Division of Microbial Technology, PG and Research Department of Zoology, Chikkanna Govt. Arts College, Tirupur – 641 602, Tamilnadu, India
Article Publishing History
Received: 20/05/2016
Accepted After Revision: 25/06/2016
Fluoride, the pivot of preventive dentistry, continuous to be the corner stone of caries prevention programmes. The success of fluoride in caries prevention of smooth surfaces has made dental caries primarily a disease of pits and fissures of teeth. Caries infection caused by predominant organism Lactobacillus spp. also a most common pathogen in ECC life, is also serious problem among caries patients. The decay samples were collected from different dental clinics from rural area of Tirupur city, from caries patient. A total of 22 multidrug resistant cariogenic strains were isolated from 50 decay samples and were analyzed and characterized. Further, the optimal conditions of pH (2 – 8) and temperature (30°C to 45°C) were determined. The potentially of Lactobacillus spp. in lactic acid production was assessed. Lactic acid production by Lactobacillus spp. is partially growth associated and about 9.008 mg of lactic acid/ml was synthesized in the oral cavity of childhood caries. Later, the antimicrobial susceptibilities of this isolates was assessed against 10 commonly used antibiotics, which inhibitory of cell wall synthesis (Amoxicillin, Cloxacillin and Vancomycin), inhibitory of protein synthesis (Azithromycin, Clarithromycin and Roxithromycin), inhibitory of nucleic acid synthesis (Ciprofloxacin and Rifampicin) and inhibitory of folate metabolism (Sulfamethizole and Trimethoprim). Among these strains 8%, 98%, 40%, 18%, 14%, 18%, 10%, 2%, 40% and 68% were found to be exhibit a significance degree of resistance to four groups of antibiotics. Further, plasmid profile were performed for the seven multi drug resistance isolates and observed the molecular weight was 1500bp, 100bp, 300bp and 100 bp respectively. NaF is currently most promising tools applied as antimicrobial agents for diagnosis the dental disease. The NaF was performed by using well diffusion assay. Different concentrations of sodium fluoride (0.25%, 0.50%, 0.75% and 1%) were used against multidrug resistance cariogenic isolates. In this present investigation NaF was used and it shows prominent resistant activity against decay causing organism. Moreover, most antimicrobial agents that are currently in use have been rendered in effective by a wide occurrence of multi drug resistant strains of microbes.
Antibiotic Resistance, Dental Caries, Lactobacillus Spp., Naf, Plasmid Profile
Vijayalakshmi S, Mohankumar A. Emerging of Sodium Fluoride Resistant Lactobacilli Isolated from Dental Caries Patients. Biosc.Biotech.Res.Comm. 2016;9(2).
Vijayalakshmi S, Mohankumar A. Emerging of Sodium Fluoride Resistant Lactobacilli Isolated from Dental Caries Patients. Biosc.Biotech.Res.Comm. 2016;9(2). Available from: https://bit.ly/2Mn0i3l
Introduction
Dental caries continuous to be significant public health problem in many parts of the world. Although the bacteria responsible for caries initiation and early caries progression have been studied extensively, the microbiology of dentine caries has been reported to show considerable diversity and has not yet been fully characterized. It is a multifactorial disease which is caused by host agents and environmental factors. The disease continues to be major problem in dentistry affecting the mankind even today. It affects persons of both genders in all races, all socioeconomic strata and every age group. A wide group of microorganisms can be isolated from carious lesions of which Streptococcus mutans, Lactobacillus acidophilus, Lactobacillus fermentum, Actinomyces viscosus are the main pathogenic species involved in the initiation and development of carious lesions (Aas et al., 2008 Rizwan Ullah and Zafar, 2015).
However, Lactobacilli are generally considered to be non-pathogenic except in dental caries (Attebery and Finegold, 1970). Historically Lactobacilli were the first microorganisms implicated in dental caries development. This genus is involved in the progression of carious and caries dentin is the main ecological site of Lactobacilli. They appear during the first years of child’s and are present in high numbers in saliva, on the dorsum of the tongue, mucous membranes, the hard plate, in dental plaque and few numbers, on tooth surface (Straetemans et al., 1998). During the last fifteen years, the Lactobacillus genus has subjected to numerous taxonomical changes and includes at present more than 80 species (Loesche et al., 1984), some of which having been found in the oral cavity. Lactobacilli seem to be mostly transient in the oral cavity of small children, and only later a resident Lactobacillus flora is established (Badet and Thebaud, 2008). Their analysis of Lactobacilli by culture under micro aerophilic conditions in 65 deep caries samples indicated that Lactobacillus acidophilus was numerically dominant, although Lactobacillus paracasei, Lactobacillus rhamnosus, and Lactobacillus fermentum were also present in many samples (Martin et al., 2002 and Bernable and Sheiham, 2014).
Lactic acid bacteria produce various compounds such as organic acids di-acetyl, hydrogen peroxide and bacteriocin or bacteria proteins during lactic fermentations (Oyetayo et al., 2003). But in the oral cavity infections caused by Lactobacillus may be under reported in the medical literature because of the failure or reluctance to recognize it as a true pathogen (Antony et al., 1996). Children receiving 200,000 U of oral penicillin daily for rheumatic fever prophylaxis have been reported to experience reduction in the incidence of dental caries of up to 56% as compared with their untreated sibling’s (Handle man et al., 1966). The oral penicillin therapy had no effect on the number of salivary Lactobacilli and that the penicillin resistances of the total flora, the Streptococci, and the Staphylococci in the saliva’s of the antibiotic users were higher than those in the untreated sibling’s (Handle man and Hawas, 1965,1964).
Antibiotics provide an invaluable tool for a control of infection in modern dentistry (Cruickshank, 1968). Development of resistance to various antibiotics makes it necessary to select logically and rationally, a drug for successful gingival therapy during orthodontic treatment. Moreover, most of the antimicrobial agents that are currently in use have been rendered ineffective by a wide occurrence of multiple drug resistant strains of microbes (Owhe – Ureghe et al., 2010).
Infectious diseases are the leading cause of death world-wide and at the same time antibiotic resistance has become a global concern (Westh et al., 2004). Therefore there has been an increasing incidence of multiple resistance in human pathogenic microorganisms in recent years, largely due to indiscriminate use of commercial antimicrobial drugs commonly employed in the treatment of infectious diseases. The development of bacterial resistance to presently available antibiotics has necessitated the search for new antibacterial agents (Mylotte et al., 1987).
Resistant bacteria which are human pathogens may cause disease that are difficult to treat. A breakthrough came with the discovery that antibiotic resistance is transmitted by mixed cultivation between drug-resistant and sensitive strains independently of the donor strain chromosome. Many studies of drug resistant and its extra chromosomal position have been carried out. Very few reports exist on drug resistance in members of Lactobacillus genus (Sozzi and Smiley, 1980) and its link with plasmid (Klaenhammer and Sutherland, 1980). Recurrently, the fluoride ion (F) has been widely used topically in the treatment of dental caries for its anticariogenic and antimicrobial properties (Rizwan Ullah and Zafar, 2015). Thorough mechanical removal of plaque twice daily with a fluoride-containing tooth paste is a commonly-taught method of caries prevention. However, studies show that incidence of dental caries remains high (Bernable and Sheiham, 2014), suggesting that such regimens are commonly not strictly adhered to. So, hence the present study has made on attempt to point out the emerging of sodium fluoride resistant cariogenic Lactobacilli which is isolated from rural childhood caries, and the multidrug resistant organism link with plasmids.
Materials And Methods
Sample Collection
The plaque samples were collected from tooth decay of rural patients using sterile forceps around Tirupur District. The sample was suspended in sterilized screw capped bottle with saline solution. It was place in ice pack box and were brought in to the laboratory. Then, the samples were incubated at 37°C for 24 hours and the samples were used for isolation of cariogenic bacteria.
Isolation Of Cariogenic Lactobacillus Spp. From Dental Plaque Samples
All the strains isolated from dental plaque samples were serially diluted from 10-1 to 10-9 and the dilutions 10-4 to 10-6 were plated onto Man, Rogosa and Sharpe media (MRS) agar it was incubated at 37°C for 48 hours. The individual colonies with different morphology were picked using sterile tooth pick and grown in MRS broth and it was incubated at 37°C for 24hours. Further it was plated to check for purity.
Phenotypic Characterization
The isolated bacteria were primarily identified on the basis of the Gram staining, IMViC test, oxidase test, nitrate reduction test, motility test, different carbohydrate fermentation test, starch hydrolysis, growth at different pH, growth at 15°C and 45°C in the Lactobacilli de Mann Rogosa and Sharpe (MRS) broth as described as Bergy’s Manual of Systematic Bacteriology (Kandler and Weiss, 1986). These isolates were used as a lactic acid producing strain. All lactobacilli strains used in this study were grown in MRS broth at 37°C for 24 hours.
Lactic Acid Production Assay
Isolates were screened for the production of lactic acid using the titrimetric estimation (AOAC, 1990). For these measurements, the test organisms were grown on MRS broth for 48 hours and supernatant was collected by centrifuging at 10,000 rpm for 15 minutes at 4°C. Phenolphalein was added in to the 20 ml of supernatant as an indicator for titrimetric estimation. One ml of 0.1M NaOH is equivalent to 90.08 mg of lactic acid.
Antibiotic Susceptibility Test
Antibiotic susceptibility was semi-quantitatively determined by disc diffusion method (Katla et al., 2001). There are different group of antibiotics were tested: Class I Group – Inhibitors of cell wall synthesis (Amoxicillin, Cloxacillin, Vancomycin), Class II Group – Inhibitors of protein synthesis (Azithromycin, Clarithromycin, Roxithromycin), Class III Group – Inhibitors of nucleic acid synthesis (Ciprofloxacin, Rifampicin), Class IV Group – Inhibitors of folate metabolism (Sulphamethizole, Trimethoprim). The culture was inoculated into the MRS broth which was incubated at 37°C for 12hours. Plates were made with Mueller Hinton agar and allowed to solidified 10 to 15 minutes. 0.1ml of this culture were inoculated in the plates by spread plate technique. The antibiotic disks of Amoxicillin (30mcg), Cloxacillin (5mcg), Vancomycin (30mcg), Azithromycin (30mcg), Clarithromycin (15mcg), Roxithromycin (30mcg), Ciprofloxacin (30mcg), Rifampicin (30mcg), Sulfamethizole (300mcg), and Trimethoprim (30mcg) were placed in the plates. Then the plates were incubated for 37°C 24 hours. The diameters of the inhibition zone were measured using a ruler under a colony counter apparatus. The results were expressed as sensitive (S), marginally susceptible (I), and resistant (R).
 |
Figure 1: Effect of NaF against Lactobacillus spp. |
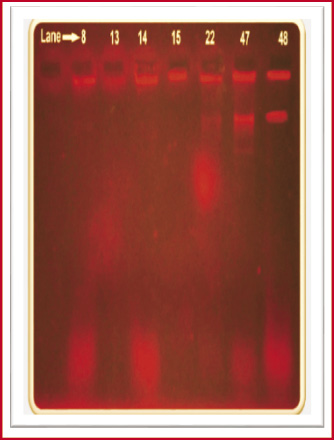 |
Figure 2: Plasmid profile of Lactobacillus spp. |
Effect Of Sodium Fluoride (Naf) Used Against Lactobacillus Spp.
The NaF was performed by using well diffusion method (Yavagal et al., 2014). About 20 ml of sterile molten Mueller Hinton agar was poured into the sterile petriplates. Triplicate plates were swabbed with the overnight culture (108cells /ml) of pathogenic bacteria Lactobacillus spp. different concentration of NaF (0.25%, 0.50%, 0.75% and 1%) were prepared and screened against fifty isolates of Lactobacillus spp. The isolates were selected on the basis of Lactobacillus spp. should have above 50% antibacterial activity against the antibiotics tested: Amoxicillin, Cloxacillin, Vancomycin, Azithromycin, Clarithromycin, Roxithromycin, Ciprofloxacin, Rifampicin, Sulphamethizole, and Trimethoprim. The solid medium was gently punctured with the help of cork borer to make a well. Different concentration of NaF: 50μl, 100μl and 150μl, were added from the stock into each well and incubated for 24h at 37±2oC. After 24 hrs of incubation, the zone of inhibition was measured and expressed as millimeter in diameter.
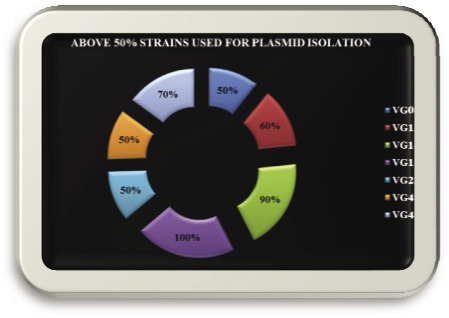 |
Figure 3: Isolated used for plasmid isolation |
Plasmid Analysis
Plasmid were isolated from cariogenic Lactobacilli, using the previous method (Holmes and Quigley, 1981) and the presence of plasmid was checked by 0.7% agarose gel was with visualized under UV light on transiluminator and photographed. Size of the plasmids was determined with the help of a calibration curve prepared using log MW (kb) of the standard molecular marker.
Results And Discussion
Totally 22 multidrug resistant isolates were identified as Lactobacillus spp. according to Bergy’s manual of Bacteriology. Isolated cariogenic Lactobacillus spp. isolates were tested invitro to determine antibiotic susceptibility patterns by antibiotic disc diffusion method. There are different group of antibiotics were tested: Class I Group – Inhibitors of cell wall synthesis (Amoxicillin, Cloxacillin, Vancomycin), Class II Group – Inhibitors of protein synthesis (Azithromycin, Clarithromycin, Roxithromycin), Class III Group – Inhibitors of nucleic acid synthesis (Ciprofloxacin, Rifampicin), Class IV Group – Inhibitors of folate metabolism (Sulphamethizole, Trimethoprim). The antibiotic discs of Amoxicillin (30mcg), Cloxacillin (5mcg), Vancomycin (30mcg), Azithromycin (30mcg), Clarithromycin (15mcg), Roxithromycin (30mcg), Ciprofloxacin (30mcg), Rifampicin (30mcg), Sulfamethizole (300mcg), and Trimethoprim (30mcg). Totally 10 antibiotic discs were used to identify drug resistant Lactobacillus spp. All the isolates showed multiple antibiotic resistances to the antibiotic used in this study. The maximum resistant pattern percentage (100%) was recorded in strain no VG22 and the minimum resistant pattern percentage (10%) was recorded in strain no VG01.
Among 22 strains, Strain No Vg22 was observed 100% resistant with the following antibiogram: SM-VA-RO-TR-RIF-AZM-AMX-CIP-CLR-COX followed by the 90% with antibiogram of SM-VA-RO-TR-AZM-AMX-CIP-CLR-COX was recorded in strain no. Vg21. Whereas strain no. Vg20 was logged 70% with antibiogram of VA-RO-TR-AZM-CIP-CLR-COX followed by the strain no. Vg19 showed 60% with antibiogram of SM-RO-TR-CIP-CLR-COX followed by the strain no. Vg16, Vg17 and Vg18, noted resistant against five antibiotics which showed 50% frequency the antibiogram was SM-VA-RO-TR-COX, VA-RO-CIP-CLR-COX and SM-VA-RO-AZM-COX followed by the strain no. Vg12, Vg13, Vg14 and Vg15, documented resistant against four antibiotics which showed 40% frequency the antibiogram was VA-RO-CLR-COX, SM-TR-COX-CLR, VA-TR-AZM-COX and TR-SM-AZM-COX followed by the strain no. Vg05, Vg06, Vg07, Vg08, Vg09, Vg10 and Vg11, showed resistant against three antibiotics which showed 30% frequency the antibiogram was TR-VA-COX, SM-VA-COX, SM-TR-COX, VA-AMX-COX, TR-AZM-COX, VA-AZM-COX and SM-AMX-COX followed by the strain no. Vg02, Vg03 and VGg4, showed resistant against two antibiotics which exhibited 20% frequency the antibiogram was SM-TR, TR-COX and VA-COX monitored by the strain no. Vg01, detailed resistant against one antibiotics which showed 10% frequency the antibiogram was COX.
| Table 1: Antibiotic Resistant Percentage of Lactobacillus spp. | ||
| S. No | Antibiotics | Percentage of resistant |
| 1. | Rifampicin | 02% |
| 2. | Azithromycin | 18% |
| 3. | Vancomycin | 40% |
| 4. | Amoxicillin | 08% |
| 5. | Sulphamethizole | 40% |
| 6. | Roxithromycin | 18% |
| 7. | Cloxacillin | 98% |
| 8. | Ciprofloxacin | 10% |
| 9. | Clarithromycin | 14% |
| 10. | Trimethoprim | 68% |
The isolates were analyzed for antibiogram as described to determine the antibiotic susceptibility pattern along with the tendency of current resistance against widely used drugs. Among 50 isolates, 22 different antibiogram were found and showed resistance against Amoxicillin (8%), Cloxacillin (98%), Vancomycin (40%), Azithromycin (18%), Clarithromycin (14%), Roxithromycin (18%), Ciprofloxacin (10%), Rifampicin (2%), Sulfamethizole (40%), and Trimethoprim (68%) (Table: 14).
Multiple Antibiotic Resistance (MAR) index was calculated according to the standard formula. Maximum MAR index 1.0 was showed by Vg15 and minimum MAR index 0.1 was showed by Vg6 and Vg07.
Seven isolates: Vg08, Vg13, Vg14, Vg15, Vg22, Vg47, and Vg48 which was showed more than above 50% resistance used for plasmid DNA isolation by boiling preparation method of (Holmes & Quigley, 1981; modified by Riggs & McLachlan, 1986).
Three fragments were obtained in Strain No Vg22, Vg47 and Vg48 with molecular weight of 1500bp, 1000bp, and 100bp respectively, followed by the Strain No. Vg08, Vg13, and Vg14 were harboured three fragments of molecular weight 1500bp, 300bp, and 100bp and other one isolate Vg15 were harboured one fragment of molecular weight 1500bp respectively. This strain was showed maximum resistant against the antibiotics tested. Molecular weight of the fragments was estimated by using 100bp DNA ladder (Medox, Chennai), Multidrug resistance bacteria were found to be carrying plasmids (Figure: 7).
The growth medium of Lactobacillus spp. was adjusted at different pH values: pH 2.0, pH 2.5, pH 3.0, pH 3.5, pH 4.0, pH 4.5, pH 5.0, pH 5.5, pH 6.0, pH 6.5, pH 7.0, pH 7.5 and pH 8.0. The results indicated that the rapid growth was obtained from pH 6 to pH 8, and declined in pH 2.0 and pH 5.5. Maximum growth was recorded in pH 6.5 it indicates the optimum pH for the growth of Lactobacillus spp. is pH 6.5.
The growth medium of Lactobacillus spp. was adjusted at different temperature: 30°C, 35°C, 40°C and 45°C. The results showed maximum growth was observed in 35°C it indicates the optimum temperature for the growth of cariogenic LAB and the growth was declined in 45°C.
Lactic acid production of above 50% resistant plasmid strains were studied by titrimetric estimation which include Vg08,Vg13,Vg14,Vg15,Vg22,Vg47 and Vg48 produce equal amount of lactic acid 9.008 mg/ml in the oral cavity of rural caries patients.
NaF was performed by using agar cup diffusion assay. Different concentrations of NaF (0.25%, 0.50%, 0.75% and 1%) were used against multidrug resistance cariogenic isolates. NaF was used and it shows prominent resistant activity against decay causing organism.
Dental caries is a complex process masked by many indirect factors which obscure the direct causes. Bacteria are through to play an integral role in this process. Cariogenic microorganisms colonize on tooth surface, causes a marked reduction of pH in the presence of sugar substrate, and consequently induce dental caries. Previous studies showed that Lactobacilli is an efficient cariogenic microorganisms the bacteria widely distributed in the nature and occurring naturally as indigenous micro flora in decay oral that play an important role in much food and feed fermentations. This special effect is due to the production of certain antimicrobial substance in the name of lactic acid. Antibiotics provide an invaluable tool for a control of infection in modern dentistry. Development of resistance to various antibiotics makes it necessary to select logically and rationally, a drug for successful gingival therapy during orthodontic treatment. Resistance in microorganisms was caused by extra chromosomal elements. Many studies of drug resistant and its extra chromosomal position have been carried out. Very few reports exits on drug resistance in members of Lactobacillus genus and its link with pla Kheadr, (2006) determined the susceptibility of 13 Lactobacillus strains to 14 antibiotics and to evaluate the impact of some gastrointestinal stressful conditions, particularly acid and bile stress, as well as acid adaptation on their antibiogram profiles. The strains tested were 2 of Lactobacillus acidophilus, 1 Lb. delbrueckii subsp. bulgaricus, 2 Lb. casei, 1 Lb. casei paracasei subsp. paracasei, 1 Lb. delbrueckii subsp. lactis, 4 Lb. plantarum and 2 Lb. rhamnosus. In control trails, the majority of the strains tested were susceptible to Ampicillin, Penicillin, Chloramphenicol, Erythromycin, Novobiocin and Nisin A, but resistant to Vancomycin, Kanamycin, Neomycin, Paromomycin, Streptomycin and Nalidixic acid. Lactobacilli strains showed variable susceptibility to Cloxacillin and Tetracycline. Acid-adaptation (strains adapted to grow at pH 4.0) resulted in increased resistance to Cloxacillin, Erythromycin and Tetracycline, in strain dependent manner. Acid- stressed (exposure to pH 2 for 90 min at 37˚C) Lactobacilli appeared to be more resistant to Ampicillin, Cloxacillin, Chloramphenicol and Tetracycline compared with un-stressed strains. In the presence of 0.3% (w/v) ox gall, Lactobacilli became more susceptible to amino glycosides and slightly resistant to cell wall-targeted antibiotics. However, Oxgall stress (exposure to 0.3% (w/v) ox gall for 90 min at 37˚C) slightly modified antibiogram profile depending on the strain tested. Results obtained in the present study showed Lactobacilli became more susceptible to ample spectrum penicillin such as Amoxicillin and more resistant to â–Lactam group of antibiotic such as Cloxacillin, glycopeptide group of antibiotic Vancomycin and slightly resistant to inhibitors of nucleic acid synthesis.
Lavanya et al., (2011) studied, the antimicrobial susceptibilities and presence of plasmids in 7 probiotics strains which had been isolated from the fermented milk were determined. Resistance to 8 commonly used antibiotics â- Lactams (Penicillin, Ampicillin), gram positive spectrum (Vancomycin), Broad spectrum (Rifampin, Trimethoprim) and Aminoglycosides’ (Kanamycin, Streptomycin, and Bacitracin) was assessed by disk diffusion. Among these strains 20, 20, 60, 70, 90 and 100% were found to be exhibit a significant degree of resistance to Kanamycin, Trimethoprim, Rifampicin, Ampicillin and Penicillin respectively. Further, plasmid profile and curing of plasmid were performed for the seven isolates. Analysis of the plasmid profiles of the 7 cured derivatives revealed loss of plasmids except 2 strains where curing was partially effective. All the strains lost Penicillin resistance after curing indicating that plasmids encodes for resistance character.
However, Vancomycin resistance is not lost upon curing which indicates that such resistance is usually intrinsic. Finally, the antimicrobial susceptibility after curing was done to check the safety aspect of the isolates for their application as probiotics and among the 7 strains, 5 were proved to be potent probiotics. In this present investigation, antimicrobial susceptibilities and presence of plasmids in 7 cariogenic strains which had been isolated from the plaque samples. Resistance to 3 commonly used antibiotics (Vancomycin, Rifampicin, and Trimethoprim) was assessed by disk diffusion. Among these strains 40%, 2% and 68% were found to be exhibit a significant degree of resistance to Vancomycin, Rifampicin and Trimethoprim respectively.
O’Sullivan and Klaenhammer, (1993) reported a simple, rapid plasmid mini-prep procedure for Lactococci and Lactobacilli which gives high yields and can be performed on overnight broth cultures is presented. Large plasmids were isolated from both Lactococci and Lactobacilli, including a 70-kb plasmid from Lactobacillus acidophilus C7. The purity of the resulting plasmid DNA makes it suitable for subsequent molecular manipulations. The convenience of the technique makes this rapid mini-prep procedure suitable for routine plasmid isolation from lactic acid bacteria. But in this research, plasmid DNA was done by boiling preparation method (Holmes & Quigley, 1981; modified by Riggs & McLachlan, 1986). Plasmids were isolated from Lactobacilli, including a range from 1500bp to 100bp respectively.
A significant reduction between pre-test and post-test was found only for salivary S. mutans count (p=0.006) in 0.5% NaF impregnated miswak sticks group but not for salivary Lactobacilli count. Sodium fluoride when impregnated to miswak sticks did not significantly add to the antimicrobial efficacy (Yavagal et al., 2014). But in this study showed dissimilar results of antimicrobial ability of sodium fluoride without miswak stick ingredients at concentration of 0.5% used against oral pathogen. The NaF was used and it shows prominent resistant activity against decay causing organism.
Conclusion
Development of resistance to antibiotics by bacteria is inevitable, not only because of their rates in mutation and transferability of drug resistant genes. Resistance in microorganisms caused by extra chromosomal elements. A breakthrough came with the discovery that antibiotic resistance is transmitted by mixed cultivation between drug-resistant and sensitive strains independently of the donor strain chromosome. It is concluded from the present study that species of cariogenic Lactobacillus isolated from plaque. The rapid emergence of drug resistant strains of microbial cariogenic pathogen especially those with multi drug resistance characteristics and the organism link with a plasmid have the ability to survive at low pH and low temperatures and had strongly to produce antimicrobial substance during fermentation process. These results also suggests that the consumption of carbonated drinks, eating of fast foods, chocolates and fruit juices from bakery shops. The products those enhances the food supply to growing plaque forming microorganisms and the condition favorable for development of pathogenic microorganisms. NaF is currently, as antimicrobial the most promising tools applied as antimicrobial agents for diagnosis the dental disease. However the present study NaF was used and it shows prominent resistant activity against decay causing organism. Moreover, most antimicrobial agents that are currently in use have been rendered in effective by a wide occurrence of multi drug resistant strains of microbes.
References
Aas J.A., A.L. Griffen, S.R. Dardis, Lee A.M. and Olsen. (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbial., Vol. 46, Issue 4, 1407-1417.
Antony, S.J., C.W. Stratton, and Dummer J.S. (1996). Lactobacillus bacteremia: description of the clinical course in adult patients without endocarditis. Clinical Infections Diseases: an official publication of the Infectious Diseases Society of America, Vol. 23, Issue 4, 773-778.
AOAC, (1990). Association of Official Analytical Chemists. Official Methods of Analysis 15th Edition (Helrick, K.ed.). AOAC, Arlington, Virginia.
Attebery HR, and Fine gold S.M. (1970). A new anaerobic blood culture system. Xth Int. Congr. Microbial. Abstract, 1970:105.
Badet C, and Thebaud N.B. (2005). Ecology of Lactobacilli in the oral cavity: a review of literature. The Open Microbial Journal, Vol. 2, 38-48.
Bernable E, and Sheiham A. (2014). Age, period and cohort trends in caries of permanent teeth in four developed countries. AM J Public Health. Vol. 104, Issue 7, 115-21.
Charteris WP, P.M Kelly, Moreli L, and Collins J.K. (1998). Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. Vol. 61, 1636 – 1643.
Cruickshank R, (1968). Medical microbiology: a guide to diagnosis and control of infection. 11th Ed. Edinburgh and London: E & S Livingstone Ltd., 50 – 97.
Handle man S.L, and Hawes R.R. (1964). The effect of long – term antibiotic therapy on the antibiotic resistance of the salivary flora. J. Oral. Ther. Pharmacol. Vol. 1, 23-44.
Handle man S.L, and Hawes R.R. (1965). The effect of
long – term systemic antibiotic administration on the number of salivary organism. Arch. Oral Biol., Vol. 10, 353-360.
Handle man S.L, J.R. Mills, and Hawes R.R. (1996). Caries incidence in subjects receiving long – term antibiotic therapy. J. Oral Ther. Pharmacol. Vol. 2, 338-345.
Holmes D, and Quigley M. (1981). A rapid boiling method for the preparation of bacterial plasmids. Anal. Biochem. Vol. 114, 193-197.
Kandler O, and Weiss N. (1986). Genus Lactobacillus beijerinck 1901. In Bergey’s Manual of systematic Bacteriology, Vol. 2, 1209 – 1234.
Katla, A.K., H. Kruse, Johnsen G, Louwers J, and Herikstad H. (2001). Antimicrobial susceptibility of starter culture bacteria used in Norwegian dairy products. Inter J Food Microbiol, Vol. 67, 147-152.
Kheadr EE, (2006). Impact of acid ox gall on antibiotic susceptibility of probiotic Lactobacilli. African Journal of Agricultural Research. Vol. 1, Issues 5, 172-181.
Klaenhammer T.R, and Sutherland S.M. (1980). Detection of plasmid deoxyribonucleic acid in an isolate of Lactobacillus acidophilus. Appl Environ Microbial. Vol. 39, 671-674.
Lavanya B, S. Sowmiya, Balaji S. and Muthuvelan B. (2011). Plasmid profiling and curing of Lactobacillus strains isolated from fermented milk for probiotic applications. Advance Journal of Food Science and Technology. Vol. 3, Issues 2, 95-101.
Loesche W.J, S. Eklund, Earnest R, and Burt B. (1984). Longitudinal investigation of bacteriology of human fissure decay: Epidemiological studies in molar shortly after eruption. Infect Immun, Vol. 46, Issue 3, 765 – 772.
Martin F.E, M.A. Nadkarni, Jacques N.A, and Hunter N. (2002). Quantitative microbiological study of human carious dentine by culture and real time PCR: association of anaerobes with histopathological changes in chronic pulpitis. J Clin Microbial, Vol. 40, 1698-1704.
Mylotte JM, Mc Dermott C, and Spooner J. (1987). Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteria. Rev Infec Dis., Vol. 9, 981.
O’Sullivan DJ, and Klaenhammer T.R. (1993). Rapid mini-prep isolation of high quality plasmid DNA from Lactococcus and Lactobacillus spp. Applied and Environmental Microbiology, Vol. 59, Issue 8, 2730-2733.
Owhe – Ureghe, U.B., Ehwarieme D.A, and Ebo D.O. (2010). Antibacterial activity of garlic and lime on isolates of extracted carious teeth. Afr J Biotech., Vol, 9, Issue 21, 3163-3166.
Oyetayo V.O, F.C. Adetuyi, Akinyosoye and F.A. (2003). Safety and protective effect of Lactobacillus acidophilus and Lactobacillus casei used as probiotic agent invivo. Afr. J. Biotech. Vol. 2, 448-452.
Rizwan Ullah and Mohammad Sohail Zafar, (2015). Oral and dental delivery of fluoride: A Review. Research review fluoride. Vol 48, Issue 3, 195-204.
Sozzi T, and Smeiley M.B. (1980). Antibiotics resistance of yoghurt culture Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbial, Vol. 40, 862-865.
Straetemans MM, C. Van Loveren, J.J De Soet, de Graaff J and ten Cate J.M. (1998). Colonization with mutans streptococci and lactobacilli and the caries experience of children after the age of five. J Dent Res, Vol. 77, Issue 10, 1851 –1853
Westh H, C.C. Zinn and Rosdahl V.T. (2004). Sarisa study group, an international multi-center study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microbial Drug Resistance, Vol.10, 169-176.
Yavagal PC, S. Kumbhar, Nagesh L, and Kulkarni A. (2014). Antimicrobial effect of miswak sticks and 0.5% sodium fluoride miswak sticks on Streptococcus mutans and Lactobacilli –a randomized controlled trial. Unique Journal of Medical and Dental Science. Vol. 02, Issue 02, 79 – 82.


