1Central Marine Fisheries Research Institute, Tuticorin Research Centre, Tuticorin- 628001, TN, India
2Udaya School of Engineering, Nagercoil, TN, India
Article Publishing History
Received: 11/04/2016
Accepted After Revision: 31/05/2016
The present study was aimed to carry out a green synthesis of Silver Nano Particless by using marine micro algae and its characterization by SEM, EDAX, Particle analyzer and Zeta potential. Microalgae samples (Isochrysis galbana and Nannochloropsis sp.) were collected from phytoplankton laboratory, Tuticorin Research Centre of ICAR-Central Marine Fisheries Research Institute. The culture media of I. galbana and Nannochloropsis sp. were taken for the preliminary synthesis of SNPs. Hence, Nannochloropsis sp. was not synthesized for its SNPs, I. galbana was selected for further study. Culture media, cell filtrate and supernatant of I. galbana were subjected to the SNPs synthesis in order to find out the maximum yield of silver with low chloride content. Among these, the supernatant of I. galbana showed maximum yield with low chloride content. It was confirmed by colorimetric reading at 450 nm. Synthesized SNPs from I. galbana containd 80.68% of silver and 15.25% of chloride as detected by EDAX analysis. Size of the SNPs was analyzed by particle analyzer and ranged from 16.6 to 205.3 nm and average size was 40.6 nm. Zeta potential of SNPs from I. galbana was -44.0 mV, which reveals the high stability of SNPs. This is the first authenticated work of I. galbana with respect to complete characterization of its SNPs.
Microalgae, Isochrysis Galbana, Nanoparticles, Characterization
Suja C.P, Senthil. S. L, Priya. S A, Preethi M. S, Renu A. Optimization and Characterization of Silver Nanoparticle Synthesis from the Microalgae, Isochrysis Galbana. Biosc.Biotech.Res.Comm. 2016;9(2).
Suja C.P, Senthil. S. L, Priya. S A, Preethi M. S, Renu A. Optimization and Characterization of Silver Nanoparticle Synthesis from the Microalgae, Isochrysis Galbana. Biosc.Biotech.Res.Comm. 2016;9(2). Available from: https://bit.ly/2IQEgUH
Introduction
Nanoparticles (NPs) are used for numerous physical, biological and pharmaceutical applications. It has been widely used in textile engineering, biotechnology and bioengineering, water treatment, electronics, antibacterial/antifungal agents in a diverse range of consumer products. It is used in DNA sequences due to the fluorescence and surface plasmon resonance characteristics of silver nanoparticles (SNPs) (Jayasree et al. 2013). The NPs have synthesized by chemical as well as electrical methods. The biosynthesis methods have many advantages and are eco friendly (Priyanga Singh et al. 2015).
Research into the natural products and drug development of algae results in the isolation of over 15,000 novel compounds of economical importance (Farnsworth 1997) such as polysaccharides, iodine organic products, macro and micro elements, vitamins and unsaturated fatty acids (Craigie 2011). Green synthesis of metal NPs using microalgae is an emerging field in nanotechnology and biotechnology. Green synthesis of silver NPs using algae and algal extract shows more advantageous over other biological samples such as bacteria and fungi (Kartikeyan et al. 2015).
Characterization of NPs is an important tool to regulate the NPs synthesis and its applications. It is performed using a variety of different techniques such as transmission and scanning electron microscopy (TEM and SEM), atomic force microscopy (AFM). These techniques are used to determine the different parameter such as particle size, shape, crystallinity, fractional dimensions, pore size and surface area (Kholoud et al. 2010)
Silver nanoparticles are used in the treatment of various diseases. It acts as a potent anti microbial agent. Role of SNPs in cancer treatment is an emerging technology but it needs further more researches in the aspect of toxicity. Nanoparticle based carrier may be an excellent anticancer drug delivery module due to their extremely small size and high permeability that facilitates to evade first-pass metabolism and increasing efficacy (Buckston 2006). In addition, NPs show anticancer activity possibly through conversion of near-infrared radiation to vibration energy, generating sufficient heat to kill selective cancer cell (West et al. 2011).
Researchers have explored the use of SNPs as carriers for delivering various payloads such as small drug molecules or large biomolecules to specific targets. Once the SNPs have sufficient time to reach its target, release of the payload could potentially be triggered by an internal or external stimulus. The targeting and accumulation of NPs may provide high payload concentrations at specific target sites and could minimize side effects (Phillips et al. 2010). The present study was aimed to green synthesize of SNPs by using different marine micro algae and characterization by SEM, EDAX, Particle analyzer and Zeta potential in order to find out the drug potential of SNPs for further research.
Materials And Methods
Collection Of Microalgae
Microalgae samples (Isochrysis galbana and Nannochloropsis sp.) were collected from phytoplankton laboratory, TRC of ICAR-CMFRI, Tuticorin. Stock cultures of microalgae were maintained in a special room adjacent to the mass culture and maintained in Walne’s medium. Mass culture was maintained by using major nutrients (ammonium sulfate: Super phosphate: urea) at 10:1:1ratio.
Synthesis Of Snps
For the synthesis of SNPs, algae with culture medium, cell filtrate and supernatant was taken. The algal biomass was collected through centrifugation by centrifuge (Remi) at 5000 rpm for 10 min. Initially all the samples were subjected to colorimetric reading at 400 to 620 nm. In culture medium, cell filtrate and supernatant of micro algae were mixed with 1mM of AgNo3 solution and OD values were taken for every 20 min by using calorimeter (EI). Silver nitrate was used as a control. The preliminary confirmation of SNPs synthesis was done based on the lambda max obtained at 425 nm. Synthesized SNPs were dried in hot air oven at 65 °C for characterization.
Characterization Of Snps
Optical properties of SNPs which are sensitive to concentration, size, shape and agglomeration state, makes colorimeter is a valuable tool for identifying these materials. The formation of unique peak at specific wavelength of light is due to the surface plasmon resonance of the electrons present on the NP surface. The surface plasmon resonance of SNPs is 425 nm.
Scanning Electron Microscopy (Sem)
For electron microscopic imaging, synthesized NPs were sputter-coated with gold palladium mixture. Subsequently, the samples were examined in field emission scanning electron microscope (Model: SIGMA VP, make: Carl Zeiss Microscopy GmbH, Germany; operated at 15 kV) equipped with X-ray energy- dispersive spectrometry system.
Particle Size Distribution And Zeta Potential
Colloidal dispersions are stabilized by electrostatic repulsion and/or steric hindrance. Electrostatic stabilization can be determined by measuring the zeta potential, which is the charge at the surface of the electrical double layer. Particle size distribution and zeta potential (indicator of dispersion and stability) was studied using Nanopartica SZ-100 nanoparticle analyzer (Horiba Ltd., Kyoto, Japan).
Results And Disscussion
The development of SNPs from natural resources is an important in contemporary technology. The development of biological and experimental processes for the synthesis of NPs is developing into an important branch of nanotechnology. There is no authenticated evidence in the synthesis of NPs from marine micro algae in the aspects of complete analysis such as SEM, EDAX, Zeta potential and particle analysis. Cell count was taken before the reaction started by haemocytometer and the cell count was 2.1× 106 and 2.1× 107 for I. galbana and Nannochlropsis sp., respectively.
Patel et al. (2005) reported that the biosynthesize of SNPs from algae by using two methods; (i) suspending the live and washed biomass of microalgae into the AgNO3 solution and (ii) by adding AgNO3 into a cell-free culture liquid. In most of the cases SNPs were developed both in the presence of biomass as well as in the cell-free culture liquid and the NPs were ranged from 13 to 31 nm in size (Patel et al. 2015). In this study, the culture medium of I. galbana and Nannochloropsis were taken for the synthesis of SNPs. Silver nanoparticles were synthesized only in the culture medium I. galbana. Then the culture medium, cell filtrate and supernatant of I. galbana were subjected to the SNPs synthesis in order to find out the maximum yield with low chloride content. Among these, the supernatant of I. galbana showed maximum yield with low chloride content (Figure 1) and it was confirmed by calorimetric reading at 450 nm (Figure 2). In cell filtrate, the cells were crashed and released larger chlorophyll content which makes the reaction mixture as green in color.
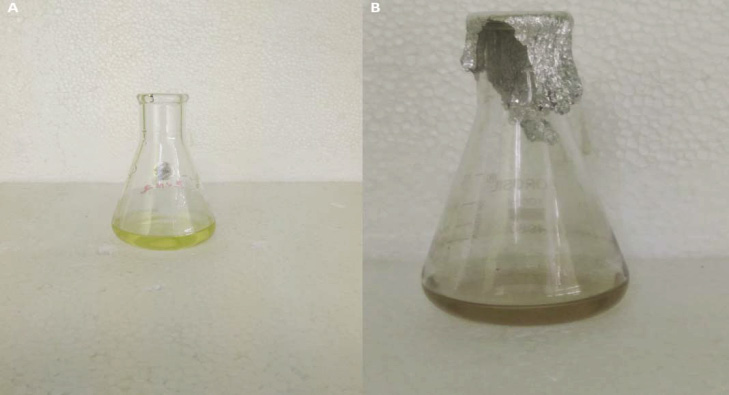 |
Figure 1: A) Supernatant of I. galbana before synthesizing SNP; B) after synthesizing SNP |
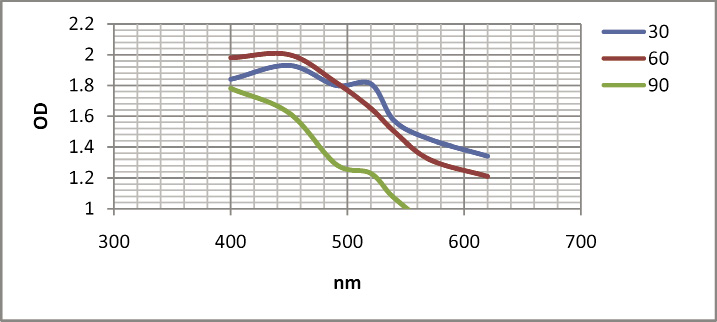 |
Figure 2: Calorimetric reading of synthesized SNPs from I. galbana |
Shiny et al. (2013) reported that seaweeds were used as bio reluctant for the reduction of the silver salt to form the SNPs. The development of the SNPs was confirmed with the dark brown color development and it was noticeable with UV-Visible spectroscopy. The Transmission electron micrographs indicate the size of the NPs which can be from 25-40 nm and the NPs were spherical in shape.
Karthikeyan et al. (2015) reported that the synthesis of SNPs fom green microalgae, Chlorella vulgaris and Chaetoceros calcitrans. The absorbance spectrum of aqueous medium containing SNPs showed a peak at 420 and 436 nm by C. vulgaris and C. calcitrans respectively. The shape and size of SNPs were studied by SEM. The spherical shaped SNPs were observed and their element was ranged from 50-70 and 30-35 nm from C. vulgaris and C. calcitrans, respectively. In this study, synthesized SNPs were spherical in shape and ranged from 157.3 to 183.8 nm which is shown in SEM image of synthesized SNPs (Figure 3).
 |
Figure 3: SEM image of synthesized SNPs from I. galbana |
In the presence of salt condition, AgNo3 can be formed AgCl2, which is the major problem for reducing AgNo3 to Ag and it is reflected in all the analysis (Yaro 2016). It is a main reason for lack of complete report about SNPs from marine micro algae and most of the statements are based on SNPs synthesized from fresh water and macro algae. In this present study, these harms were considered and standardized different protocol for SNPs synthesis from marine micro algae. Presence of SNPs was confirmed by EDAX analysis with 80.68% silver with less chloride (15.25%) as shown in Figure 4.
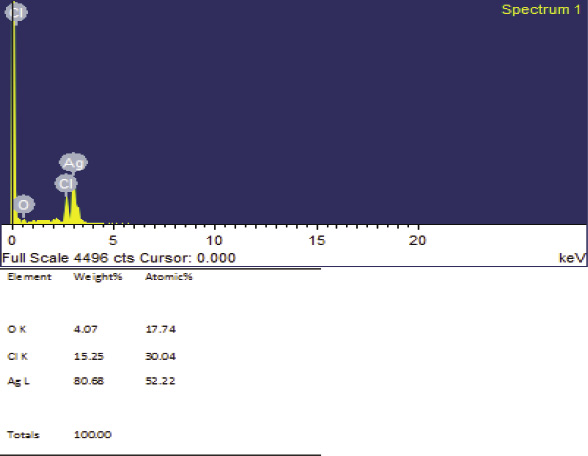 |
Figure 4: EDAX image of synthesized SNPs from I. galbana |
Particle size distribution and zeta potential micrograph of SNPs is shown in Figure 5 and 6. Particle size distribution graph shows that the synthesized SNPs are in the range of 14.3-63.3 nm. The mean size of SNPs was found to be 40.6 nm.
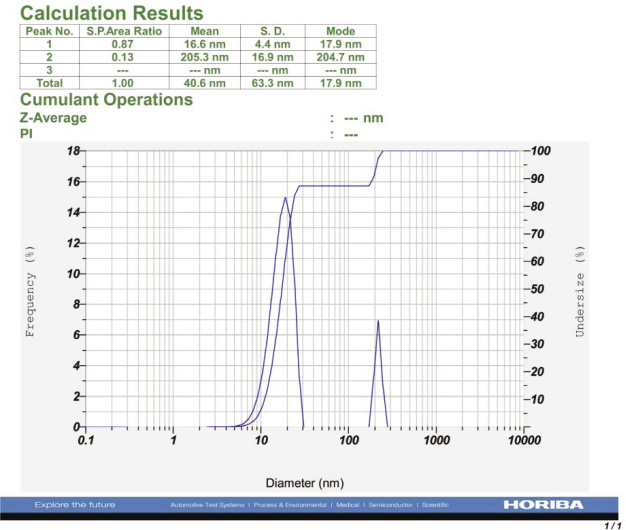 |
Figure 5: Particle analyzer report of synthesized SNPs from I. galbana |
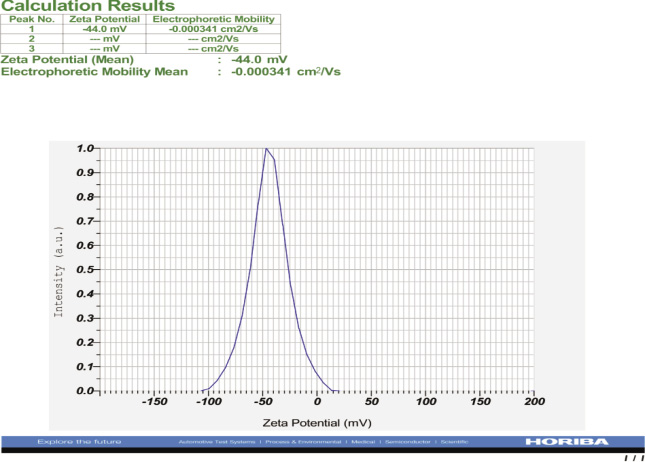 |
Figure 6: Zeta potential report of synthesized SNPs from I. galbana |
In this present study, zeta potential of SNP is -44.0 mv. If the zeta potential of SNP is greater than ±25mv, It means that the moderate colloidal stability of SNPs, and If it is greater than ±40 mv revealed the high colloidal stability of SNPs (Greenwood and Kendall 1999).
According to Karthikeyan et al. (2015), the green microalgae, Chlorella vulgaris and Chaetoceros calcitrans were tested for synthesis of SNPs. The absorbance spectrum of aqueous medium containing SNPs showed a peak at 420 and 436 nm by C. vulgaris and C. calcitrans respectively. The shape and size of SNPs were studied by SEM. The spherical shaped SNPs were observed and their dimension was ranged from 50-70 and 30-35 nm from C. vulgaris and C. calcitrans, respectively.
The SNPs exhibit good activity of antimicrobial and hence their potential application in medicine as wound dressing or for sterile clothing in hospital environment. Electron micrographs were taken to study the surface morphology and size of the NPs. The graphs indicate spherical NPs in the size range of 25-40nm. Specific surface plasmon resonance peak at 410 nm exhibited by UV-Visible spectroscopy. The particle size was proved through transmission electron microscopy. The particles were within the range of 25-40 nm (Shiny et al.
2013).
Similarly, Davina Merin et al. (2010) reported that synthesis of SNPs from normal and microwave irradiated mixed marine micro algae including I. galbana by using AgNO3 as supplement with medium. The size of SNPs is 53-79 nm. Previous reports have revealed only the size and morphology of SNPs synthesized from marine micro algae but there is no confirmation about percentage of silver, chloride and other particles. From the present study, the synthesized SNPs from I. galbana contain 80.68% of silver and 15.25% of chloride which is very less. Size of the SNPs also ranged from 16.6 to 205.3 nm and average size is 40.6 nm. Zeta potential of SNPs from I. galbana is -44.0 mV, which reveals the high stability of SNPs. Therefore, the data of the present study show that the SNPs from I. galbana could be developed as a potential agent against human cancer, microbial infections and other aquatic diseases.
Acknowledgements
The authors are grateful to Dr. A. Gopalakrishnan, Director, Dr. P. Vijayagopal, Head (MBTD), Dr. P.P. Manojkumar, Scientist-in-Charge, Tuticorin Research Centre of Central Marine Fisheries Research Institute for providing necessary facilities for the work.
References
Buxton. I (2006). Pharmacokinetics and pharmacodynamics: the dynamics of drug absorption, distribution, action, and elimination. In: Brunton L, Lazo J, Parker K, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill Medical Publishing Division; NY, USA. pp. 1–40.
Craigie, JS (2011). Biology of the red algae. Cambrige University Press, Cambridge.
Devina Merin, S. Prakash, B. Valentine Bhimba. (2010). Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pacific Journal of Tropical Medicine:797-799.
E.J. Farnsworth, EJ and A.M. Ellison. (1997). The global conservation status of mangroves. Ambio 26 (6): 328–334.
Greenwood, R and K. Kendall. (1999). Electroacoustic studies of moderately concentrated colloidal suspensions. Journal of the European Ceramic Society 19 (4): 479–488.
Jena J, Nilotpala Pradhan, Bisnu Prasad Dash, Lala Behari Sukla, Prasanna Kumar Panda. (2013). Biosynthesis and characterization of silver Nanoparticles using microalga Chlorococcum humicola And its antibacterial activity. International Journal of Nanomaterials and Biostructure 3(1):1-8
Karthikeyan,P D. Mohan, G. Abishek, P. Priya. (2015). Synthesis of silver nanoparticles using Phytoplankton and its characteristics. International Journal of Fisheries and Aquatic Studies 2(6): 398-401.
Kholoud, MM Abou EI-Nour, Ala Eftaiha, Abdulrhman Al-Warthan, A.A. Reda A.A. Ammar. (2010). Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry 3(3):135-140.
Patel, V., David Berthold, Pravin Puranik, Miroslav Gantar. (2015). Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnology Reports 5: 112–119.
Phillips,MA M.L. Gran, N.A. Peppas. (2010). Targeted nanodelivery of drugs and diagnostics. Nano Today 5:143–159.
Shiny., PJ M. Amitava, N. Chandrasekran. (2013). Marine algae mediated synthesis of the silver nanoparticles and its antibacterial efficiency. International Journal of Pharmacy and Pharmaceutical Sciences 5 (2): 239-241.
Singh, P., Yeon Ju Kim, Hina Singh, Chao Wang, Kyu Hyon Hwang, Mohamed El-Agamy Farh, Deok Chun Yang (2015) Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. International Journal of Nanomedicine 10: 2567–2577.
West JL , L.C. Kennedy, L.R. Bickford, N.A. Lewinski, A.J. Coughlin, Y. Hu, E.S. Day, R.A. Drezek (2011) A New Era for Cancer Treatment: Gold-Nanoparticle-Mediated Thermal Therapies. Small 7:169–183.
Yaro, MN (2016). Effect of Concentration of AgNO3 on the Rate of Formation of a Complex Salt, [Co(H3)6](NO3)3 and Estimation of Molar Concentration of the Complex. Chemistry and Materials Research 8: 8-12


