1Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai Tamil Nadu, India.
2Lingam Microbiological Laboratory, Kanchipuram, Tamil Nadu, India;
3Central Research Laboratory Meenakshi Medical College Hospital & Research Institute, Meenakshi Academy of Higher Education and Research Institute, Kanchipuram, Tamil Nadu, India
4Sathyabama Institute of Science & -Technology, Chennai – 600119
5California University of Science & Medicine, School of Medicine, CA, USA
6Musculoskeletal Disease Research Laboratory US Department of Veteran Affairs, Loma Linda, CA, USA.
Corresponding author email: kpm_suresh@yahoo.com
Article Publishing History
Received: 18/04/2020
Accepted After Revision: 16/06/2020
Incorporation of chemicals in the antiseptics and cosmetic products has increased. Natural antiseptics are non-toxic and ideal for human skin; they are eco-friendly and biodegradable. Numerous researches are focusing on herbal plant-based studies for biomedical therapeutic application. This study was designed to examine antiseptic potential of certain essential oils (Lemon grass oil, Cinnamon oil, Citronella, Cedar wood oil, Lemon oil, Lavender oil, Tea tree oil, Sandal wood oil, Basil oil. Essential oils (n = 9) were selected based on the previous studies, the selected essential oils were purchased from Naturoman Pvt. Ltd., Antimicrobial testing of the essential oils was done by CLSI standard, further the antiseptic application of essential oil was tested by American Society for Testing and Materials (ASTM) standards. In addition, Drug resistant clinical pathogens were used to screen antiseptic activity. The following drug resistant pathogens were isolated from our previous studies, Such as Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Candida albicans. Antioxidant property of essential oil by 2,2-diphenyl-1-picrylhydrazyl (DPPH). 9 essential oils were subjected to antimicrobial testing with drug resistant isolates from DFU, Cinnamon oil, Lavender oil, and Tea tree oil have broad-spectrum activity of 0.125-2µg/ml against drug-resistant S. aureus E. coli, and C. albicans. And it has 0.6-0.8µg/ml of antioxidant property done by DPPH method. ASTM standard such as time-kill assay, Anti-biofilm, and Real-life disinfectant efficacy testing showed promising activity for Tea tree oil and Cinnamon amalgamation. The present study showed the importance of essential oil alone and mixture in antiseptic and cosmetic applications. Thus, essential oil blend might be a prospective source of alternative antimicrobial agents and may play a vital role in antiseptic and skin care product development.
Antiseptic, Essential Oils, Multidrug Resistant Pathogens
Joseph J, Rajesh D, Suresh A, Radhakrishnan M, Rajasekar T, Gopikrishnan V, Suresh S, Aruni W. Antiseptic Efficacy of Combination of Certain Essential Oils Against Multi Drug Resistant Pathogens. Biosc.Biotech.Res.Comm. 2020;13(2).
Joseph J, Rajesh D, Suresh A, Radhakrishnan M, Rajasekar T, Gopikrishnan V, Suresh S, Aruni W. Antiseptic Efficacy of Combination of Certain Essential Oils Against Multi Drug Resistant Pathogens. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2ZRNVUm
Copyright © Joseph et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Antibiotic resistance is documented to be a serious problem that affects the choice of appropriate antibiotic therapy and increases the probability of unfavorable infection outcome. One of the proposed methods to cope with multidrug-resistant (MDR) bacteria is the use of alternative antibacterial treatments, which include natural antimicrobial substances such as plant essential oils (EOs) (Kon et al., 2012). In addition to dramatic events of this type is the ongoing struggle to identify new and effective antibiotics, especially against Gram-negative bacteria; new classes of effective antibiotics have not yet emerged and the prospects of this happening in the near future are low (Piddock et al.,). Clinicians frequently reach a therapeutic limit when treating patients with serious bacterial infections and efforts are being made worldwide to find new treatments for resistant bacteria. New approaches include educating the public, improving sanitation infrastructures, imposing strict regulations on antibiotic prescription, developing new antibiotics, reevaluating old and rejected antibiotics, abolishing the nontherapeutic use of antibiotics (e.g., in agriculture and animal feed), and developing nonantibiotic approaches that can successfully prevent and protect against infectious diseases, (Carlet et a., 2011 Elcocks (2020).
Herbal plants are considered as good antioxidant since ancient times. Aromatic and medicinal plants, through their secondary metabolism, provide a complex mixture of volatile molecules known as essential oils. These volatile molecules exert antibacterial activity that has been used in folk medicine for centuries. During the last few decades, the emergence of antibacterial resistance has forced us to search for new and efficient antimicrobial agents. Moreover, the use of essential oils and their components in combination with antibiotics may increase bacterial susceptibility, thus limiting resistance (Faleiro and Miguel 2013). Essential oils are known to possess potential as natural agents for food preservation. Many of them recently have been qualified as natural antioxidants and proposed as potential substitutes for synthetic antioxidants in specific sectors of food preservation where their use is not in contrast with their aroma (Ruberto & Baratta, 2000). An antioxidant can be broadly defined as any substance that delays or inhibits oxidative damage to a target molecule (Yamagishi et al., 2011). The main characteristic of an antioxidant is its ability to trap free radicals. Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases, (Wu et al., 2011 Elcocks (2020).
The different antioxidant assays is available and, because results rely on different mechanisms, they strictly depend on the antioxidant models employed and on lipophilic/hydrophilic balance. A single-substance/single-assay produces relative results, and it is perceived as a reductive approach whenever a phytocomplex is involved. Therefore, a multiple-test and a simultaneous chemical characterization must be taken into account whenever assays of essential oils are performed to allow a balance between the sensory acceptability and functional properties (Saccheti et al., 2005).
Hence this present study was undertaken to evaluate the antiseptic efficacy of essential oils
MATERIAL AND METHODS
Essential oils: The following nine essential oils (Lemon grass oil, Cinnamon oil, Citronella, Cedar wood oil, Lemon oil, Lavender oil, Tea tree oil, Sandal wood oil, Basil oil) Purchased from Naturoman Pvt. Ltd(table-1).
Antimicrobial Susceptibility test:Preliminary Screening of essential oils for antimicrobial characterization was done by the agar well diffusion method. All the test pathogen with appropriate standard cultures (Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Pseudomonas aeruginosa ATCC 27853, Escherichia coli, ATCC 35218, Klebsiella pneumoniae ATCC 700603 and Candida albicans ATCC 90028), were prepared according to McFarland standard. The 18 h bacterial cultures were adjusted to approximately 105CFU/ml with sterile saline solution. Lawn culture were made on Mueller-Hinton agar using a sterile cotton swab for uniform microbial growth. The essential oils were diluted in DMSO with 0.5% Tween 80. Well were made at 6mm diameter in inoculated MHA agar plate. A standard mineral oil and 5μg concentration of Ciprofloxacin was used as positive control. Then the plates were incubated at 37°C for overnight. After incubation, the zone of inhibition was measured with standard procedures.
Minimum Inhibitory concentration were performed 9 oils based on preliminary antimicrobial results such as Cinnamon, lemon, lemon grass, and tea tree oil were identified to have potent antimicrobial activity and their Minimum Inhibitory Concentrations (MIC) were determined. The standard broth dilution method followed by the Clinical Laboratory standard institute, 2018. Standard two fold dilution method followed for each oil, ranging from 1 to 512 µg/ml, was prepared in Muellur Hinton broth. 0.5 McFarland standard broth culture suspension were prepared and inoculated in individual MIC tube, Inoculated tubes were incubated at 37°C for overnight for bacteria for fungi 24-72 hrs based. The MIC were determined as the lowest concentration of oil inhibiting visible growth of each organism on the tube.
Organisms used : The bacterial test strains used in this study were Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, Candida krusei ATCC 6258, Candida albicans ATTC 10231. clinical pathogens were used to screen antiseptic efficacy, the pathogens were isolated from my previous studies, it was stored and maintained, Drug resistant S. aureus (31), E. coli (11), K. pneumoniae (07) and C. albicans (08). The bacterial strains were maintained on 60% glycerol BHI broth at -200C in the Lingam Microbiological Laboratory, Kanchipuram.
Synergistic effect of Essential oil: Effect of Tea tree oil and cinnamon oil; Tea tree oil and Lemon grass oil mixture were tested for synergistic activity against routine bacterial, viral and fungal pathogens. This study could serve as a baseline data to investigate new combination of essential oil for antiseptics.
Antioxidant activity of essential oil by DPPH method:The hydrogen atoms or electrons donation ability of the corresponding extracts and some pure compounds were measured by bleaching the purple colored methanolic solution of DPPH. The effects of methanolic extract and essential oil on DPPH radicals were evaluated according to a method described elsewhere (21). 4 mL samples of various concentrations of the extracts in methanol were separately added to a 1 mL solution of DPPH radical in methanol (final concentration of DPPH was 0.2 mM). The mixture was shaken vigorously and allowed to stand for 30 min after which the absorbance of the resulting solution was measured at 517 nm with a spectrophotometer.
Inhibition of free radical DPPH as percentage [I(%)] was calculated as follows:
I (%) = 100 × (Ablank -A sample) / Ablank
where A blank is the absorbance of the control (containing all reagents except the test compound) and A sample is the absorbance of the test compound. EC50 value (μg /mL) is the effective concentration at which DPPH radicals are scavenged by 50%. This was obtained by interpolation and using linear regression analysis.
Time kill assay for the essential oil blend: Sterile 96 well microtitre plates were used test with Muller Hinton broth for the determination of the time kill assay. Briefly, 180 μl of sterile freshly prepared MH broth was added in each well. 10μl of essential oil were added to the media. 10 μl of the standard bacterial strains was inoculated in test medium. Every 30mins, killing activity was measured by spot culture in new plate MHA, this procedure repated every 20mins for 6 hrs.
Anti-biofilm assays: Antibiofilm activity was carried out in polystyrene microtitre plate method. An overnight culture of standard strains was diluted to the McFarland standard 0.5. After the addition of different concentrations of Essential oil1-128 μl/ml of Muller Hinton broth, 10-μl broth culture was inoculated in all tube except negative control. incubated for 48 to 72 h at 370C. Thereafter Planktonic cells of Standard strains is discarded, 100μl of 1% (w/v) aqueous solution of crystal violet will be added to microtitre plate and incubated at room temperature for 20 minutes. Then crystal violet solution is removed from the microtitre plate and it will be washed thoroughly with sterile water. For quantification of attached cells, the bound crystal violet solubilized in dimethyl sulfoxide (DMSO) and spot culture method.
Table 1. Selected Essential oil for antimicrobial studies
| s.no. | Common name | Botanical Name | Part used |
| 1 | Lemon | Citrus limonum | Fruit skin |
| 2 | Basil | Ocimum basilicum | Leaf |
| 3 | Cinnamon Bark | Cinnamomum zeylanicum | Bark |
| 4 | Lavender | Lavandula officinalis | Flower |
| 5 | Citronella | Cymbopogon nardus | Grass |
| 6 | Pepper Mint | Mentha piperita | Leaf |
| 7 | Lemon grass | Cymbopogon citratus | Grass |
| 8 | Cedar wood | Cedrus libani | Wood |
| 9 | Eucalyptus | Eucalyptus polybractea | Leaf |
RESULTS AND DISCUSSION
Antimicrobial assay: Agar well Diffusion method:
Lemon grass oil showed highest antimicrobial activities against C. krusei_ATCC 6258 (19 mm) followed by E. faecalis_ATCC 29212 (18mm), E. coli_ATCC 25922 and P. aeruginosa_ATCC 27853 (17mm), S. aureus_ATCC 29213 (13mm) and minimum inhibition against C. albicans_ATTC 10231 (12mm).Cinnamon oil showed highest antimicrobial activities against S. aureus_ATCC 29213 (30mm), E. coli_ATCC 25922 and E. faecalis_ATCC 29212 (25mm), C. albicans_ATTC 10231 (20mm), C. krusei_ATCC 6258 (18 mm) and minimum inhibition against P. aeruginosa_ATCC 27853 (16mm).Citronella oil showed highest antimicrobial activities against E. faecalis_ATCC 29212 (12mm) followed by P. aeruginosa_ATCC 27853 (11mm) and minimum inhibition against S. aureus_ATCC 29213 (7mm). other organisms not shown any activities against other organisms .Cedar wood oil showed highest antimicrobial activities against E. coli_ATCC 25922 (11mm) and minimum activities against S. aureus_ATCC 29213 (10mm).Lemon oil showed highest antimicrobial activities against S. aureus_ATCC 29213 (24mm), P. aeruginosa_ATCC 27853 (21mm) E. coli_ATCC 25922 (20mm), E. faecalis_ATCC 29212 (17mm), C. albicans_ATTC 10231 (15mm) and minimum inhibition activities against C. krusei_ATCC 6258 (13 mm).Lavender showed highest antimicrobial activities against E. faecalis_ATCC 29212 (12mm), P. aeruginosa_ATCC 27853 and C. krusei_ATCC 6258 (11 mm), E. coli_ATCC 25922 and C. albicans_ATTC 10231 (10mm) and minimum inhibition activities against S. aureus_ATCC 29213 (9mm).Tea tree oil showed highest antimicrobial activities against S. aureus_ATCC 29213 (35mm), followed by C. albicans_ATTC 10231 (32mm), C. krusei_ATCC 6258 and E. coli_ATCC 25922 ( 28mm) E. faecalis_ATCC 29212 (26mm) and minimum inhibition activities against P. aeruginosa_ATCC 27853 (25mm).Sandal wood oil showed highest antimicrobial activities against E. coli_ATCC 25922 (11mm), E. faecalis_ATCC 29212 (10mm) and minimum inhibition activities against S. aureus_ATCC 29213 (7mm).Basil oil showed highest antimicrobial activities against S. aureus_ATCC 29213 (11mm), C. krusei_ATCC 6258 (10 mm) and minimum inhibition activities against P. aeruginosa_ATCC 27853 (9mm) (table-2)
Table 2. Antimicrobial property of essential oils
| Microbes | Agar well diffusion assay | Minimum Inhibitory Concentration(µL) | |||||||||||
| Lemon grass oil | Cinnamon oil | Citronella
oil |
Cedar wood oil | Lemon oil | Lavender | Tea tree oil | Sandal wood oil | Basil oil | Lemon grass oil | Cinnamon oil | Lemon oil | Tea tree oil | |
| S. aureus ATCC 29213 | 13 | 30 | 7 | 10 | 24 | 9 | 35 | 7 | 11 | 64 | 1 | 32 | 0.5 |
| E. coli ATCC 25922 | 17 | 25 | – | 11 | 20 | 10 | 28 | 11 | – | 16 | 1 | 64 | 1 |
| P. aeruginosa ATCC 27853 | 17 | 16 | 11 | – | 21 | 11 | 25 | – | 9 | 64 | 2 | 128 | 1 |
| E. faecalis ATCC
29212 |
18 | 25 | 12 | – | 17 | 12 | 26 | 10 | – | 32 | 1 | 64 | 0.12 |
| C. krusei ATCC 6258 | 19 | 18 | – | – | 13 | 11 | 28 | – | 10 | – | – | – | – |
| C. albicans ATTC 10231 | 12 | 20 | – | – | 15 | 10 | 32 | – | – | 16 | 4 | 128 | 1 |
| Multi drug Resistant diabetic foot ulcer isolates | |||||||||||||
| MRSA | 11 | 32 | – | – | 18 | 7 | 28 | – | 10 | 128 | 0.5 | 64 | 0.5 |
| P. aeruginosa | 14 | 30 | 6 | – | 21 | 8 | 28 | 15 | – | 64 | 1 | 64 | 2 |
| E. coli | 13 | 20 | 11 | – | 20 | 10 | 25 | – | 9 | 32 | 0.5 | 128 | 0.5 |
| K. pneumoniae | 12 | 22 | – | 8 | 14 | 8 | 26 | – | – | 64 | 1 | 128 | 0.25 |
| C. albicans | 14 | 19 | – | – | 13 | 9 | 28 | 9 | 10 | 32 | 8 | 64 | 1 |
Effect of essential oil with Multi drug Resistant isolates : Lemon grass oil showed highest antimicrobial activities against P. aeruginosa and C. albicans (14mm) followed by E. coli showed (13mm), K. pneumonia (12mm) and minimum inhibition activities against MRSA (11mm).Cinnamon oil showed highest antimicrobial activities MRSA (32m), followed by P. aeruginosa (30mm), K. pneumonia (22mm), E. coli (20mm) and minimum inhibition activities against C. albicans (19mm).Citronella oil showed highest antimicrobial activities E. coli (11mm) and minimum inhibition activities against P. aeruginosa (6mm).Cedar wood oil showed highest antimicrobial activities K. pneumonia (8mm). Other organisms not shown any activities. Lemon oil showed highest antimicrobial activities P. aeruginosa (21mm) followed by E. coli (20mm), MRSA (18mm), K. pneumonia (14mm) and minimum inhibition activities against C. albicans (13mm).Lavender oil showed highest antimicrobial activities E. coli (10mm) followed by C. albicans (9mm), P. aeruginosa and K. pneumonia (8mm) and minimum inhibition activities against MRSA (7mm).Tea tree oil showed highest antimicrobial activities MRSA, P. aeruginosa and C. albicans (28mm), followed by K. pneumonia (26mm), minimum inhibition activities against E. coli (25mm).Sandal wood oil showed highest antimicrobial activities P. aeruginosa (15mm) and minimum inhibition activities against C. albicans (9mm).Basil oil showed highest antimicrobial activities MRSA and C. albicans (10mm) and minimum inhibition activities against E. coli (9mm)
Table 3. Anti-biofilm activity of essential oil by viability
|
Microbes |
Essential oil (µL/mL) | |||
| Lemon grass oil | Cinnamon oil | Lemon oil | Tea tree oil | |
| S. aureus ATCC 29213 | 16 | 2 | 128 | 4 |
| E. coli ATCC 25922 | 16 | 2 | 128 | 2 |
| P. aeruginosa ATCC 27853 | 128 | 2 | 128 | 4 |
| E. faecalis ATCC 29212 | 64 | 2 | 32 | 2 |
| C. krusei ATCC 6258 | – | – | – | – |
| C. albicans ATTC 10231 | 64 | 8 | 128 | 4 |
Oil Blend: Cinnamon oil: Tea tree oil=1:0.25; 1:0.5; 1:1; 0.5:1; and 1:0.25
Minimum Inhibitory Concentration: Based on the antimicrobial activities Lemon grass oil, Cinnamon oil, Lemon oil and Tea tree oil taken for the Minimum Inhibitory Concentration. Lemon grass oil showed maximum Minimum Inhibitory Concentration values against S. aureus_ATCC 29213 and P. aeruginosa_ATCC 27853 (64 µL) followed by E. faecalis_ATCC 29212 (32 µL), E. coli_ATCC 25922 and C. albicans_ATTC 10231 (16 µL).Cinnamon oil showed maximum Minimum Inhibitory Concentration values against C. albicans_ATTC 10231 (4µL), P. aeruginosa_ATCC 27853 (2µL), S. aureus_ATCC 29213, E. coli_ATCC 25922 and E. faecalis_ATCC 29212 (1µL),Lemon oil showed maximum Minimum Inhibitory Concentration values against P. aeruginosa_ATCC 27853 and C. albicans_ATTC 10231 (128 µL), E. coli_ATCC 25922 and E. faecalis_ATCC 29212 (64µL), S. aureus_ATCC 29213 (32 µL).Tea tree oil showed maximum Minimum Inhibitory Concentration values E. coli_ATCC 25922, P. aeruginosa_ATCC 27853 and C. albicans_ATTC 10231 (1 µL), S. aureus_ATCC 29213 (0.5 µL), E. faecalis_ATCC 29212 (0.12µL) (table-2)
Effect of essential oil with Multidrug resistant isolates
Based on the antimicrobial activities of Multi drug Resistant diabetic foot ulcer isolates.
Lemon grass oil, MRSA (128 µL) P. aeruginosa and K. pneumonia (64 µL) , E. coli and C. albicans (32 µL))
Cinnamon oil C. albicans (8µL), P. aeruginosa and K. pneumonia (1µL), MRSA and E. coli (0.5 µL) Lemon oil– E. coli and K. pneumonia (128µL), MRSA, C. albicans and P. aeruginosa (64 µL) Tea tree oil- P. aeruginosa (2 µL), C. albicans (1 µL) MRSA and E. coli (0.5 µL), K. pneumonia (0.25 µL)
Table 4. Minimum Inhibitory concentration of essential oil Blend-
|
Microbes |
Tea tree oil: cinnamon oil Essential oil blend (µL/mL) | ||||
| 1:0.25 | 1:0.5 | 1:1 | 0.5:1 | 0.25:1 | |
| S. aureus ATCC 29213 | 16 | 1 | 1 | 2 | 2 |
| E. coli ATCC 25922 | 16 | 0.5 | 0.5 | 1 | 0.5 |
| P. aeruginosa ATCC 27853 | 128 | 1 | 0.5 | 4 | 2 |
| E. faecalisATCC 29212 | 64 | 2 | 0.12 | 0.5 | 1 |
| C. krusei ATCC 6258 | – | – | 0.5 | 2 | 2 |
| C. albicansATTC 10231 | 64 | 4 | 0.25 | 2 | 4 |
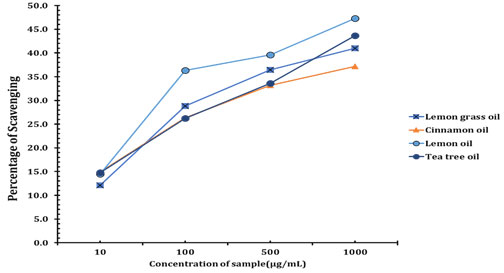
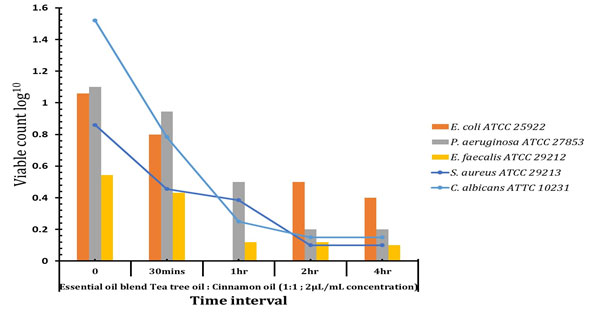
Antioxidant and Time kill assay: Antioxidant of essential oil was shown in figure 1. Lemon oil showed Highest percentage of anti-oxidant activities when compare with other oil. Time kill assay for the essential oil blend show in figure 2. Better growth suppression or killing assay was started on 30 mins above at the concentration of 2 µL of 1:1 Cinnamon oil and Tea tree oils. Recent study found cinnamon oil alone exhibited a broad‐spectrum activity against Gram‐negative and Gram‐positive bacteria and showed bacteriostatic and bactericidal effects against P. aeruginosa PAO1 at 2%, including known multidrug resistant species. The number of scientific studies on the antimicrobial activity of plant essential oils has strongly grown over the last three decades. Due to the use of many different microbiological methods for susceptibility testing and different definitions of antimicrobial activity, the comparability of studies on essential oils is often critical. Many studies focus on selected EOs, providing insight into their activity against one or more microorganisms, but only few publications compress information by testing multiplicities of essential oils with a defined single method. Therefore, this study was conducted to investigate as many EOs as possible with a single quantitative microbiological method, to achieve maximum comparability.
Figure 1: Antioxidant activity of essential oil by DPPH method
Figure 2: Time kill assay for the essential oil blend
CONCLUSION
The present study showed the importance of essential oil alone and mixture in antiseptic and cosmetic applications. Thus, essential oil blend might be a prospective source of alternative antimicrobial agents and may play a vital role in antiseptic and skin care product development.
Authors Contributions: All authors have equal contribution in bringing out this research work.
Conflict of Interest: None.
REFERENCES
Carlet J, Jarlier V, Harbath S, Voss A, Goossens H, Pittet D.(2012) Ready for a world without antibiotics? The Pensieres antibiotic resistance call to action. Antimicrob Res Inf Cont 2012;1:11.
Elcocks ER (2020) Spencer‐Phillips PTN, Adukwu EC. Rapid bactericidal effect of cinnamon bark essential oil against Pseudomonas aeruginosa. J App Microbiol 128(4): 1025-1037
Faleiro ML, Miguel MG.(2013) Use of essential oils and their components against multidrug-resistant bacteria. In Fighting multidrug resistance with herbal extracts, essential oils and their components 2013 Jan 1 (pp. 65-94). Academic Press.
Kon KV, Rai MK.(2012) Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert review of anti-infective therapy Jul 1;10(7):775-90.
Piddock LJ. (2011)The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis 12:249-53
Ruberto G, Baratta MT (2000): Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 69: 167–174.
Saccheti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R (2005): Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91: 621–632.
Wu YY, Li W, Xu Y, Jin EH, Tu YY.(2011) Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J Zhejiang Univ Sci B. 12: 744–751.
Yamagishi S, Matsui T.(2011) Nitric oxide, a Janus-faced therapeutic target for diabetic microangiopathy-Friend or foe? Pharmacol Res. 64:187–194.