1Nano-Biology Laboratory, School of Studies in Life Science, Pt. Ravishankar
Shukla University, Raipur 492010, Chhattisgarh, India
2Microbiology Research Laboratory, School of Studies in Life Science, Pt.
Ravishankar Shukla University, Raipur 492010, Chhattisgarh, India
3Nova Nature Club, Raipur, Chhattisgarh, India
Corresponding author email: manojkpatel@prsu.ac.in
Article Publishing History
Received: 20/06/2025
Accepted After Revision: 07/09/2025
The unfolding of bacterial drug resistance poses a significant threat to public health globally, requiring novel and effective antimicrobial strategies. Knowing the necessity of this situation, the current study explores the biosynthesis of silver nanoparticles as potent antibacterial agents. The biosynthesis was explored by a green approach mediated by the endophytic actinomycete Streptomyces rochei, 16S rRNA sequencing helped identification of the actinomycete and assigned the GenBank accession number ON142045. The synthesized silver nanoparticles (AgNPs) were characterized for their formation by using various techniques, which included FE-SEM, EDS, FT-IR, UV-Vis spectroscopy, and DLS. UV-Vis analysis confirmed nanoparticle formation with an absorption peak at 400 nm. The FT-IR analysis shows existence of multiple functional groups such as O–H, C–N, and C=C, which explains biomolecules in nanoparticle stabilization. The biosynthesized AgNPs exhibited notable antimicrobial and bactericidal activity against both MTCC strains and clinically isolated Gram-negative bacterial pathogens. The above results show that the biosynthesized AgNPs possess significant potential as alternative antibacterial agents, offering a promising approach for combating multidrug-resistant bacterial infections. Utilizing endophytic actinomycetes for nanoparticle synthesis promotes environmentally sustainable methods while offering promising opportunities for biomedical use, particularly in antimicrobial treatments. This study highlights the effectiveness of biosynthesized silver nanoparticles in inhibiting bacterial growth, underscoring their potential role in addressing the ongoing challenge of antibiotic resistance through green nanotechnology-based solutions.
Antimicrobial activity, Biosynthesis, bacterial Pathogen, Silver nanoparticles, Streptomyces rochei.
Yadav S, Singh R. R, Sahu A. K, Gupta A. K, Patel M. K. Biosynthesis of Silver Nanoparticles from Streptomyces rochei and its Antibacterial Efficacy against Bacterial Pathogens. Biosc.Biotech.Res.Comm. 2025;18(3).
Yadav S, Singh R. R, Sahu A. K, Gupta A. K, Patel M. K. Biosynthesis of Silver Nanoparticles from Streptomyces rochei and its Antibacterial Efficacy against Bacterial Pathogens. Biosc.Biotech.Res.Comm. 2024;18(3). Available from: <a href=”https://shorturl.at/q982y“>https://shorturl.at/q982y</a>
INTRODUCTION
In the recent era of nanomaterials, metallic nanoparticles (NPs) are becoming increasingly significant due to their distinctive properties based on form, size, and dispersion (El‐Baz et al., 2016; Mourdikoudis et al., 2018). Among these novel qualities are excellent physicochemical, electrical conductivity, catalytic, and mechanical stability ( Srikar et al 2016, Crisan et al., 2021; Rania et al., 2021). Researchers have focused a lot of attention on different metal nanoparticles because of their effective physics, chemistry, and biological characteristics. Nanoparticles have proven useful in a variety of fields, including bioaugmentation of environmental contaminants, delivery of gene for the treatment and circumvention of genetic diseases, imaging, catalysis, and medical applications such as vaccine antigen administration, novel bioelectronics devices like nano-biosensors, metal optics, or enhanced drug delivery methods. Since multi-drug resistant bacteria are currently poorly managed, this is a serious worldwide problem. Therefore, the need for innovative antibacterial agents is essential. The lack of efficacious treatments for multi-drug resistance bacteria poses a serious worldwide threat. Finding new antimicrobial medications is therefore essential. In this regard, AgNPs have been suggested as a novel class of antibacterial agents.
Current treatments do not have the capacity to completely eradicate multi-drug resistant bacteria, posing a severe global threat (Wypij et al., 2018). Therefore, the need to develop innovative bactericides is very high. In many healthcare institutions, Gram-negative bacteria have been seen as the most frequent pathogens, there has been an upsurge in the incidence of nosocomial infection causing multidrug-resistance pathogens ( Saeid et al 2011, Ahmed Elsadek Fakhr & Fayza Fathy, 2019; Rania et al., 2021).The antibiotic resistance among patients revealed the prevalence of methicillin-resistant S. aureus (MRSA), extended-spectrum beta-lactamase bacteria, cephalosporin resistant K. pneumoniae, carbapenem resistant K. pneumonia, and multidrug resistant P. aeruginosa, E. coli, and Acinetobacter baumannii spp. (Ahmed Elsadek Fakhr & Fayza M. Fathy, 2019; Rania et al., 2021).
The antibacterial properties of AgNPs have led to their increasing popularity and even the suggestion that they represent a new class of antimicrobial drugs. As a result, numerous uses for Ag-NPs have been discovered, such as medical device coatings, household, wound dressings, and healthcare products, and cosmetics (Martínez et al., 2020). Majorly, two techniques for employed for synthesizing metal nanoparticles: “bottom-up” and “top-down.” The methods involved are carried out by a number of chemical, biological, and physical processes. Many physical and chemical techniques are essential for the production of metal nanoparticles in view of their selectivity and capacity to produce monodisperse nanoparticles (Iravani & Zolfaghari, 2013).
The usage of a biological system is required for nanoparticles biological synthesis. Biosynthesis is a bottom-up strategy in which nanoparticles are formed as a consequence of metal reduction/oxidation and enzymes released by microbial systems and different metabolites from plants are responsible (Prabhu & Poulose, 2012). The bioproduction mechanism of AgNPs was reported according to which the enzyme of nitrogen cycle i.e., nitrate reductase is in charge of converting nitrate to nitrite. The nitrate reductase enzyme, is known to play a noteworthy role in the bioproduction of AgNPs. The silver ions are formed due to enzymatic metal reduction mechanism. Nitrate reductase reduces silver ions to metallic silver and therefore silver nanoparticles is formed (Karthik et al., 2014).
Many attempts have been made to synthesise metal nanoparticles utilising microorganisms and plants. In regards of biological synthesis, there is an entire list of bacteria and plants. Many kinds of bacteria, including Bacillus sp. and Pseudomonas sp., are being used to reduce silver ions to silver nanoparticles. AgNPs have been manufactured using various actinomycetes which include Streptomyces sp. (Alani et al., 2012), Streptomyces sp. JAR1 (Chauhan, 2013), Nocardiopsis sp. MBRC-1 (Manivasagan et al., 2013), Rhodococcus sp. (Otari et al., 2012), Streptomyces albidoflavus CNP10 (Prakasham, 2012) Streptomyces hygroscopicus BDUS 49 (Sadhasivam et al., 2010), Streptomyces sp.VITPK1 (Sanjenbam et al., 2014), Streptomyces rochei (Kim et al., 1998), Streptomyces sp. BDUKAS10 (Sivalingam et al., 2012), Streptomyces sp. VITBT7 (Yingst et al., 1998), Streptomyces sp. I, Streptomyces sp. II, (Sukanya et al., 2013), Streptomyces glaucus 71MD (Tsibakhashvili et al., 2011), Streptomyces aureofaciens MTCC356 (Liossis et al., 1998) , Streptomyces sp. ERI-3 (Faghri Zonooz & Salouti, 2011), and Streptomyces sp. LK3 (Karthik et al., 2014).
Because of their promising bioactivities, such as antioxidant, antifungal, antibacterial, and anticancer effects, AgNPs have proved to be efficient metallic nanoparticles. The potential antimicrobial properties of these nanoparticles can be used in medical device industry to reduce microbial infections and prevent bacteria colonisation on medical equipment such as dental materials, catheters, vascular grafts, stainless steel materials, and human skin (“The Clinician and the Microbiology Laboratory,” 2015). The present work involves the green biosynthesis of AgNPs by endophytic actinomycete Streptomyces rochei as an excellent alternative route, its characterization and evaluates the antimicrobial and anti-biofilm activities of the synthesized silver nanoparticles.
MATERIAL AND METHODS
Isolation of the actinomycete Streptomyces rochei was done from root tissue of Bryophyllum pinnatum (Lam.) Oken and the GenBank accession number ON142045 was assigned after 16S rRNA sequencing (Yadav et al., 2023). Himedia, Mumbai, India provided the chemicals used for preparing media (ISP-4 broth and NAM) and 2,3,5- Triphenyl tetrazolium chloride (TTC). Silver nitrate (AgNO3) was bought from Merck, Mumbai, India.
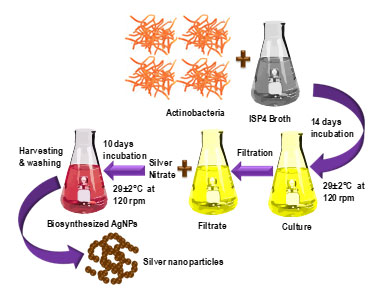
Silver nanoparticles biosynthesis: The endophytic actinomycete Streptomyces rochei was isolated (GenBank accession no. ON142045) (Yadav et al., 2023) and incubated in 250 ml of ISP-4 broth (pH 7.0) for 21 days at 29°C at 120 rpm. The obtained culture was filtered twice using a Whatman filter paper to remove any actinobacterial cell content, and the bio-extract was obtained. 200 mM AgNO3 was added to of above extract (1:1); to make a concentration of 100 mM of solution. Centrifugation of the incubated solution was done at 10000 rpm at 4°C for 10 days, during which time it was surveilled for colour changes each day. After 10 days, pellet of the incubated solution was taken by centrifugation at 10000 rpm at 4°C. The pellet was then washed 3 times with ethanol and distilled water mixture and air dried. The obtained dried pellet was crushed in a mortar pestle to obtain fine powder and was further characterized. The detailed biosynthesis procedure of AgNO3 has shown in Scheme 1.
Scheme 1. Biosynthesis procedure of silver nanoparticles (AgNPs).
Characterization of biosynthesized silver nanoparticles: FESEM imaging: The biosynthesized AgNPs were ultra-sonicated in an ultrasonic cleaner (Aczet, Mumbai) with ethanol and drop casted on ITO glass plates to increase their conductivity for visualizing using Field Emission Scanning Electron Microscopy (FESEM). At a magnification of 60.00 K X, FESEM images were recorded using FESEM (Carl Zeiss UHR, Gemini SEM 500 Kmat). Energy Dispersive X-Ray Spectroscopy (EDS) was also executed on the same sample to determine elemental composition.
UV-Vis spectroscopy: Using ethanol at a concentration of 1.0 mg/ml the biosynthesized AgNPs were ultra-sonicated in an ultrasonic cleaner (Aczet, Mumbai) and the genesis of AgNPs was analysed using UV-Vis Spectrophotometer (Thermo Scientific, Evolution 201) against ethanol as blank at a wavelength range of 300-700 nm.
Dynamic light scattering (DLS): The biosynthesized AgNPs were ultra-sonicated in an ultrasonic cleaner (Aczet, Mumbai) with double distilled water to for a clear aqueous solution and was analysed using the DLS method to estimate the hydrodynamic size and the zeta potential of AgNPs using zeta sizer pro (Malvern).
Fourier transform infrared spectroscopy: FT-IR spectra of the dried biosynthesized AgNPs sample was performed on an FT-IR spectrometer (Perkin-Elmer Spectrum IR Version 10.7.2), with KBr in the wave number region of 4000–400 cm−1. The identification of functional groups was done by collation of the spectral data recorded with the available reference database.
Antimicrobial activity screening: The antimicrobial activity screening of the biosynthesised AgNPs was conducted against MTCC and clinical cultures of four Gram-negative bacteria (Escherichia coli, Proteus mirabilis, Salmonella typhimurium, and Pseudomonas aeruginosa) . Every bacterial strain was cultivated on nutritional broth. In order to create fresh stock inoculum suspensions of the pathogenic strains, colonies from 24-hour cultures grown at 37°C were selected, and the colonies were then suspended in sterile saline solution (0.9% NaCl, w/v). By adjusting the optical density of pathogens, the turbidity was brought to the 0.5 McFarland standard or approximately 1×108 CFU/mL for bacteria. Bacterial cultures were inoculated on Nutrient Agar Media plates and wells were created with 0.1 mg/ml of the bacteria. Next, three copies of the silver nanoparticle were inoculated to all the wells and the incubation was done at 37 °C for 24 h and observed.
MIC, MBC method, and tolerance level: In ELISA plates, the lowest concentration of AgNPs that can stop bacterial growth known as minimum inhibitory concentration, or MIC was measured. A silver nanoparticle was used against the bacterial cultures of Escherichia coli, Proteus mirabilis, Salmonella typhimurium, and Pseudomonas aeruginosa in an ELISA plate and incubation was processed for 24 hours at 37 °C after adding a serial dilution by 2-fold ranging from 1 mg/ml to 0.0019 mg/ml to each well. To quantify the amount of inhibition, 2,3,5-Triphenyl tetrazolium chloride (TTC) was added after incubation. Both negative control and positive control with bacteria but no AgNPs were examined.
The least concentration at which bacteria are eliminated is the minimum bactericidal concentration (MBC) of AgNPs. The procedure involved inoculating a sample from every well in the previously conducted experiment onto a Nutrient Agar plate, followed by a 24-hour incubation period at 37 °C. The substance’s bacteriostatic and bactericidal qualities are assessed in relation to the tolerance level. The material is bacteriostatic if the tolerance level is greater than 16, and bactericidal if it is ≤4. The tolerance threshold is calculated using the MBC/MIC method.
RESULTS AND DISCUSSION
In this work, the culture supernatant of fresh Streptomyces rochei was successfully used to manufacture AgNPs extracellularly, within 6 hours of incubation, the biosynthesis of AgNPs was mediated by the culture supernatant treated with 100 mM AgNO3. The reaction mixture’s colour changed from yellow to a dark reddish-brown colour, signifying the creation of AgNPs. In contrast, neither the uninoculated media with AgNO3 as a control nor the culture supernatant without AgNO3 showed any colour change. After ten days, the colour remained constant, signifying that AgNO3 had entirely decreased and the reaction had come to a stop which is depicted in Figure 1.
Figure 1: Biosynthesized of nanoparticles: (a) control; (b) and (c) colour change observed after 10 days of incubation.

Because AgNPs are environmentally friendly, there has been a great deal of interest in their cost-effective biological production. Ag-NPs are surface-coated with various biomolecules from the Streptomyces supernatant, which increases their biocompatibility and safety. Consequently, intriguing uses of biological synthesis can be advantageous for the fields of biology and other related sciences.
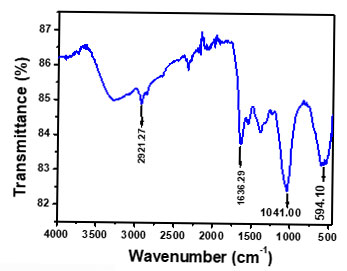
At a magnification of 60.00 KX the FESEM images showed the nanoparticles formed were nanosized (1-100 nm) and the shape observed was spherical as depicted in Figure 2a. The EDS showed the elemental composition which confirms the existence of Silver shown in Figure 2b. At 400 nm, a sharp and wide UV-Vis band maxima peak was seen shown in Figure 3a, which is typical for AgNPs. The DLS represents the value of how the particle diffuses within water. Generally, the hydrodynamic size of a particle is larger than its original diameter. The DLS pattern showed the Z-average diameter of the Ag-NPs biosynthesized by S. rochei was 146.17 nm, and its Zeta potential was 0.4 displayed in Figure 3b&3c respectively. Multiple functional groups were seen on the surface of biosynthesized Ag-NPs was verified by the FT-IR analysis displayed in Figure 4. Results revealed distinct, strong absorption bands at 2921.27 cm-1 (O-H stretch), 1636 cm-1 (C=C alkenyl stretch), 1041 cm-1 (C-N stretch), and 594.10 cm-1 (wagging region), which indicated that phenols and alcohols were the capping agents. The increased ability of proteins to bond with metals and to create a surface layer on metallic AgNPs via their amide and aromatic group residues proves that they serve as a binder for metals.
Figure 2: (a) SEM Images of biosynthesized AgNPs; (b) Energy Dispersive X-Ray spectroscopy of biosynthesized AgNPs.
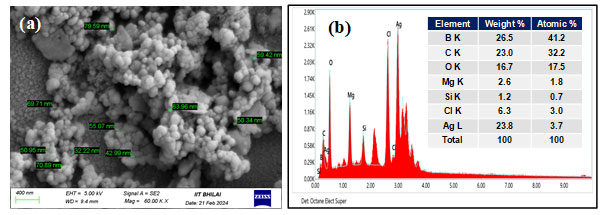
Figure 3: Characterization of biosynthesized silver nanoparticles: (a) UV-Vis spectra; (b) zeta size; (c) zeta potential.
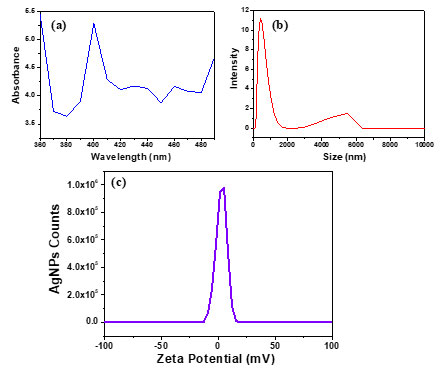
Figure 4: FT-IR Spectra of biosynthesized silver nanoparticles
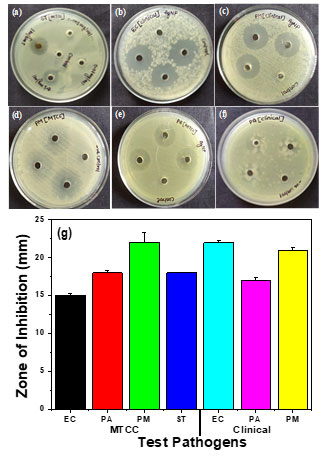
Resistance mechanisms have been observed in bacteria for many generations; chemical antimicrobial treatments are limited in the current situation. When it comes to the problem of multidrug resistance, AgNPs are a great improvement over conventional chemical antimicrobial agents since metallic NPs are less likely than traditional antibiotics for bacterial resistance. The biosynthesized AgNPs showed exceptional dose-dependent antibacterial effectiveness in MTCC and clinical cultures of numerous different Gram-negative bacteria, Escherichia coli, Salmonella typhimurium, Proteus mirabilis, and Pseudomonas aeruginosa shown in Figure 5(a-f). With an inhibitory zone diameter of 24 mm for Escherichia coli and 22 mm for Proteus mirabilis in the MTCC culture, antimicrobial activity was demonstrated by biosynthesized Ag-NPs against all pathogens shown in Figure 5 (g). MTCC cultures of Escherichia coli and Salmonella typhimurium exhibited the least activity, with inhibitory zone diameters of 14 and 18 mm, respectively. The biosynthesized Ag-NPs partially inhibited the advancement of the clinical strain of Pseudomonas aeruginosa.
Figure 5: Antibacterial activity of biosynthesized silver nanoparticles against (a) Salmonella typhimurium (MTCC-98), (b) Escherichia coli (clinical), (c) Proteus mirabilis (clinical), (d) Proteus mirabilis (MTCC- 425), (e) Pseudomonas aeruginosa (MTCC-424), (f) Pseudomonas aeruginosa (clinical) and (g) Graphical representation of antibacterial activity silver nanoparticles against Gram negative pathogenic bacteria.
To find out if the biosynthesized AgNPs have a bacteriostatic or bactericidal impact against tested pathogenic strains, the intensity of tolerance should be assessed. According to (Das et al., 2014), an antibacterial agent is classified as bacteriostatic when the ratio of MBC/MIC is ≥16 and bactericidal when the ratio is ≤ 4. According to National Clinical Committee for Laboratory Standards, if an agent reduces CFU/mL by 99.9% after a day of incubation in broth media it is deemed bactericidal. Also, if the microbe’s MBC is 32 times or more greater than its MIC, it is deemed tolerant (Woods and Washington 1995).
Figure 6: Minimum inhibitory concentration of biosynthesized silver nanoparticles against (a) Escherichia coli (MTCC- 40), (b) Escherichia coli (clinical), (c) Proteus mirabilis (MTCC- 425), (d) Proteus mirabilis (clinical), (e) Pseudomonas aeruginosa (MTCC-424), (f) Pseudomonas aeruginosa (clinical) and (g) Salmonella typhimurium (MTCC-98).
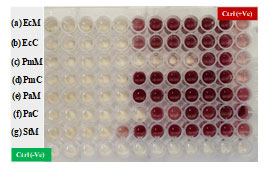
Figure 6 (a-g) and Table 1 displays the Minimum Inhibitory Concentration (MIC) of silver nanoparticles against the MTCC and clinical cultures of Salmonella typhimurium, Proteus mirabilis, Escherichia coli, and Pseudomonas aeruginosa. 2,3,5-Triphenyl tetrazolium chloride (TTC) for MIC measures the metabolic activity that bacteria are undergoing and reacts by turning red if metabolites are present. AgNPs’ bactericidal action is indicated by absence of red colouring. The Minimum Bactericidal Concentration (MBC) of AgNPs against MTCC and clinical cultures of Escherichia coli, Salmonella typhimurium, Proteus mirabilis, Pseudomonas aeruginosa, and both positive and negative controls is displayed in Figure 7 (a-g) and Table 2. While the negative control shows no bacterial growth, the positive control shows bacterial growth in the absence of AgNPs. Salmonella typhimurium, Escherichia coli, and Proteus mirabilis have tolerance levels between 1-4, indicating bactericidal activity, while Pseudomonas aeruginosa has a tolerance level of >16, indicating bacteriostatic activity.
Figure 7: Minimum bactericidal concentration of biosynthesized silver nanoparticles against (a) Escherichia coli (Clinical), (b) Escherichia coli (MTCC-40), (c) Proteus mirabilis (MTCC- 425), (d) Proteus mirabilis (clinical), (e) Pseudomonas aeruginosa (MTCC-424) (f) seudomonas aeruginosa (clinical) and (g) Salmonella typhimurium (MTCC-98).
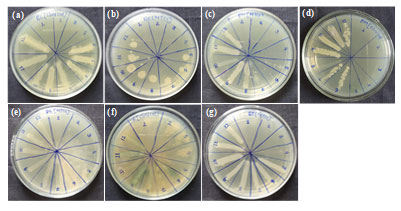
Table 1. Minimum inhibitory concentration (MIC) of biosynthesized silver nanoparticles.
| Name of micro-organisms | MIC | |
| a. | Escherichia coli (MTCC- 40) | 0.0625 mg/ml |
| b. | Escherichia coli (clinical) | 0.0625 mg/ml |
| c. | Proteus mirabilis (MTCC- 425) | 0.0312 mg/ml |
| d. | Proteus mirabilis (Clinical) | 0.0625 mg/ml |
| e. | Pseudomonas aeruginosa (MTCC-424) | 0.0625 mg/ml |
| f. | Pseudomonas aeruginosa (clinical) | 0.0312 mg/ml |
| g. | Salmonella typhimurium (MTCC-98) | 0.1250 mg/ml |
Table 2. Minimum bactericidal concentration (MBC) of biosynthesized silver nanoparticles.
| Name of micro-organisms | MBC | |
| a. | Escherichia coli (MTCC- 40) | 0.1250 mg/ml |
| b. | Escherichia coli (clinical) | 0.1250 mg/ml |
| c. | Proteus mirabilis (MTCC- 425) | 0.1250 mg/ml |
| d. | Proteus mirabilis (Clinical) | 0.0625 mg/ml |
| e. | Pseudomonas aeruginosa (MTCC-424) | 1.000 mg/ml |
| f. | Pseudomonas aeruginosa (clinical) | >1.000 mg/ml |
| g. | Salmonella typhimurium (MTCC-98) | 0.125 mg/ml |
CONCLUSION
In the studies mentioned above, the biosynthesized silver nanoparticles (AgNPs) were spherical in shape, nanosized, and demonstrated elevated antimicrobial activity against Gram-negative bacteria. The tolerance range of 2-4 for Escherichia coli, Salmonella typhimurium, and Proteus mirabilis indicates their bactericidal properties, while a tolerance level of 16 indicates their bacteriostatic effect against Pseudomonas aeruginosa.
Author’s Contributions: RRS and SY planned, designed, and performed the experiments. RRS and AKS prepared the draft and participated in manuscript writing. MKP corrected the draft and finalized the manuscript with RRS. MKP and AKG provided the experimental facilities, analysed, and supervised the work. All authors read and approved the final manuscript before submission.
Declaration of competing interest: There is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
RRS is grateful to the Department of Science and Technology (DST), New Delhi, India for the award of INSPIRE fellowship (No. DST/INSPIRE Fellowship/2020/IF200007) and SY is thankful to CSIR, New Delhi for the award of Junior Research Fellowship. MKP acknowledge the Science and Engineering research Board (SERB), New Delhi, India for the financial support from major research project (File No. SRG/2019/000754). Authors are also thankful to Dr. Manmohan Lal Satnami, SoS in Chemistry and Dr. Amber Vyas, University Institute of Pharmacy, PRSU, Raipur (C.G.) for providing the instrumentation facility.
Declaration of generative AI in scientific writing: Generative AI tools such as QuillBot were used solely for language enhancement and paraphrasing; all scientific content, analysis, and conclusions were developed independently by the authors.
Data availability: Data will be made available on request.
REFERENCES
Alani, F., Moo-Young, M., & Anderson, W. (2012). Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World Journal of Microbiology and Biotechnology, 28(3), 1081–1086. https://doi.org/10.1007/s11274-011-0906-0
Chauhan, R. (2013). A Biological approach to synthesis of silver nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity. Scientia Pharmaceutica, 81(2), 607–621. https://doi.org/10.3797/scipharm.1302-02
Crisan, C. M., Mocan, T., Manolea, et al. (2021). Review on silver nanoparticles as a novel class of antibacterial solutions. Applied Sciences, 11(3), 1120. https://doi.org/10.3390/app11031120
Das, V. L., Thomas, R., Varghese, et al. (2014). Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech, 4(2), 121–126. https://doi.org/10.1007/s13205-013-0130-8
El‐Baz, A. F., Sorour, N. M., & Shetaia, Y. M. (2016). Trichosporon jirovecii–mediated synthesis of cadmium sulfide nanoparticles. Journal of Basic Microbiology, 56(5), 520–530. https://doi.org/10.1002/jobm.201500275
Faghri Zonooz, N., & Salouti, M. (2011). Extracellular biosynthesis of silver nanoparticles using cell filtrate of Streptomyces sp. ERI-3. Scientia Iranica, 18(6), 1631–1635. https://doi.org/10.1016/j.scient.2011.11.029
Fakhr, A. E. & Fathy, F. M. (2019). Bacterial pattern and risk factors of hospital-acquired infections in a tertiary care hospital, Egypt. The Egyptian Journal of Medical Microbiology, 27(1), 9–16. https://doi.org/10.12816/0054166
Iravani, S., & Zolfaghari, B. (2013). Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed Research International, 2013, 1–5. https://doi.org/10.1155/2013/639725
Karthik, L., Kumar, G., Kirthi, A. V., Rahuman, A. A., et al. (2014). Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess and Biosystems Engineering, 37(2), 261–267. https://doi.org/10.1007/s00449-013-0994-3
Kim, N. H., Chung, H. M., Cha, K. Y. et al. (1998). Microtubule and microfilament organization in maturing human oocytes. Human Reproduction (Oxford, England), 13(8), 2217–2222. https://doi.org/10.1093/humrep/13.8.2217
Liossis, S. N., Hoffman, R. W., & Tsokos, G. C. (1998). Abnormal early TCR/CD3-mediated signaling events of a snRNP-autoreactive lupus T cell clone. Clinical Immunology and Immunopathology, 88(3), 305–310. https://doi.org/10.1006/clin.1998.4569
Manivasagan, P., Venkatesan, J., Senthilkumar, K., et al. (2013). Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Research International, 2013, 1–9. https://doi.org/10.1155/2013/287638
Martínez, G., Merinero, M., Pérez-Aranda, M. et al. (2020). Environmental impact of nanoparticles’ application as an emerging technology: A Review. Materials, 14(1), 166. https://doi.org/10.3390/ma14010166
Mourdikoudis, S., Pallares, R. M., & Thanh, N. T. K. (2018). Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale, 10(27), 12871–12934. https://doi.org/10.1039/c8nr02278j
Otari, S. V., Patil, R. M., Nadaf, N. H., et al. (2012). Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Materials Letters, 72, 92–94. https://doi.org/10.1016/j.matlet.2011.12.109
Prabhu, S., & Poulose, E. K. (2012). Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International Nano Letters, 2(1). https://doi.org/10.1186/2228-5326-2-32
Prakasham, R. S. (2012). Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. Journal of Microbiology and Biotechnology, 22(5), 614–621. https://doi.org/10.4014/jmb.1107.07013
Rania, H., Abdel-Hady, E.-G., Amina M Abd, E., Noha, et al. (2021). Antibiotic resistance pattern of bacteria causing hospital acquired infections in the New Mansoura General Hospital, Egypt. Archives of Community Medicine, 3(1). https://doi.org/10.36959/547/645
R Yadav, S., Wag, G., & Gupta, A. K. (2023). Endophytic Streptomyces rochei BpR-2 GER (ON142045) from Bryophyllum pinnatum (Lam.) Oken with antimicrobial and antibiofilm potential. Indian Journal of Science And Technology, 16(11), 775–784. https://doi.org/10.17485/ijst/v16i11.1458
Sadhasivam, S., Shanmugam, P., & Yun, K. (2010). Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids and Surfaces B: Biointerfaces, 81(1), 358–362. https://doi.org/10.1016/j.colsurfb.2010.07.036
Saied, T., Elkholy, A., Hafez, S. F., et al. (2011). Antimicrobial resistance in pathogens causing nosocomial bloodstream infections in university hospitals in Egypt. American Journal of Infection Control, 39(9), e61–e65. https://doi.org/10.1016/j.ajic.2011.04.009
Sanjenbam, P., Gopal, J. V., & Kannabiran, K. (2014). Anticandidal activity of silver nanoparticles synthesized using Streptomyces sp.VITPK1. Journal de Mycologie Médicale, 24(3), 211–219. https://doi.org/10.1016/j.mycmed.2014.03.004
Sivalingam, P., Antony, J. J., Siva, D., et al. (2012). Mangrove Streptomyces sp. BDUKAS10 as nanofactory for fabrication of bactericidal silver nanoparticles. Colloids and Surfaces B: Biointerfaces, 98, 12–17. https://doi.org/10.1016/j.colsurfb.2012.03.032
Srikar, S. K., Giri, D. D., Pal, D. B., et al. (2016). Green synthesis of silver nanoparticles: A Review. Green and Sustainable Chemistry, 06(01), 34–56. https://doi.org/10.4236/gsc.2016.61004
Sukanya, M. K., Saju, K. A., Praseetha, P. K., et al. (2013). Therapeutic potential of biologically reduced silver nanoparticles from Actinomycete cultures. Journal of Nanoscience, 2013, 1–8. https://doi.org/10.1155/2013/940719
The clinician and the microbiology laboratory. (2015). In P. R. Murray, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (pp. 191–223). Elsevier. https://doi.org/10.1016/b978-1-4557-4801-3.00016-3
Tsibakhashvili, N. Y., Kirkesali, E. I., Pataraya, D. T., et al. (2011). Microbial synthesis of silver nanoparticles by Streptomyces glaucus and Spirulina platensis. Advanced Science Letters, 4(11), 3408–3417. https://doi.org/10.1166/asl.2011.1915
Wypij, M., Czarnecka, J., Świecimska, M., et al. (2018). Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World Journal of Microbiology and Biotechnology, 34(2). https://doi.org/10.1007/s11274-017-2406-3
Yingst, D. R., Yang, S. Y., & Schiebinger, R. (1998). Purification of active Na+-K+-ATPase using a new ouabain-affinity column. The American Journal of Physiology, 275(4), C1167-1177. https://doi.org/10.1152/ajpcell.1998.275.4.C1167.


