Department of Oral and Maxillofacial Surgery, College of Dentistry, King Saud University, Riyadh, Saudi Arabia.
Corresponding author email: Malkindi@ksu.edu.sa
Article Publishing History
Received: 09/01/2020
Accepted After Revision: 29/02/2020
How the application of photodynamic therapy (PDT) is clinically efficacious in the rapid treatment of oral squamous cell carcinoma (OSCC)?. This focused question has been designed for the present study, which is comprised of the theme: Does PDT offer effective treatment in the regression of OSCCs ? Three indexed databases were searched (PubMed, EMBASE and CENTRAL) from May 1965 up to and including September 2019 for pertinent literature. Articles were selected if they had prospective design, published in English language and reported efficacy of PDT in the treatment of OSCCs in adult patients. Thirteen studies were included. A total of 447 patients with OSCCs were included. Their mean age ranged between 60.8 years to 69.6 years. The follow-up period of the clinical trials ranged from 3 months to 144 months. All studies showed statistically significant improvement in the complete regression of OSCCs on follow-up. Several clinical trials categorized their outcomes as complete, partial or no response to therapy. For PDT, the complete response ranged from 16% to 100% in the OSCCs. PDT shows to be a clinically efficient therapeutic modality for OSCCs. PDT is equally effective as surgery with regards to rates of recurrence.
Oral cancer, photodynamic therapy, photosensitizers, literature review
Alkindi M. Therapeutic Efficacy of Photodynamic Therapy in Oral Squamous Cell Carcinoma: A Systematic Review. Biosc.Biotech.Res.Comm. 2020;13(1).
Alkindi M. Therapeutic Efficacy of Photodynamic Therapy in Oral Squamous Cell Carcinoma: A Systematic Review. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2T779Am
Copyright © Alkindi, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Oral cancer is ranked sixth among all cancers and is considered a wide scale global health crisis distributed among diverse geographical areas (highest recorded in the South-East Asia) (Petti, Masood, & Scully, 2013). Among all the oral cancers, oral squamous cell carcinoma (OSCC) cover almost 90% of all oral malignant lesions (Johnson, Jayasekara, & Amarasinghe, 2011). Oral cancers are about twice as common in men as in women and are slightly more common in blacks than in whites (Kachuri, De, Ellison, & Semenciw, 2013). Worldwide, OSCC is a major health-care problem, and is the most frequently diagnosed cancer in some countries (Parkin, Bray, Ferlay, & Pisani, 2005). Great improvements in surgical techniques, radiotherapy, and chemotherapy have been achieved (Cooper et al., 2004), but the 5-year survival rate for OSCC is still between 40% and 60% and has not greatly improved over the last 30 years (Fonseca, 2017 Siegel et al 2019, Hung et al., 2020).
Oral squamous cell carcinoma is a malignant tumour that commonly invades the jawbone. Treatment often requires a surgical resection that compromises the patient’s quality of life, function, and aesthetic. Intraorally, it occurs most commonly in the tongue (20-30%), floor of the mouth (15-20%), retro-molar and tonsillar pillar areas (15%), soft palate (10-15%), buccal mucosa (10%), gingiva (10%), alveolar bone (10%), and maxillary sinus (5-10%) (Marx & Stern, 2012). About 40% of head and neck SCC mortality is due to locoregional recurrence, and about 30% develop a distant metastasis in the 5-year period following the diagnosis (de Bree, Deurloo, Snow, & Leemans, 2000).A comprehensive number of factors with probable impact on the outcome of the diseases are well-established. These include patient related factors that involve sex predilection, age, use of tobacco and alcohol, sociodemographic conditions, diagnostic delays, organic stress and other miscellaneous factors (Massano, Regateiro, Januário, & Ferreira, 2006). Early detection and rapid treatment modality of OSCC are essential for high survival rate (Hassona, Scully, Shahin, Maayta, & Sawair, 2016 Li et al 2018, Siegel et al., 2019. Hung et al., 2020).
There is a wide range of therapeutic modalities for OSCC. The most common includes surgical excision of the lesion. Other pharmacological treatment modalities include topical application of drugs such as vitamin A, steroids, herbal medicines, aloe vera, curcumin and turmeric which requires a steady 3-5 months of healing and recovery (Singh et al., 2016; Triesscheijn, Baas, Schellens, & Stewart, 2006). In addition, there are several systemic drugs that are useful for the regression of tumor mass (Karemore & Motwani, 2012). Moreover, certain other therapeutic modalities include radiotherapy which possesses several unwanted side effects such as oral mucositis, neuro-sensory disturbances, infections and fibrosis that could compromise the patient’s quality of life (Sroussi et al., 2017, Hung et al., 2020).
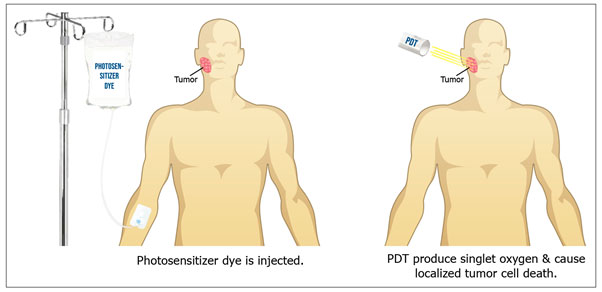
Nevertheless, surgery and radiation are widely used because these modalities work effectively. Photodynamic or photodynamic therapy (PDT) has gained a major popularity in the field of oral health. Such type of treatment utilizes photo/light therapy that activates a photosensitizer dye in the presence of oxygen. Several types of photosensitizers have been used for photobiomodulation depending on their mode of action (Castano, Demidova, & Hamblin, 2004). These include intravenous injections, orally ingested or topical application. The introduction of light on the photosensitizer at tumor site creates an array of destructive oxygen species including singlet oxygen and damaging free radicals producing localized cell death (Allison & Moghissi, 2013; Allison & Sibata, 2010).
The illustration in Figure 1 shows the use of an injected photosensitizer in combination with a photodynamic light to treat a facial tumor.
Figure 1: Photodynamic Therapy
Such modality has been widely used in a set of oral diseases including periodontal diseases, lichen planus, fungal infections, red and white leukoplakia (Akram et al., 2016; Akram et al., 2018; Baltazar et al., 2015; Li, Wang, Zheng, & He, 2018). Ample data confirms that PDT has been used to treat more robust types of cancers of head and neck origin including OSCC (Grant, Hopper, Speight, Macrobert, & Bown, 1993; Alexander Kübler, Haase, Rheinwald, Barth, & Mühling, 1998). However, it is very important to understand that OSCC lesions are of various types including primary or recurrent, invasive or non-invasive. This does put a great impact on the choice of therapy. On the other hand, research indicates that there are several number of recurrence rates found with the use of PDT (Schweitzer, 2001; Schweitzer & Somers, 2010). However, to our familiarity from the published data, no systematic review has been published that evaluated the therapeutic efficacy of PDT in the treatment of OSCC lesions in adult patients. Considering the contrary results and novel idea, the aim of the present study was to assess how the application of PDT is clinically efficacious in the rapid treatment of OSCC.
MATERIAL AND METHODS
Focused question: This systematic review was designed in accordance with the general guidelines set by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher, Liberati, Tetzlaff, & Altman, 2009). The focused question designed for the present study comprised of: “Does PDT offer effective treatment in the regression of OSCC lesions?”
Data search and eligibility criteria: Three ISI-indexed databases were searched (PubMed, EMBASE and CENTRAL) from May 1965 up to and including September 2019 for pertinent literature. Articles were selected if they had prospective design, published in English language and reported efficacy of PDT in the treatment of OSCC lesions in adult patients. Initially screening and evaluation of pertinent studies was performed and those studies not compliant with the selection criteria were omitted. The exclusion criteria involved review studies, case reports/series, in-vitro settings, animal studies and letters to the editor. Original articles were manually sought in journals including Lasers Med Sci, Photobiomodul Photomed Laser Surg, Photochem Photobiol Sci, and Photodiagnosis Photodyn Ther to recognize articles that may had missed from electronic database search.
Data search and abstraction: The combination of following key words were used to search for included literature: ‘Photodynamic therapy’, ‘photochemotherapy’, ‘oral cancer’, ‘oral squamous cell carcinoma’, ‘invasive’, ‘non-invasive’, ‘primary tumors’, ‘recurrent tumors’, ‘malignancy’, ‘therapy’, ‘treatment’. Once the relevant literature search was accomplished, the articles were then subjected for data extraction. Important evidence from all the articles were extracted that included study design, subject demographics, cancer site, follow-up duration, final outcome, recurrence rate, laser and PDT related parameters.
Quality assessment:The appropriate method to evaluate quality in non-randomized controlled trials (NRCTs) is controversial. For the purpose of this review, we decided to use a modified scale method that allowed us to rank selected reports according to a previously established score system. The Methodological Index for Nonrandomized Studies (MINORS) is an instrument that was developed by a group of practicing surgeons in France and validated specifically for NRCT evaluation (Slim et al., 2003). Some modifications were introduced to the MINORS to meet the needs of our study.
Data analysis: Meta-analyses could not be performed due to high rate of heterogeneity in the study design methods, lasers used, and cancer sites in the oral cavity.
RESULTS AND DISCUSSION
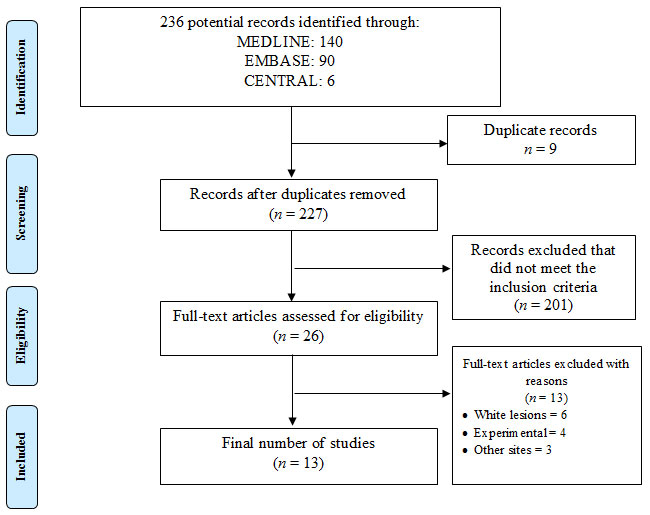
Search result: Initial screening of the titles and abstracts gave a total of 236 potential articles. Removal of the duplicates (n=9) and articles that did not comply with the focused question (n=201) were later excluded from the study search. Out of twenty-six potential articles that underwent full-text reading, thirteen articles were further removed. After the final selection, thirteen studies were included and processed for data extraction (Fan et al., 1996; Fan, Hopper, Speight, Buonaccorsi, & Bown, 1997; Feyh, 1996; Grant et al., 1993; Hopper et al., 2004; AC Kübler, De Carpentier, Hopper, Leonard, & Putnam, 2001; Alexander Kübler et al., 1998; N. Rigual et al., 2013; N. R. Rigual et al., 2009; Schuller, McCaughan, & Rock, 1985; Schweitzer, 2001; Schweitzer & Somers, 2010; Toratani et al., 2016). These studies were performed either in health care setups or universities. The complete flow of study selection is illustrated in Figure 2 according to PRISMA standard.
Figure 2: PRISMA flow chart representing study search.
Description of included studies: All clinical studies were prospective longitudinal trials. Five studies were performed in United States and United Kingdom, two studies were performed in Germany, while one study was performed in Japan. A total of 447 patients with OSCCs were included for the treatment of PDT. Their mean age ranging between 60.8 years to 69.6 years. Cancer sites included in the clinical trials comprised of tongue, floor of the mouth, alveolus, gingiva, buccal mucosa, lips, larynx, neck, oropharynx and palate. The follow-up period of the clinical trials ranged from 3 months to 144 months. Four studies reported about recurrence of the OSCC to be 0% to 20% only (Table 1).
Table 1. General characteristics of the studies.
| Author et al. Year | Country/Patients | Sample size | Male/Female ratio | Mean age (age range) | Cancer site | Follow-up (mos) | Recurrence (%) | Main conclusion | Quality of studies |
| Schuller et al.22 1985 | United States | 24 | 14/10 | 67 (NA) | FM, T, L, F, N, TO, LA, PH | NR | NA | PDT is feasible for oral cancer with well-tolerable and low toxicity. | Low |
| Fyeh 23 1996 | Germany | 83 | NA | NA | PH, F, LA | 50 | NA | PDT is an adequate treatment for early stage superficial cancers | Low |
| Grant et al.17 1993 | United Kingdom | 11 | NA | NA | T, BM, A, L, P | 19 | 0 | PDT offers an effective repeatable treatment option, whether on its own or as adjunct to local excision | Moderate |
| Kübler et al.18 1998 | Germany | 12 | 11/1 | NA | FM, BM | 16 | NA | PDT offers an effective repeatable treatment option without causing harm | Moderate |
| Fan et al.24 1996 | United Kingdom | 18 | 11/7 | 62.6 (NA) | BM, FM, T, A | 48 | NA | PDT is an adequate treatment for superficial cancers | Moderate |
| Hopper et al.25 2004 | United Kingdom | 114 | NA | 64 (30-99) | BM, T, FM, P, L, PH | 24 | NA | PDT offers an effective
alternative treatment for early oral squamous cell carcinoma |
Moderate |
| Schweitzer19 2001 | United States | 20 | NA | NA | OC, PH, LA | 6-115 | 20 | PDT offers a curative treatment of early stage oral cavity and laryngeal malignancies with minimal side effects. | High |
| Fan et al.26 1997 | United Kingdom | 20 | 16/4 | 60.8 (30-82) | BM, T, FM, P | 15 | NA | PDT is a promising new treatment for patients with oral cancer | Low |
| Rigual et al.28 2009 | United States | 20 | 14/6 | 61.2 (NA) | OC, L | 53 | NA | PDT is an adequate treatment for oral and laryngeal cancers | Moderate |
| Rigual et al.29 2013 | United States | 40 | 28/13 | 65 (39-88) | P, BM, T, FM, | 3 | NA | PDT is safe for the treatment of early stage cancer of the oral cavity. | High |
| Toratani et al.27 2016 | Japan | 30 | 12/22 | NA | A, T, BM, FM, G | 6 | NA | PDT is an effective treatment modality for superficial oral carcinomas, with excellent healing and minimal side effects | Low |
| Schweitzer and Somers20 2010 | United States | 30 | 15/15 | NA (35-82) | OC, L, OP | 3-144 | 20 | PDT provides a surgical oncologic modality for potentially curative treatment of early stage oral cavity and oropharyngeal malignancies | Moderate |
| Kübler et al.30 2001 | United Kingdom | 25 | 19/6 | 69.6 (NA) | L | 3 | 8 | PDT is an effective treatment modality for small primary tumours of the lips. | Low |
A, alveolus; BM, buccal mucosa; FM, floor of mouth; G, gingiva; LA, larynx; L, lips; N, neck; OC, oral cavity; P, palate; PH, OP; oropharynx; T, tongue; PDT – photodynamic therapy, NA – not available
Photodynamic related parameters: A total of nine studies used argon pumped dye laser, while two studies used diode laser. One study each used excimer dye laser and gold vapour laser, respectively. The wavelength ranged from 628 to 665 nanometres (nm). Energy fluence and power density were reported in ten and eight studies, that ranged from 20–150 joules per centimetre square (Jcm-2) and 100 to 250 milliwatts per centimetre square (mW cm-2), respectively. Only two studies reported the power output. Duration of irradiation was reported in four studies that ranged from 1.8 to 143 minutes (min). Optic fibre diameter was mentioned in only three studies that ranged from 400 to 600 micrometre (μm). Five studies used photofrin, two studies used hematoporphyrin derivative (HPD), 5-aminolevulinic acid (ALA) and Foscan as photosensitizer (PS), respectively. One study used metatetrahydroxyphenylchlorin (mTHPC) and 3-(1’-hexyloxyethyl) pyropheophorbide a (HPPH) as PS, respectively. Pre-irradiation time of PS ranged from 48 to 240 hours in the clinical studies. Dose of the PS ranged from 0.15 mg/kg to 60 mg/kg. None of the studies reported the number of laser sessions except one study that reported only once (Table 2).
Table 2. Laser and photosensitizer related parameters of the studies.
| Investigators | Type of laser | Wavelength (nm) | Energy fluence (J cm-2) | Power output (W) | Power density (mW cm-2) | Duration of irradiation (min) | Optic fibre diameter (μm) | Types of PS | Pre-irradiation time (hours) | Dose of PS (mg/kg) | Number of laser sessions |
| Schuller et al.22 | Argon pumped dye | 630 | NA | 3 | NA | NA | NA | HPD | 72 | 3-5 | NA |
| Fyeh 23 | Argon pumped dye | 630 | NA | NA | 100 | NA | 600 | HPD | 48 | NA | NA |
| Grant et al.17 | Argon pumped dye | 630 | 50-100 | NA | 150 | NA | NA | Photofrin | 48 | 2.0 | NA |
| Kübler et al.18 | Argon pumped dye | 630 | 100 | NA | 100 | 16.6 | NA | ALA | 120 | NA | NA |
| Fan et al.24 | Gold vapour laser | 628 | NA | NA | 250 | 143 | 400 | ALA | 150-240 | 60 | NA |
| Hopper et al.25 | LED | 652 | 20 | NA | 100 | 3.33 | NA | mTHPC | 96 | 0.15 | NA |
| Schweitzer19 | Argon pumped dye | 630 | 50-100 | NA | 100-500 | NA | NA | Photofrin | 48-60 | 2.0 | NA |
| Fan et al.26 | Argon pumped dye | 652 | 5-20 | NA | 250 | 1.8-8.0 | 400 | Foscan | 72-96 | 0.15 | NA |
| Rigual et al.28 | Argon pumped dye | 630 | 75 | NA | NA | NA | NA | Photofrin | 48 | 2.0 | NA |
| Rigual et al.29 | Argon pumped dye | 665 | 50-140 | NA | NA | NA | NA | HPPH | NA | 4.0 | 1 |
| Toratani et al.27 | Excimer dye laser | 630 | 100-150 | 0.16 | NA | NA | NA | Photofrin | 48 | 2.0 | NA |
| Schweitzer and Somers 20 | Diode laser | 630 | 50-100 | NA | NA | NA | NA | Photofrin | 48-60 | 2.0 | NA |
| Kübler et al.30 | Argon pumped dye | 652 | 20 | NA | 100 | NA | NA | Foscan | 96 | 0.15 | NA |
ALA – 5-aminolevulinic acid; HPD – hematoporphyrin derivative; HPPH – 3-(1’-hexyloxyethyl) pyropheophorbide a; mTHPC – metatetrahydroxyphenylchlorin; LED – light emitting diode; nm – nanometer; J cm-2 – joules per centimetre square ; mW – milliwatts; mW cm-2 – milliwatts per centimetre square; mm – millimetre; PTC – Phenothiazine chloride; PS – photosensitizer; mg/mL – milligram per millilitre; NA – not available
Quality Assessment: In general, they suffer from methodologic drawbacks, mainly difficulties in concealing the allocation of patients and the inherent complexity of blinding between PDT and surgical cases. Older clinical trials also are limited by the small number of patients included (Table 1).
Main outcome of the studies: All PDT studies showed statistically significant improvement in the complete regression of OSCCs on follow-up. Several clinical trials categorized their outcomes as complete, partial or no response to therapy. For PDT, the complete response ranged from 16% to 100% in the OSCCs.
To achieve high survival rate with sound quality of life, minimally invasive intervention is preferred over radical surgical therapy. This goes for all the type of head and neck cancers including OSCC lesions (Maxwell et al., 2014). Although surgical therapy and radiotherapy are suitable therapeutic modalities, they often compromise the significant functional roles of the oral environment. PDT is a noninvasive method that maintains speech and deglutition. The laser light application to stimulate the PS does not interrupt the sound adjacent tissue structures. Most importantly, PDT does not disrupt the underlying fibrotic structures including collagen and elastin fibres; therefore the level of scarring is reduced (Hopper et al., 2004).
Repetitive surgical therapy is mainly problematic due to limitations in the access and advanced deterioration in the tissue structures. Moreover repeating radiotherapy is generally unfeasible due to a maximum permitted dose to the areas of the head and neck (Dilkes, Benjamin, Ovaisi, & Banerjee, 2003; Hopper et al., 2004). In addition, surgical intervention at an already radiation induced conveys a major possibility of higher disease rates secondary to slow healing of the wound and formation of fistula and warrants an increased doses that may cause disturbances in the angiogenic component of the cancer cells, making them low radiosensitive (Hopper et al., 2004).
The additional benefit of PDT is in the procedure being a simple and PDT has the benefit of being an outpatient method. This suggests that PDT is completed within a short time, also entails a short healing time, and involving a small cost (AC Kübler et al., 2001). These features till date, characterise key factors when opting between surgical therapy and radiation therapy for other HNCs. However, the main limitation of PDT is the complexity of use with regards to its direction of phototherapy on the exposure area, that suggests its fundamental use in treating shallow and easy to reach and manageable cancers. Momentary photosensitization has highly deterring problems, although novel PS are curbing the duration of action of PDT (Fan et al., 1997; Grant et al., 1993; Alexander Kübler et al., 1998).
It should be noted from the included clinical studies that laser parameters were either missing or had meaningful differences. Characteristics related to PDT including wavelength, energy fluence, and power density either had a large variation or data not reported. It is well-known that multiple number of laser sessions has a significant effect on the clinical efficacy of phototherapy (Wang et al., 2001). In the reported studies, the number of sittings were not mentioned. It is evident that by applying a single application of PDT to sustain anti-proliferative effect of cancer, it is assumable that one laser session is equally effective. Moreover, diameter of fibre produces an effect on total power density and output that may alter the genuine energy released during the process, thereby affecting the anticancer efficacy. None of the studies described the power output of the laser used. These missing parameters of laser protocols may put some effect on the therapeutic efficacy of PDT on cancer treatment. However, since most included studies were incomplete, in terms of basic items such as drug and light dose, number of treatment sessions, recurrence rate, this proves the poor quality of the studies making a valid conclusion impossible. Therefore, future research with consistent laser dimensions are needed to interpret the efficacy of PDT in treating OSCC.
Our systematic literature review does have some limitations. Firstly, no meta-analyses could be performed to interpret the overall odds ratios across different studies. Studies being performed in different countries suggest the inclusion of different ethnic group patients whose level of severity and hence outcomes are critically affected which may have produced potential bias with regards to a high degree of selectivity. Presently, photobiomodulation for treating OSCC is only being carried out in only a limited health care centre globally. Moreover, several studies on PDT included a limited number of study cohorts. For instance, the total number of studies that were included consisted only 447 patients with OSCC treated with PDT. Furthermore, to achieve a high survival rate with quality of life requires complete elimination or at least control of tumor. It was observed that PDT studies did not demonstrate these findings. Moreover, plenty of scarring occurred in several trials in which functional loss was also noted. All these measures do have an impact on the overall quality of life.
With future studies with long period of follow-up, PDT could be reflected as a valid and acceptable adjunctive therapeutic modality in the future. It is well tolerable by patients, that could additionally serve as a substitute therapy for patients with medical problems who may not be able to bear the unwanted complications of radiation therapy or those patients who may be too hampered to undergo surgery. It is indicated that PDT is associated with lower morbidity rates and less side effects.
CONCLUSION
PDT shows to be a clinically efficient therapeutic modality for OSCCs. PDT is equally effective as surgery with regards to rates of recurrence. However, extreme caution should be made while interpreting the findings of this study as number of parameters including laser parameters, type of patients and number of treatment sessions may affect the overall outcome of PDT in the treatment of OSCC.
Competing interests: None declared.
REFERENCES
Akram, Z., Al-Shareef, S. A. A., Daood, U., Asiri, F. Y., Shah, A. H., AlQahtani, M. A., . . . Javed, F. (2016) Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomedicine and Laser Surgery Vol. 34 No 4: Pages 137-149.
Akram, Z., Javed, F., Hosein, M., Al‐Qahtani, M. A., Alshehri, F., Alzahrani, A. I., & Vohra, F. (2018) Photodynamic therapy in the treatment of symptomatic oral lichen planus: A systematic review. Photodermatology, Photoimmunology & Photomedicine Vol. 34 No 3: Pages 167-174.
Allison, R. R., & Moghissi, K. (2013) Photodynamic therapy (PDT): PDT mechanisms. Clinical Endoscopy Vol. 46 No 1: Pages 24-29.
Allison, R. R., & Sibata, C. H. (2010) Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagnosis & Photodynamic Therapy Vol. 7 No 2: Pages 61-75.
Baltazar, L. M., Ray, A., Santos, D. A., Cisalpino, P. S., Friedman, A. J., & Nosanchuk, J. D. (2015) Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Frontiers in microbiology Vol. 6: Pages 202.
Castano, A. P., Demidova, T. N., & Hamblin, M. R. (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis & Photodynamic Therapy Vol. 1 No 4: Pages 279-293.
Cooper, J. S., Pajak, T. F., Forastiere, A. A., Jacobs, J., Campbell, B. H., Saxman, S. B., . . . Rotman, M. (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. New England Journal of Medicine Vol. 350 No 19: Pages 1937-1944.
de Bree, R., Deurloo, E. E., Snow, G. B., & Leemans, C. R. (2000) Screening for distant metastases in patients with head and neck cancer. The Laryngoscope Vol. 110 No 3: Pages 397-401.
Dilkes, M., Benjamin, E., Ovaisi, S., & Banerjee, A. (2003) Treatment of primary mucosal head and neck squamous cell carcinoma using photodynamic therapy: results after 25 treated cases. The Journal of Laryngology & Otology Vol. 117 No 9: Pages 713-717.
Fan, K. F., Hopper, C., Speight, P. M., Buonaccorsi, G., MacRobert, A. J., & Bown, S. G. (1996) Photodynamic therapy using 5‐aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer: Interdisciplinary International Journal of the American Cancer Society Vol. 78 No 7: Pages 1374-1383.
Fan, K. F., Hopper, C., Speight, P. M., Buonaccorsi, G. A., & Bown, S. G. (1997) Photodynamic therapy using mTHPC for malignant disease in the oral cavity. International Journal of Cancer Vol. 73 No 1: Pages 25-32.
Feyh, J. (1996) Photodynamic treatment for cancers of the head and neck. Journal of Photochemistry and Photobiology B: Biology Vol. 36 No 2: Pages 175-177.
Fonseca, R. J. (2017) Oral and Maxillofacial Surgery-E-Book: 3-Volume Set: Elsevier Health Sciences.
Grant, W., Hopper, C., Speight, P., Macrobert, A., & Bown, S. (1993) Photodynamic therapy of malignant and premalignant lesions in patients with’field cancerization ‘of the oral cavity. The Journal of Laryngology & Otology Vol. 107 No 12: Pages 1140-1145.
Hassona, Y., Scully, C., Shahin, A., Maayta, W., & Sawair, F. (2016) Factors influencing early detection of oral cancer by primary health-care professionals. Journal of Cancer Education Vol. 31 No 2: Pages 285-291.
Hopper, C., Kübler, A., Lewis, H., Tan, I. B., Putnam, G., & Group, F. S. (2004) mTHPC‐mediated photodynamic therapy for early oral squamous cell carcinoma. International Journal of Cancer Vol. 111 No 1: Pages 138-146.
Hung, Li Chen , Pei-Tseng Kung, Chi-Hsuan Lung, Ming-Hsui Tsai, Shih-An Liu, Li-Ting Chiu (2020) Assessment of the Risk of Oral Cancer Incidence in A High-Risk Population and Establishment of A Predictive Model for Oral Cancer Incidence Using A Population-Based Cohort in Taiwan Int. J. Environ. Res. Public Health 17(2), 665; https://doi.org/10.3390/ijerph17020665
Johnson, N. W., Jayasekara, P., & Amarasinghe, A. H. K. (2011) Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 2000 Vol. 57 No 1: Pages 19-37.
Kachuri, L., De, P., Ellison, L., & Semenciw, R. (2013) Cancer incidence, mortality and survival trends in Canada, 1970-2007. Chronic Diseases and Injuries in Canada Vol. 33 No 2: Pages 69-80.
Karemore, T. V., & Motwani, M. (2012) Evaluation of the effect of newer antioxidant lycopene in the treatment of oral submucous fibrosis. Indian Journal of Dental Research Vol. 23 No 4: Pages 524.
Kübler, A., De Carpentier, J., Hopper, C., Leonard, A., & Putnam, G. (2001) Treatment of squamous cell carcinoma of the lip using Foscan-mediated photodynamic therapy. International Journal of Oral and Maxillofacial Surgery Vol. 30 No 6: Pages 504-509.
Kübler, A., Haase, T., Rheinwald, M., Barth, T., & Mühling, J. (1998) Treatment of oral leukoplakia by topical application of 5-aminolevulinic acid. International Journal of Oral and Maxillofacial Surgery Vol. 27 No 6: Pages 466-469.
Li, Y., Wang, B., Zheng, S., & He, Y. (2018) Photodynamic therapy in the treatment of oral leukoplakia: a systematic review. Photodiagnosis & Photodynamic Therapy Vol. 25: Pages 17-22.
Marx, R. E., & Stern, D. (2012) Oral and maxillofacial pathology: a rationale for diagnosis and treatment: Hanover Park, IL: Quintessence Pub. Co.
Massano, J., Regateiro, F. S., Januário, G., & Ferreira, A. (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontology, Vol. 102 No 1: Pages 67-76.
Maxwell, J. H., Mehta, V., Wang, H., Cunningham, D., Duvvuri, U., Kim, S., . . . Ferris, R. L. (2014) Quality of life in head and neck cancer patients: impact of HPV and primary treatment modality. The Laryngoscope Vol. 124 No 7: Pages 1592-1597.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine Vol. 151 No 4: Pages 264-269.
Parkin, D. M., Bray, F., Ferlay, J., & Pisani, P. (2005) Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians Vol. 55 No 2: Pages 74-108.
Petti, S., Masood, M., & Scully, C. (2013) The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. PloS One Vol. 8 No 11: Pages e78999.
Rigual, N., Shafirstein, G., Cooper, M. T., Baumann, H., Bellnier, D. A., Sunar, U., . . . Tan, W. (2013) Photodynamic therapy with 3-(1′-hexyloxyethyl) pyropheophorbide a for cancer of the oral cavity. Clinical Cancer Research Vol. 19 No 23: Pages 6605-6613.
Rigual, N. R., Thankappan, K., Cooper, M., Sullivan, M. A., Dougherty, T., Popat, S. R., . . . Henderson, B. (2009) Photodynamic therapy for head and neck dysplasia and cancer. Archives of otolaryngology–head & neck surgery Vol. 135 No 8: Pages 784-788.
Schuller, D. E., McCaughan, J. S., & Rock, R. P. (1985) Photodynamic therapy in head and neck cancer. Archives of Otolaryngology Vol. 111 No 6: Pages 351-355.
Schweitzer, V. G. (2001) PHOTOFRIN‐mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignancies. Lasers in Surgery and Medicine Vol. 29 No 4: Pages 305-313.
Schweitzer, V. G., & Somers, M. L. (2010) PHOTOFRIN‐mediated photodynamic therapy for treatment of early stage (Tis‐T2N0M0) SqCCa of oral cavity and oropharynx. Lasers in Surgery and Medicine Vol. 42 No 1: Pages 1-8.
Siegel, Rebecca L., Kimberly D. Miller Ahmedin Jemal (2019) CA: A Cancer Journal for Clinicians Volume 69, Issue 1 https://doi.org/10.3322/caac.21551
Singh, N., Hebbale, M., Mhapuskar, A., Ul, S. N., Thopte, S., & Singh, S. (2016) Effectiveness of Aloe Vera and Antioxidant along with Physiotherapy in the Management of Oral Submucous Fibrosis. The Journal of Contemporary Dental Practice Vol. 17 No 1: Pages 78-84.
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., & Chipponi, J. (2003) Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ Journal of Surgery Vol. 73 No 9: Pages 712-716.
Sroussi, H. Y., Epstein, J. B., Bensadoun, R. J., Saunders, D. P., Lalla, R. V., Migliorati, C. A., . . . Zumsteg, Z. S. (2017) Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Medicine Vol. 6 No 12: Pages 2918-2931.
Toratani, S., Tani, R., Kanda, T., Koizumi, K., Yoshioka, Y., & Okamoto, T. (2016) Photodynamic therapy using Photofrin and excimer dye laser treatment for superficial oral squamous cell carcinomas with long-term follow up. Photodiagnosis Photodynamic Therapy, Vol. 14: Pages 104-110.
Triesscheijn, M., Baas, P., Schellens, J. H., & Stewart, F. A. (2006) Photodynamic therapy in oncology. The Oncologist Vol. 11 No 9: Pages 1034-1044.
Wang, I., Bendsoe, N., Klinteberg, C. A. f., Enejder, A., Andersson‐Engels, S., Svanberg, S., & Svanberg, K. (2001) Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. British Journal of Dermatology Vol. 144 No 4: Pages 832-840.