used to treat many agricultural pests in the field and post-harvest to protect the crops but with time insect developed a resistance to the synthetic used pesticides. Resistant to Malathion, DDT, deltamethrin and bio-pesticides (Bacillus thuringiensis) was increased which affect human health and need high operational cost. Moreover, these synthetic chemical pesticides cause environmental pollution and many health hazards to warm-blooded animals. There is a need for developing alternative materials to inhibit insect and pests (Isman, 2000 a, b; Yang et al., 2005).Environmental factors may affect the shelf life of nanoemulsion and phyto-nanoemulsions are eco-friendly and effective formulation to combat insects (Sharma et al., 2020).
Therefore, one of the alternative materials with low impact on environment, and safe for human health is natural products or secondary metabolites of plants. More than 2400 plants belonging to 189 families were found in nature (Rao et al., 2005) and contained thousands of essential oils and active compounds like phenolics, terpenoids and alkaloids which may control insects. Some plants natural products can be used as pest control agents instead of the used and unsafe synthetic pesticides. Generally plant essential oils are considered broad-spectrum insectside that are safe for the environment due to high biodegradation rate by soil microbes (Rajendran and Sriranjini, 2008; Devi & Maji, 2011Rodríguez-González et al., 2019).
Lipophilic characters of essential oils made it suitable to be easy used as toxins, feeding deterrents, repellent, oviposition deterrents to broad diversity of insect pests. The aims of this study was to detect biological activities of some essentials oils and their nanoemulsion against Artemis salina as test organism for their potential uses as alternative materials to control insects pests. A wide range of insects were affected by essential oils and they found that essential oils of Pinus brutia, Laurus nobilis, Cupressus sempervirens, Lavandula stoechas, L. angustifolia, Eucalyptus camaldulensis and Thymus vulgaris were active against many dangerous insects (Kanat and Hakki Alma, 2003). Also, Sampson et al. (2005) reported the insecticidal activities of 23 oil plants as Bifora, Satureja, Coridothymus, Thymbra and Pimpinella using dosage-mortality bioassays and turnip aphids as test organisms.
According to Raina et al. (2007) orange oil (containing ~92% d-limonene) caused 96 % mortality to Coptotermes formosanus within 5 days due to feeding reduction. Upadhyay et al. (2007) tested 14 oils from Azadirachta indica, Cinnamomum cassia, Cleome gynandra, Cuminum cyminum, Carum copticum,, Cymbopogon narudus, Eugenia aromaticum and Nigella sativa and LC50 values were ranged from 0.85-1.25 µl/ml due to inhibition of oviposition and repellent activity. Ayvaz et al. (2010) reported that essential oils of Origanum onites, Satureja thymbra and Myrtus communis can be used as efficient insecticides. After 24 hr of application, the essential oils of O. onites and S. thymbra at 9 and 25 µl/ml showed inhibitory effects on some insects with 100% mortality level.
Also, the same effect was recorded by Ebadollahi and Ashouri (2011) for Achillea millefolium, Artemisia dracunculus and Heracleum persicum with mortality levels of 100% at 50, 65 and 80 µl/ml. The LC50 values of some essential oils from A. dracunculus, A. millefolium and H. persicum were 22.24, 34.80 and 36.96 µl/ml after 24 h and the LC50 values decreased with increasing exposure times. Essential oils obtained from Carum carvi and Thymus vulgaris had LD50 of 197 and 250 µg/ cm2, respectively. The Insecticidal action of Lavandula hybrida, Rosmarinus officinalis and Eucalyptus globulus oils and of their major constituents was reported by Papachristos et al. (2004) and with LC50 values was ranged from 0.8 to 47.1 mg/l.
Application of EOs in industrial, agriculture is difficult due to their no solubility in water but formation of nanoemulsions from these oils entranced the solubility. The use of nanotechnology in agriculture and crop protection (Khot et al., 2012; Pavela and Benelli, 2016) is needed to overcome the EOs low water solubility (Donsi et al., 2012). Encapsulation of EOs in the form of oil-in-water nanoemulsions (oil droplet <100 nm) could be an effectively possible solution for the mentioned difficulty (Suresh-Kumar et al., 2013).The prepared organic nanoemulsions have drawn a great deal of interest to different sectors with a great number of applications in drugs, pharmaceutical, nutraceuticals, food and agriculture products, and cosmetics formulation. This study aimed to screening of some essential oil or their prepared nanoemulsions for insecticidal activities against using Artimia salina as test organism.
MATERIAL AND METHODS
The used essential oils :The essential oils of Neem, Clove, basil and Eucalyptus were purchased from local markets, Jeddah.
Preparation of Nanoemulsions: Nanoemulsions from EOs Neem (Azadirachta indica) Clove (Syzygium aromaticum), Basil (Ocimum basilicum) and Eucalyptus (Eucalyptus globulus) were prepared. The NEs were prepared using the High Pressure Homogenization (HPH) technique (Nenaah, 2015). The oil samples were diluted with a large amount of water (ratio 1:100) followed by the addition of the surfactant mixtures of Tween 80 and span 20 at a constant ratio of 2:1, respectively. Pre-emulsions were obtained by high speed stirring using an Ultra Turrax T25 (IKA Labortechnik, Jahnke und Kunkel, Germany) at 24,000 rpm for 5 min. Then, the pre-emulsions were passed 5 times through an orifice high pressure homogenizer Nano DeBEE Electric Benchtop Laboratory (BEE International, USA) at 300 MPa. The resulting Nanoemulsions formulas were stored at room temperature, 25°C until used.
Detection of size and morphological characterization of nanoemulsion droplets using Transmission Electron Microscope (TEM):The particle size analysis and morphology was determined using Transmission Electron Microscope at King Abdulaziz University, Faculty of Science. Three different diameters of each particle were determined and mean value was calculated and standard deviation (SD) was determined (Nenaah, 2015).
Preparation a mixture of 2 oils:By mixing the Neem oil with the other tested oils (Clove, Basil, and Eucalyptus ) at a ratio of 1:1, v/v.
Preparation a mixture of 2 nanoemulsions: Proper fraction of 2 essential oils at ratio 1.1 and distilled water were mixing and heated, followed by the addition of the surfactant mixtures of Tween 80 and span 20 at a constant ratio of 2:1, respectively. By the addition of the warm T80 drop wise along with vortexing. The resulted mixture was heated at high temperature up to 80°C with continuous mixing until one phase emulsion was produced. After that, the warm S20 was added drop wise to the previous mixture with a continuous mixing. This resulted solution was mixed and heated continuously until a clear and transparent solution gets formed (Al-Sowayigh et al., 2019).
Characterization of the prepared NE formulation: Samples were quantified at 25 ± 0.2°C by Zetasizer (3000 HS, Malvern Instruments, Malvern, UK). The oil phase was assumed to have a refractive index of 1.45 and the water phase of 1.33. All the produced formulas were acidic in nature (Al-Sowayigh et al., 2019).
Toxicity test: The cytotoxicity of the purified metabolites were determined using brine shrimp lethality test (Meyer et al., 1982). The tested oil in DMSO at varying concentrations were incubated with the brine shrimp larvae in sea water, and control brine shrimp larvae were incubated in a mixture of sea water and DMSO only. After 24 hr, 48hr or 72 hr the average number of larvae that survived at each concentration was determined and LD50 was calculated (Aly and Gumgumji, 2011).
Statistical analysis: All data were expressed as mean ± standard deviation (±SD). Statistical analysis was performed with two-way analysis of variance (ANOVA) and t-test. The statistical significance difference was considered when p-value≤0.05.
RESULTS AND DISCUSSION
Four tested plant oils named Neem, Clove, Basil and Eucalyptus were collected and their nanoemulsions were prepared and characterized. The average diameter of particles were measured using Zetasizer and compared. The average diameters were 200.1, 211.9, 218,7 and 288.7 nm for Neem, Clove, Basil and Eucalyptus, respectively (Table 1, Figure 1). All the particles had negative charge. Similarly, the mixtures of either Clove, Basil or Eucalyptus with Neem were prepared and their nanoemulsion were formed and characterized. The average diameters were 266.2, 425.1, and 316.1 for Neem+Clove, Neem +Basil and Neem +Eucalyptus, respectively (Table 2) and al the prepared nanoemulsion particles have negative charge. The average diameters of two mixed nanoemulsions were higher compared to control oil at p≤ 0.05.
Table 1.The physical characterization of the different NE formulations measured by the Zetasizer.
| Formulation | z-
average diameter (nm) |
CV%=
Coefficient of variation |
Zeta potential (mV)’ |
| Neem | 200.1 | 11.1 | -5.25±1.23 |
| Clove | 211.9 | 7.31 | -3.09±1.53 |
| Basil | 218.7 | 13.39 | -1.27±1.02 |
| Eucalyptus | 288.7 | 19.39 | -1.11±1.09 |
Figure 1: Particle size and zeta potential distribution of neem oil NE determined by the Zetasizer.
Table 2. The physical characterization of the mixed NE formulations measured by the Zetasizer.
|
Formulation |
z-
average diameter (nm) |
CV%=
Coefficient of variation |
Zeta potential (mV) |
| Neem + Clove | 266.2 | 9.29 | -3.22 ±1.54 |
| Neem + Basil | 425.1 | 10.40 | -1.12±1.24 |
| Neem + Eucalyptus | 316.1 | 41.16 | -1.19±1.45 |
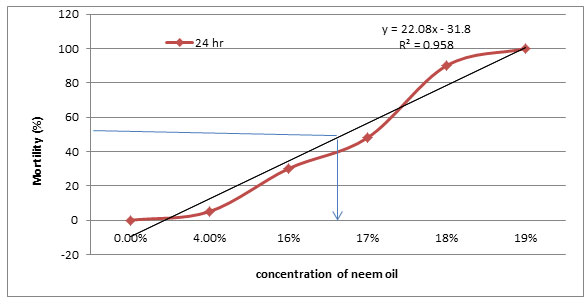
The effect of different concentrations of neem oil on percentage of mortality of Artemia salina nauplii after 24, 48 and 72 hr were determined and compared (Figure 2). It was found that increasing concentrations increased mortality percentage up to 100 %. Moreover, increasing time increased percentage of mortality. The mortality levels were 100 at concentrations of 24, 20 and 16 % after 24, 48 and 72 hr, respectively. The LD50 of the tested neem oil was calculated at 50% mortality level (Figure 3). Similarly, effect of different concentrations of neem oil nanoemulsion on percentage of mortality of Artemia salina nauplii after 24, 48 and 72 hr were determined and compared (Figure 4). As shown in Figure 4, it is clear that increasing concentration of neem oil nanoemulsion increased percentage of mortality and increasing time increased also the percentage of mortality. From the previous results, LD50 doses for neem oil nanoemulsion were calculated and they were 12, 10 and 8 % after 24, 48 ad 72 hr.
Figure 2: Effect of different concentrations of neem oil on percentage of mortality of Artemia salina nauplii after 24, 48 and 72 hr.
Figure 3: LD50 of neem oil detected using Artemia salina after 24 hr of incubation.
For each tested oil or nanoemulsion, different concentrations were prepared and LD50 was calculated after 24 hr for each oil or nanoemulsion prepared from single or mixed oils. Concerning the tested oil or their mixture with neem oil, the LD50 values were ranged from 16-46 % and the LD50 calculated values for nanoemulsion of essential oils were decreased to be ranged from 12-41%. The differences between lethal concentrations of the tested essential oils alone or mixed of two oils and their prepared nanoemulsions was significant at p≤ 0.05. Furthermore, there is a significant difference between the activities of the tested oils (LSD 11.08) and their prepared nanoemulsion (LSD 9.9).
There is an increasing request for new active materials for pest control with low adverse effects on human health and the environment. Essential oils played significant roles in plant protection against insect pests and they were simply extracted, biodegradable, ecofriendly, safe for the environment, soil and water with little or no toxicity for man, fishes and birds (Misra and Pavlostathis, 1997; Isman, 2000a, 2006, Isman and Machial, 2006; Bakkali et al., 2008). Essential oils was prepared in dimethyl sulfoxide (DMSO) which is non-toxic emulsifying agent, enhance the penetration of the waxy layer (cuticle) of the insect.
Thus, essential oils can act as rapid contact material for insects. The oil of Eucalyptus possesses a wide spectrum of biological activity including anti-microbial, fungicidal, insecticidal/ insect repellent, herbicidal, acaricidal and nematicidal (Batish et al., 2008). Neem oil is a vegetable oil obtained from the fruits or seeds of the Azadirachta indica which is known as neem tree. The evergreen tree of neem is endemic to many areas in the tropical region. The previous oil has many uses in agriculture and medicines. Ocimum basilicum (Basil) and Cymbopogon winterianus (Citronella) essential oils were used for best control at doses 60 -120 μl/l and Basil and Citronella oils exhibited similar patterns of insecticidal activity over the insects (Rodríguez-González et al., 2019).
Figure 4: Effect of different concentration of neem oil Nanoemusions on the percentage of mortality of Artemia salina nauplii after 24, 48 and 72 hr.
Table 3. The calculated LD50 of the tested oils and their nanoemulsions after 24 hr of incubation
| Tested oil (control) | LD50 ( %) | Tested oil nanoemulsion (NEs) | LD50 (%) |
| Neem | 16.7±1.8 | Neem | 12.4±3.1* |
| Clove | 32.33±1.4 | Clove | 23.9±2.9* |
| Basil | 59.1±4.6 | Basil | 41.2±1.9* |
| Eucalyptus | 46.2±6.8 | Eucalyptus | 33.3±7.0* |
| Neem + clove | 14.2±1.1 | Neem + clove | 10.1±1.9* |
| Neem + Basil | 40.1±3.5 | Neem + Basil | 30.9±2.9* |
| Neem + Eucalyptus | 40.1±7.9 | Neem + Eucalyptus | 23.7±5.1* |
| LSD : 11.0897 | LSD: 9.9812 | ||
*: significant results compared to control oil
Eugenol was the major compound of Clove essential oil which act as a promising alternative insecticidal material under storage conditions for control insect pests with 100% mortality level after 48 h with 17.9 – 35 μl/g. The LC50 was calculated to be 9.45 μl/g for A. obtectus (Jairoce et al., 2016). Many of the widely used pesticides which are applied in agricultural had low water solubility and highly hydrophobicty, thus, they must be loaded within suitable carrier before use. Several natural products with insecticidal activity have poor water solubility, including triterpenes, and nanotechnology has emerged as a good alternative to solve this main problem Thus, oil-in-water nanoemulsions were used to prepare some pesticides to ensure the efficacy, strong surface adhesion, high penetrability, and many applications. Nano size of droplets amplifies and affect the biological behavior of the nanoemulsions; characteristics of ideal nanoemulsion are low diameter, low viscosity and high zeta potential. Higher stability is dependent on the quantity and composition of surfactants. Environmental factors may affect the shelf life of nanoemulsion and phyto-nanoemulsions are eco-friendly and effective formulation to combat insects (Sharma et al., 2020).
Mentha piperita EO nanoemulsion could be easily prepared through high-energy ultrasonication process and the formulated nanoemulsions were physically stable over 12 months, which makes them interesting candidates for practical applications. EO of M. piperita could be used to kill cotton aphid A. gossypii pest and improve food production. The higher concentration of M. piperita nanoemulsion caused significantly higher mortality in the adult cotton aphid. Contact toxicity LC50 of the synthesized nanoemulsion in all formulation concentrations varied between 3852 and 3941 ppm, under laboratory conditions (Fernandes et al., 2014).
REFERENCES
Al-Sowayigh M, Aly M M. and Abounasef S (2019). Inhibitory effects of novel formula of oil in water Nanoemulsions on some pathogenic bacteria associated with wound infections. IOSR Journal of Pharmacy And Biological Sciences Volume 14, Issue 5 Ser. III, PP 51-57.
Aly MM, Gumgumjee N (2011) Antimicrobial efficacy of Rheum palmatum, Curcuma longa and Alpinia officinarum extracts against some pathogenic microorganisms. Afr J Biotechnol 10:12058–12063
Ayvaz, A., Sagdic, O., Karaborklu, S. and Ozturk, I. (2010). Insecticidal activity of the essential oils from different plants against three stored-product insects. Journal of Insect Science, 10: 21.
Bakkali, F., Averbeck, S., Averbeck, D., Idaomar, M., 2008. Biological effects of essential oils.. Food Chem. Toxicol. 46, 446–475.
Batish D. R, Singh H P , Kohli R K, Kaur S .(2008). Eucalyptus essential oil as a natural pesticide. Forest Ecology and Management 256: 2166–2174
Devi N. and Maji T. K. (2011). Neem seed oil: Encapsulation and controlled release – search for a greener alternative for pest control, pesticides in the modern world – pesticides use and management, Dr. Margarita Stoytcheva (Ed.), ISBN: 978-953-307-459-7, InTech, 520 page.
Donsi F, Annunziata M, Vincensi M, Ferrari G (2012) Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol 159(4):342–350.
Ebadollahi, A. and Mahboubi, M. (2011). Insecticidal activity of essential oil isolated from Azilia eryngioides (Pau) Hedge Et Lamond against two beetle pests. Chilean Journal of Agricultural Research, 71(3): 406–411.
Fernandes, C.P., de Almeida, F.B., Silveira, A.N. et al. (2014). Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J Nanobiotechnol 12, 22: 22-26.
Gomah NE, Ibrahim SIA, Al-Assiuty B A (2015). Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). Volume 61, Pages 9-16.
Isman, M.B. (2000a). Pesticides based on plant essential oils. Pestic. Outlook., 10: 68–72.
Isman, M.B. (2000b). Plant essential oils for pest and disease management. Crop Prot. 19, 603–608.
Isman, M.B., 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66.
Isman, M.B., Machial, C.M., 2006. Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai, M., Carpinella, M.C. (Eds.), Naturally Occurring Bioactive Compounds. Advances in Phytomedicine, vol. 3. Elsevier, pp. 29–44.
Jairoce C F., Teixeira C M., Nunes C F. P, Nunes A M, Pereira CMP and Garcia F R M (2016). Insecticide activity of clove essential oil on bean weevil and maize weevil. Rev. Bras. Eng. Agríc. Aambient., Vol. 20 No.1 : 23-29.
Kanat,. M. and Hakki Alma, M. (2003). Insecticidal effects of essential oils from various plants against larvae of pine processionary moth (Thaumetopoea pityocampa Schiff) (Lepidoptera: Thaumetopoeidae). Pest Manag Sci., 60: 173– 177.
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlan JL (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Plant Med 45:31–34.
Misra, G., Pavlostathis, S.G., 1997. Biodegradation kinetics of monoterpenes in liquid and in soil-slurry system. Appl. Microbiol. Biotechnol. 47, 572–577.
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21(12):1000–1007.
Raina, A.K., Bland, J., Dollittle, M., Lax, A., Boopathy, R. and Lolkins, M. (2007). Effect of orange oil extract on the formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol., 100: 880–885.
Rajendran, S. and Sriranjini, V. (2008). Plant products as fumigants for storedproduct insect control. J. Stored. Prod. Res., 44: 126–135.
Rao, N.V., Maheswari, T.U. and Manjula, K. (2005). Review on Botanical Pesticides as Tools of Pest Management, pp: 1–16. Narosa Publishing House Pvt., Ltd.
Rodríguez-González Á, Álvarez-García S, González-López Ó, Silva FD, Casquero PA (2019). Insecticidal properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, insect pest of the common bean (Phaseolus vulgaris, L.). Insects, 25;10(5):15
Sampson, B.J., Tabanca, N., Kirimer, N., Demirci, B., Husnu Can Baser, K., Khan, I.A., Spiers, J.M. and Wedge, D.E. (2005). Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag Sci., 61: 1122–1128.
Sharma S. Loach N and Gupta S (2020). Phyto-nanoemulsion: An emerging nano-insecticidal formulation. Environmental Nanotechnology, Monitoring and Management. Volume 14, December, 100331.
Suresh-Kumar RS, Shiny PJ, Anjali CH, Jerobin J, Goshen KM, Magdassi S, Mukherjee A, Chandrasekaran N (2013) Distinctive effects of nano-sized permethrin in the environment. Environ Sci Pollut Res Int 20(4):2593–2602.
Suresh-Kumar RS, Shiny PJ, Anjali CH, Jerobin J, Goshen KM, Magdassi S, Mukherjee A, Chandrasekaran N (2013) Distinctive effects of nano-sized permethrin in the environment. Environ Sci Pollut Res Int 20(4):2593–2602.
Upadhyay, R.K., Gayatri, J. and Neeraj, Y. (2007). Tocixity, repellency and oviposition inhibitory activty of some essential oils against Callosobruchus chinensis. J. Appl. Biosci., 33(1): 21–26.
Yang, P., Yajun, M.A. and Shuiqing, Z. (2005). Adulticidal activity of five essential oils against Culex pipiens quinquefasciatus. J. Pestic Sci., 30: 84–9.