1*Shri R.R. Lahoti Science College, Morshi, Amravati, Maharashtra, India
2,4Shri Shivaji Science College, Amravati, Maharashtra, India
3Jagdamba Mahavidyalaya, Achalpur, Amravati, Maharashtra, India
Corresponding author email: agl20class@gmail.com
Article Publishing History
Received: 25/07/2023
Accepted After Revision: 15/09/2023
Birds have ecological value and constitute a vital element of the natural system, being important bio-indicators of the ecosystem. They also play an important role in seed dispersion, pest control and food chain. As there is a lack of data regarding avian diversity and its abundance in large reservoirs, the present study was carried out to determine the diversity and abundance of avian fauna at the Upper Wardha Reservoir of Maharashtra State, India. Five stations were selected and data were recorded from each station. Each site was visited four times in a month and point count method was used to collect the data. Total 151 bird species were recorded. Out of which, 84 species (belonging to 20 families), were of wetland birds and 67 species (belonging to 22 families) were wetland associated. The diversity indices, species evenness, relative density and abundance were studied. In this study, probably for the first time, we have recorded the significant sighting of Greater white-fronted goose. We also recorded occasional sighting of Grey lag goose, Barr headed goose, Oriental pratincole and Glossy ibis. It was also found that freshwater reservoir acts as a suitable habitat for significant avian diversity and species richness.
Abundance, Avian, Amravati, Diversity, Wetland, Upper Wardha Reservoir.
Ashwin L, Gajanan W, Amol R, Pratik C. Diversity of Avian species in Upper Wardha Reservoir Amravati, Maharashtra. Biosc.Biotech.Res.Comm. 2023;16(3).
Ashwin L, Gajanan W, Amol R, Pratik C. Diversity of Avian species in Upper Wardha Reservoir Amravati, Maharashtra. Biosc.Biotech.Res.Comm. 2023;16(3). Available from: <a href=”http://surl.li/lskqm“>http://surl.li/lskqm</a>
INTRODUCTION
The Indian subcontinent is very rich in biodiversity. India hosts 1340 species (13 %), of birds out of the total 9000 birds species that are found in the world. Ali and Ripley (1987) considered 176 species endemic to the Indian subcontinent. Grasslands, Wetlands and wetland associated habitat provide appropriate dwelling places for these organisms. Out of 1340 species of the Indian subcontinent more than 577 species have been reported from Maharashtra State. In Vidarbha, a total of 417 species have been reported and overall Amravati district has 392 birds, species (Anon 2009, Kasambe 2016, Wadatkar et al., 2016, Praveen et al 2022).
Wagh and Tiwari, (2019) Wagh et al (2020) have studied the diversity and abundance of avian fauna in MIDC area of Amravati, similarly (Puri et al., 2020) surveyed water birds from Bodalkasa, Chorkhamara and Khairbandha Lakes of Gondia district, Maharashtra state (India). The diversity of birds in Simbhora Reservoir, Morshi, Amravati has been studied by Fule (2017). Simbhora reservoir inhabits several local and migratory bird. The biodiversity in the wetland is not studied in depth. Comparative studies of avian community composition in different habitats including wetlands, wetland associated habitat, forests, grasslands and even in urban and sub-urban areas can improve our knowledge of general patterns and processes that characterize bird species and communities.
Birds that depend on wetland and wetland associated vegetation have experienced a greater decline than any other habitats. Habitat loss and degradation of winter foraging and breeding ground were observed as leading causes of this decline. (Mankadan 2014, West, 2016, Johnson et al., 2019). As this Wardha reservoir is one of the largest one, supporting many bird species, the present study been made for the documentation of diversity, species richness, abundance and evenness wetland birds and wetland associated birds to know the present status of avian fauna.
MATERIAL AND METHODS
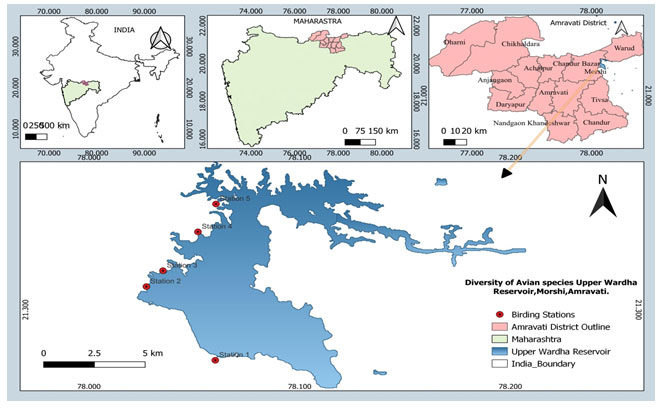
The present study was carried out from January 2022 to May 2022 in the Upper Wardha reservoir, Morshi. Five stations were selected in the study area and data were recorded from each station (Dam gate site, Fishing area near Nariman point, ITI Morshi backside, Durgwada village site and MIDC Morshi site). Monthly four visits were made at each station (Table 1).
Upper Wardha reservoir is an open water body that holds tremendous potential for optimizing fish production in the country. Upper Wardha reservoir, also known as ‘Nal Damayanti Sagar,’ is constructed on the River Wardha, a tributary of the Godavari River, near the Simbhora village in Morshi taluka of Amravati district, Maharashtra. Although being one of the large reservoirs of Maharashtra (Total area at FRL 9748 ha; latitude-longitude at 21.2764° N and 78.0572° E) supporting the livelihood of thousands of villagers residing around the reservoir (noted during primary field data collection). It can be classified as a lacustrine wetland. It has periodically open mudflats and serves as the best foraging and breeding ground for residents as well as migratory wetland birds, (Fig.1).
Table 1. The Co-ordinates of Study area.
| Station Numbers | Birding Sites | Coordinates | |
| Latitude (N) | Longitude (E) | ||
| Station 1 | Dam gate site | 21.272310o | 78.059963 o |
| Station 2 | Fishing area near Nariman point | 21.309220 o | 78.024225 o |
| Station 3 | ITI Morshi backside | 21.316585 o | 78.028042 o |
| Station 4 | Durgwada village site | 21.342680 o | 78.051739 o |
| Station 5 | MIDC site | 21.357937 o | 78.060284 o |
Figure 1: Map showing the study area (Upper Wardha Reservoir) Amravati M.S.
Birds Survey and Identification: Point Count and Line Transects methods were used to study avian diversity in wetland and wetland associated habitat (periphery of the reservoir). This methodology also helps to determine their abundance in a given research area. Potential sites were selected. Observations were made during 6:30 am to 10:00 am and 5:00 pm to 6:30 pm. At each site birds were counted using a binocular. Birds were identified and listed with the help of available resources, books and checklist Ali and Ripley (1987), Grimmet et al., (2000). Rasmussen, and Anderton, (2012), (Sangha, 2021) and book of Wetland and water birds of Amravati district (Wagh 2019). Observation were done using Nikon 8-16×40 mm Binocular. Photographic observations were taken by using Nikon D90, D5600 DSLR Camera with 70-300mm and 80-400mm Zoom lenses and 18-105mm normal lens. A Garmin TM 60 geographic positioning system (GSP) was used to mark each coordinates point of the study area.
Statistical Analysis: Biological diversity indices were calculated to compare studied sites. Various species diversity indices including Shannon–Wiener species diversity index (H), and Simpson index (D) were calculated (considering January to May for monitoring). The diversity indices were calculated and compared for both the habitats. Equally, the number of species, families, and abundance were calculated and compared through one-way ANOVA (Kruskal-Wallis Test) all analysis were performed using Software PAST version 4.03.
RESULT AND DISCUSSION
In the present study total 151 bird species were recorded from the study area. Out of which, 84 species (n = 795; belonging to 20 families) of wetland birds and 67 species (n = 915; belonging to 22 families) of wetland associated birds were recorded. The study area is richly diversified with patches of wetland associated habitat and wetland all over the Upper Wardha reservoir (Table 2&3). In the wetland habitat, maximum 16 Species from Anatidae family and in the wetland associated habitat, maximum 08 Species from Accipitridae family were recorded ( Fig 2 and Fig. 3). Recorded birds species were also categorized as per the recent IUCN’s list of threatened species (Bird life 2020) Table:5 and Fig.5.
Data Analysis: The diversity indices, species evenness, relative density, species abundance were studied. For the wetland birds diversity, Simpson Diversity Index was 0.9539, Shannon Diversity Index was 3.775, relative density was 10.56 and evenness was 0.519. Similarly in wetland associated area Simpson Diversity Index was 0.9654, Shannon Diversity Index was 3.761, relative density was 07.322 and evenness was 0.6416. Whereas abundance on the Upper Wardha reservoir was 1710 (Table 4). Kruskal-Walli’s test for equal medians H (chi2): 11.16 Hc (tie corrected): 11.24 p (same):0.0008014. There was a significant difference in the avian diversity between wetland habitat and wetland associated habitat in the Upper Wardha Reservoir. (Fig.4).
Table 2. Systematic checklist of Wetland avian fauna recorded from study area
| Sr. No. | Common Name | Scientific Name | Family | ST | IUCN Status |
| 1 | Lesser Whistling Duck | Dendrocygna javanica | DENDROCYGNDIAE (1) | R | LC |
| 2 | Bar-Headed Goose | Anser indicus | ANATIDAE (16) | W | LC |
| 3 | Northern Pintail | Anas acuta | W | LC | |
| 4 | Common Teal | Anas crecca | W | LC | |
| 5 | Indian Spot -Billed Duck | Anas pocilorhyncha | R | LC | |
| 6 | Gadwall | Anas strepera | W | LC | |
| 7 | Garganey | Anas querquedula | W | LC | |
| 8 | Northern Shoveller | Anas clypeata | W | LC | |
| 9 | Eurasian Wigeon | Anas penelope | W | LC | |
| 10 | Ruddy (Brahminy) duck | Tadorna ferruginea | W | LC | |
| 11 | Comb Duck | Sarkidiornis melanotos | W | LC | |
| 12 | Red-Crested Pochard | Rhedonessa rufina | W | LC | |
| 13 | Common Pochard | Aythya ferina | W | VU | |
| 14 | Ferruginous Pochard | Aythya nyroca | W | NT | |
| 15 | Tufted Duck | Athya fuligula | W | LC | |
| 16 | Cotton Pigmy goose | Nettapus coromandelianus | R | LC | |
| 17 | Greater White-Fronted Goose | Anser albifront | V | LC | |
| 18 | Common Kingfisher | Alcedo atthis | ALCEDINIDAE (1) | R | LC |
| 19 | White-Throated Kingfisher | Halcyon smyrnensis | HALCYONIDAE (1) | R | LC |
| 20 | Pied Kingfisher | Ceryle rudis | CERYLIDAE(1) | R | LC |
| 21 | Blue-Tailed Bee-eater | Merops philippinus | MEROPIDAE(1) | BM | LC |
| 22 | White- breasted Waterhen | Amanrornis phoenicurus | RALLIDAE (4) | R | LC |
| 23 | Purple Swamphen | Porphyrio porphyrio | R | LC | |
| 24 | Common Moorhen | Gallinula chloropus | R | LC | |
| 25 | Common Coot | Fulica atra | R | LC | |
| 26 | Black-tailed Godwit | Limosa limosa | SCOLOPACIDAE (13) | W | NT |
| 27 | Pintail Snipe | Gallinago stenura | W | LC | |
| 28 | Common Greenshank | Tringa nebularia | W | LC | |
| 29 | Spotted Redshank | Tringa Erythropus | W | LC | |
| 30 | Wood Sandpiper | Tringa glareola | W | LC | |
| 31 | Green Sandpiper | Tringa Ochropus | W | LC | |
| 32 | Common Sandpiper | Actitis hypoleucos | W | LC | |
| 33 | Eurasian Curlew | Numenius orauata | PV | LC | |
| 34 | Little Stint | Calidris minuta | W | LC | |
| 35 | Ruff | Calidris pugnax | PW | LC | |
| 36 | Curlew Sandpiper | Calidris ferruginea | PV | NT | |
| 37 | Marsh Sandpiper | Tringa stagnatilis | W | LC | |
| 38 | Dunlin | Calidris alpina | W | LC | |
| 39 | Pheasant-Tailed Jacana | Hydrophasianus chirurgus | JACANIDAE (2) | R | LC |
| 40 | Bronze-winged Jacana | Metopidius indicus | R | LC | |
| 41 | Indian Stone Curlew | Burhinus indicus | BURHINIDAE (2) | R | LC |
| 42 | Great Thick-knee | Esacus recurvirostris | R | LC | |
| 43 | Black-winged Stilt | Himantopus himantopus | CHARADRIDAE (5) | RM | LC |
| 44 | Little-Ringed Plover | Charadrius dubius | W | LC | |
| 45 | Kentish Plover | Charadrius aleandrinus | BM | LC | |
| 46 | Yellow-Watteled Lapwing | Vanellus malabaricus | R | LC | |
| 47 | Red wattled Lapwing | Vanellus indicus | R | LC | |
| 48 | Small Platincole | Glareola lactea | GLAREOLIDAE (2) | R | LC |
| 49 | Oriental Platincole | Glareola maldivarum | BM | LC | |
| 50 | Brown Headed Gull | Larus brunnicephalu | LARIDAE (7) | W | LC |
| 51 | Black Headed Gull | Larus ridibundus | W | LC | |
| 52 | Palas’s Gull | Larus ichthyaetus | W | LC | |
| 53 | Gull-billed Tern | Gelochelidon nilotica | PV | LC | |
| 54 | River Tern | Sterna aurantia | RM | NT | |
| 55 | Little Tern | Sterna albifront | BM | LC | |
| 56 | Whiskered Tern | Chlidonias hybridus | W | LC | |
| 57 | Little Grebe or (Dabchick) | Tachybaptus ruficollis | PODICIPEDIDAE (1) | R | LC |
| 58 | Darter | Anhinga melanogaster | ANHINGIDAE (1) | R | NT |
| 59 | Little Cormorant | Phalacrocorax niger | PHALACROCORACIDAE(3) | R | LC |
| 60 | Indian Cormorant | Phalacrocorax fuscicollis | R | LC | |
| 61 | Great Cormorant | Phalacrocorax carbo | R | LC | |
| 62 | Little Egret | Egretta garzetta | ARDEIDAE(11) | R | LC |
| 63 | Great Egret | Casmerodius albus | R | LC | |
| 64 | Intermediate Egret | Mesophoyx intermedia | R | LC | |
| 65 | Cattle Egret | Bubulcus ibis | R | LC | |
| 66 | Grey Heron | Ardea cinerea | R | LC | |
| 67 | Purple Heron | Ardea purpurea | R | LC | |
| 68 | Striated Heron | Butorides striatus | R | LC | |
| 69 | Indian Pond Heron | Ardeola grayii | R | LC | |
| 70 | Cinnamon Bittern | Ixobrychus cinnamomeus | R | LC | |
| 71 | Yellow Bittern | Ixobrychus sinensis | R | LC | |
| 72 | Black Bittern | Dupetor flavicollis | R | LC | |
| 73 | Glossy Ibis | Plegadis falcinellus | PHOENICOPTERIDAE(4) | W | LC |
| 74 | Black Headed Ibis | Threskiornis melanocephalus | R | NT | |
| 75 | Black Ibis | Pseudibis papillosa | R | LC | |
| 76 | Eurasian Spoonbill | Platalea leucorodia | RM | LC | |
| 77 | Painted Stork | Myeteria leucocephala | CICONIDAE (3) | RM | NT |
| 78 | Asian Openbill | Anastomus oscitans | W | LC | |
| 79 | Woolly-Necked Stork | Ciconia episcopus | R | VU | |
| 80 | White Wagtail | Motacilla alba | PASSERIDAE (5) | W | LC |
| 81 | White-browed Wagtail | Motacilla maderaspatensis | R | LC | |
| 82 | Citrine Wagtail | Motacilla citreola | W | LC | |
| 83 | Yellow Wagtail | Motacilla flava | W | LC | |
| 84 | Paddy-field pipit | Anthus rufulus | R | LC |
R– Widespread Resident, W– Widespread Winter Visitor, PV– Passage visitors, RM– Resident Migrant, BM- Breeding Migrant, V– Vagrant or irregular visitors. IUCN’s list of Threatened species (2020), categorized as Least Concerned (LC), Near Threatened (NT) and Vulnerable (VU).
Table 3. Systematic checklist of Wetland associated Avian Fauna recorded from study area.
| Sr. No. | Common Name | Scientific Name | Family | ST | IUCN Status |
| 1 | Grey Francolin | Francolinus pondicerianus | PHASIANIDAE (5) | R | LC |
| 2 | Common Quil | Coturnix coturnix | W | LC | |
| 3 | Rain Quail | Coturnix coromandelica | R | LC | |
| 4 | Painted Bush Quil | Turnix suscitator | R | LC | |
| 5 | Indian Peafowl | Pavo cristatus | R | LC | |
| 6 | Yellow-Crowned Woodpecker | Dendrocopos mahrattensis | PICIDAE(3) | R | LC |
| 7 | Golden-rumped Flameback | Dinopium benghalense | R | LC | |
| 8 | Common Flame Black | Dryocopus javensis | R | LC | |
| 9 | Coppersmith Barbet | Megalaima haemacephala | MEGALAIMIDAE (1) | R | LC |
| 10 | Indian Grey Hornbill | Ocyceros birostris | BUCEROTIDAE (1) | R | LC |
| 11 | Green Bee-Eater | Merops orientalis | MEROPIDAE(1) | R | LC |
| 12 | Common Hawk Cuckoo | Hierococcy varius | CUCULIDAE (2) | R | LC |
| 13 | Asian Koel | Eudynamys scolopacea | R | LC | |
| 14 | Greater Coucal | Centropus (senensis) parroti | CENTROPODIDAE (1) | R | LC |
| 15 | Barn Owl | Tyto alba | TYTONIDAE(1) | R | LC |
| 16 | Spotted Owlet | Athene brama | STRIGIDAE(2) | R | LC |
| 17 | Eurasian Collared Dove | Streptopelia decaocto | COLUMBIDAE (4) | R | LC |
| 18 | Red Collared –Dove | Streptopelia tranquebarica | R | LC | |
| 19 | Spotted Dove | Streptopelia chinensis | R | LC | |
| 20 | Laughing Dove | Streptopelia senegalensis | R | LC | |
| 21 | Black-shouldered Kite | Elanus caeruleus | ACCIPITRIDAE (8) | R | LC |
| 22 | Black Kite | Milivus migrans | R | LC | |
| 23 | Shikra | Accipiter badius | R | LC | |
| 24 | Osprey | Pandion haliaetus | W | LC | |
| 25 | Montagu’s Harrier | Circus pygarguss | W | LC | |
| 26 | Eurasian Marsh Harrier | Circus aeruginosus | W | LC | |
| 27 | Pied Harrier | Circus melanoleucos | W | LC | |
| 28 | Short toed Snake Eagle | Circaetus gallicus | R | LC | |
| 29 | Common Kestrel | Falco tinnunculus | FALCONIDAE (1) | R | LC |
| 30 | Bay-backed Shrike | Lanius vittatus | LANIIDAE (3) | R | LC |
| 31 | Long-tailed Shrike | Lanius schach | R | LC | |
| 32 | Brown Shrike | Lanius cristatus | W | LC | |
| 33 | House Crow | Corvus splendens | CORVIDAE (5) | R | LC |
| 34 | Large Billed (Jungle) Crow | Crow Corvus macrorhynchos | R | LC | |
| 35 | Common WoodShrike | Tephrodomis pondicerianus | R | LC | |
| 36 | Small Minivet | Pericrocotus cinnamomeus | R | LC | |
| 37 | Black Drongo | Dicrurus macrocercus | R | LC | |
| 38 | Bluethroat | Luscinia svecica | MUSCICAPIDAE (5) | W | LC |
| 39 | Orientle Magpie Robin | Copsychus saularis | R | LC | |
| 40 | Indian Robin | Saxicoloides fulicata | R | LC | |
| 41 | Common Stonechat | Saxicola torquata | W | LC | |
| 42 | Pied Bush Chat | Saxicola caprata | R | LC | |
| 43 | Brahminy Starling | Sturnus pagodarum | STURNIDAE (4) | R | LC |
| 44 | Rosy Starling | Sturnus roseus | W | LC | |
| 45 | Asian Pied Starling | Sturnus contra | R | LC | |
| 46 | Common Myna | Acridotheres tristis | R | LC | |
| 47 | Dusky Crag Martin | Hirundo concolor | HIRUNDINIDAE (4) | R | LC |
| 48 | Barn Swallow | Hirundo rustica | W | LC | |
| 49 | Wire tailed Swallow | Hirundo smithii | R | LC | |
| 50 | Red Rumped Swallow | Hirundo daurica | R | LC | |
| 51 | Red vented Bulbul | Pycnonotus cafer | PYCNONOTIDAE (1) | R | LC |
| 52 | Zitting Cisticola | Cisticola juncidis | CISTICOLIDAE (4) | R | LC |
| 53 | Jungle Prinia | Prinia sylvatica | R | LC | |
| 54 | Ashy Prinia | Prinia socialis | R | LC | |
| 55 | Plain Prinia | Prinia inornata | R | LC | |
| 56 | Indian Bush Lark | Mirafra erythroptera | ALAUDIDAE (5) | R | LC |
| 57 | Ashy- Crowned Sparrow Lark | Eremopeteri grisea | R | LC | |
| 58 | Rufous-tailed Lark | Ammomanes phoenicurus | R | LC | |
| 59 | Greater Short-toed Lark | Calandrella brachydactyla | W | LC | |
| 60 | Sykes’s Lark | Galerida deva | R | LC | |
| 61 | Purple Sunbird | Nectarinia asiatica | NECTARINIDAE (1) | R | LC |
| 62 | House Sparrow | Passer domesticus | PASSERIDAE (6) | R | LC |
| 63 | Baya Weaver | Ploceus philippinus | R | LC | |
| 64 | Red Avadavat | Amandava amandava | R | LC | |
| 65 | Sliver-bill Munia | Lonchura striata | R | LC | |
| 66 | Black headed Munia | Lonchura malacca | R | LC | |
| 67 | Scaly-breasted Munia | Lonchura punctulata | R | LC |
R– Widespread Resident, W– Widespread Winter Visitor, PV– Passage visitors, RM– Resident Migrant, BM- Breeding Migrant, V– Vagrant or irregular visitors. IUCN’s list of Threatened species (2020), categorized as Least Concerned (LC), Near Threatened (NT) and Vulnerable (VU).
In this study, probably for the first time, we have reported Greater white-fronted goose from the study area and it was a significant record for the Amravati district of Maharashtra state. The study site serves as suitable habitat for diversity and species richness of avian fauna (Plate1). As per the IUCN status, in the wetland habitat, out of the total 89 % birds species were least concerned, 8 % were near threatened and 03% were vulnerable species. While in wetland associated habitat, all the recorded avian species were least concerned. The recorded bird’s species were also classified according to their migration status. In the wetland habitat, 38 wide spread resident, 34 winter visitors, 4 breeding migrants, 3 passage visitors and 01 vagrant or irregular visitor’s species of birds were recorded. In wetland associated habitat, 56 resident species and 10 winter visitor species of birds were recorded (Table 6 & Fig.6). The Upper Wardha reservoir have extensive wetland associated region that supports the large number of birds species including wetland birds, which provides places for feeding and roosting and hence, it is a preferred habitat for various migratory and resident birds.
Birds like Green Bee-eater, Red-vented Bulbul, Indian Robin, Common Myna, House Sparrow, Black Drongo, House Crow, Grey Francolin, Bluethroat were amongst the most sighted species in the wide spread wetland associated area. This area also hosts a wide-ranging duck population. This wetland also hosts a variety of other migrating ducks which migrate from the colder regions like Siberia and Mongolia, Russia for various reasons. Migratory birds recite here for definite period and one of such very rare fascinating migratory bird is Greater-White Fronted Goose (Anser albifront) which visits the Upper Wardha reservoir. Barr headed geese is winter visitor for upper Wardha reservoir.
In the Wetland habitat; Storks, Sandpipers, Plovers, Ducks, Water Hens, Gulls, Terns and many other waders were recorded. The wetland patches also host the large number of migratory birds such as Bar-headed Geese which migrate in the study site in large numbers. Apart from these, little waders including Stints, Plovers, Pratincole and Sandpipers also sighted in large numbers (Fig.4).
Figure 2: Graph showing family-wise birds species number recorded from wetland habitat.
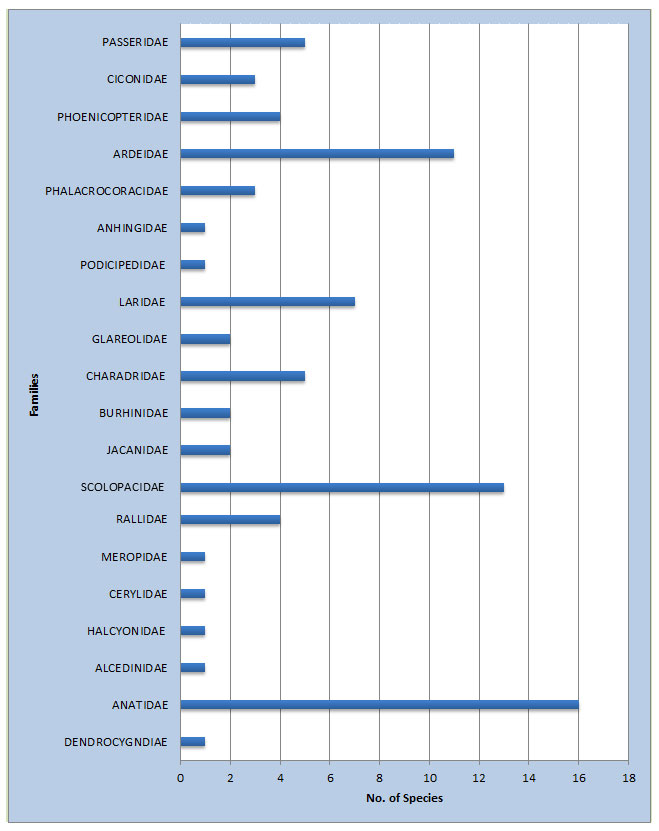
Figure 3: Graph showing family-wise birds species number recorded from wetland associated area.
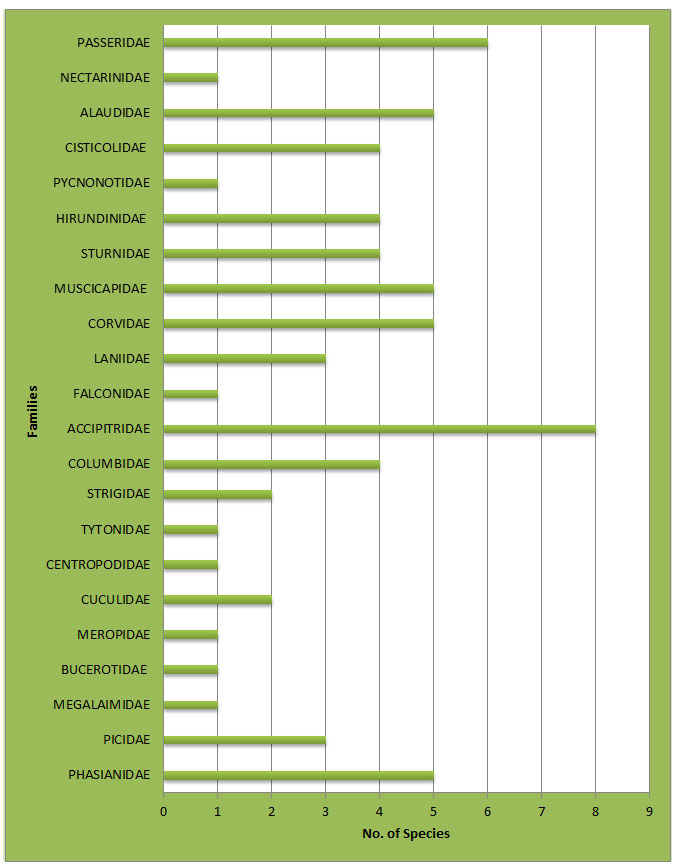
Table 4: Summary of Data Analysis.
| S.N. | Observations | Wetland
Bird Species |
Wetland Associated Bird Species |
| 1. | Species numbers | 84 | 67 |
| 2. | Total Individuals | 795 | 915 |
| 3 | Simpson Diversity Index [D] | 0.9539 | 0.9654 |
| 4 | Shannon Diversity Index [H] | 3.775 | 3.761 |
| 5 | Evenness | 0.519 | 0.6416 |
| 6 | Relative density | 10.56 | 07.322 |
| 7 | Fisher alpha | 23.72 | 16.65 |
Figure 4: Diversity of Avian species in Upper Wardha Reservoir, Morshi, Amravati, Maharashtra.
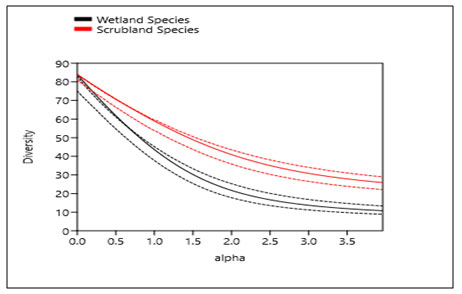
The shape of the curve indicates its diversity profile, with the shallow shape reflecting a high diversity and the steeper shape reflecting a low diversity. Note: The dash lines indicate the confidence intervals (95%).
Figure 5: Diagram showing IUCN status of birds in Upper Wardha Reservoir.
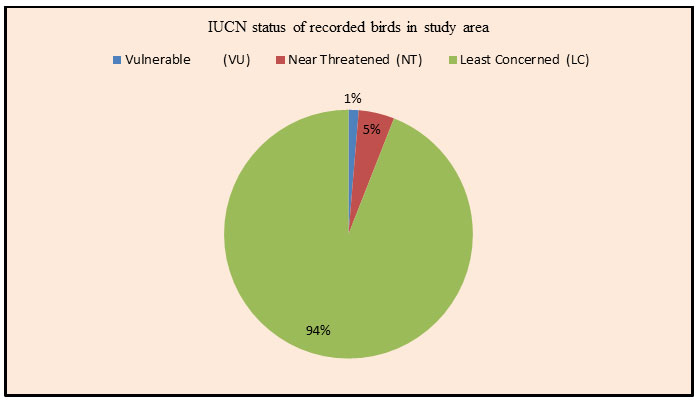
Figure 6: Diagram showing percentage of resident (R) and migratory (M) birds in Study Area.
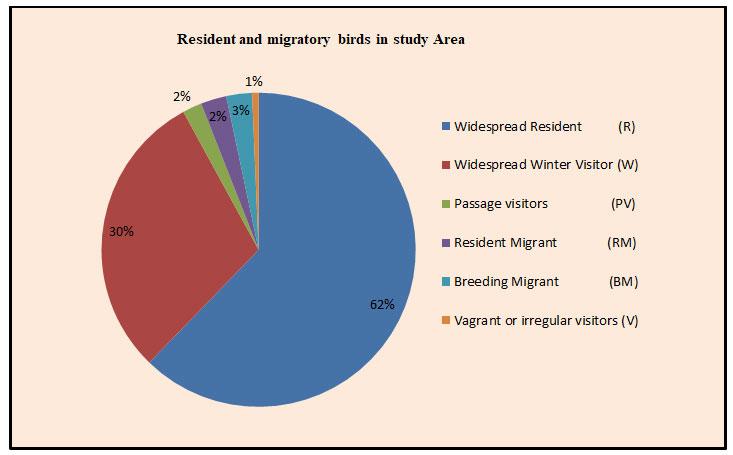
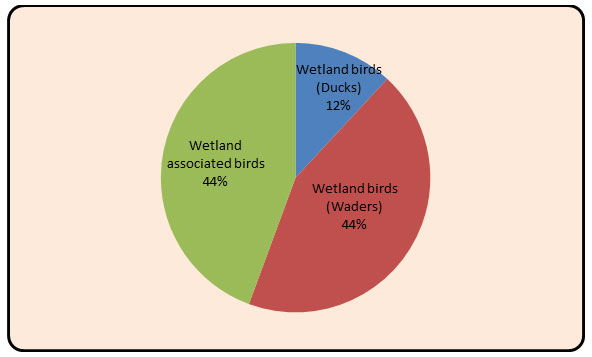
Figure 7: Diagram showing the percentage of in Wetland (Duck, Waders) and Wetland associated birds.
In the Wetlands habitat, maximum number of bird’s species was recorded from the Scolopacidae and Anatidae family which include Snipes, Sandpipers, Shanks, and Stints. Their abundant number was due to the Food and foraging preference they exhibit. Bar-headed Goose prefers small lush green grasses around the water bodies. Other regular visitors were Painted Stork, Asian Openbill stork and Woolly Necked Stork is a member of the Stork family, which was found in the wetlands, and prefers fishes as a major food item. Hence, their presence in the studied wetlands was justifiable. While wading across the banks of the water body, these birds constantly search for small fishes, snails, plants and invertebrates. Regular surveys for diversity study and awareness among the people should be conducted for a real assessment of environmental conditions prevailing in the area. The number of birds recorded during this study period was compared with earlier records (Wagh et.al .2015), and it was found in decreasing trend, particularly the abundance of waders.
Monthly variation was recorded in birds diversity and its was found that avifaunal diversity was more in January, February and March, as there was good water storage as well as open mudflat, availability of abundant food, increased vegetation and the arrival of migratory birds. During the present study, it was observed that this wetland was under serious threats due to widespread network of nylon fishing nets in the dam area, extensive fishing, cultivation and anthropogenic activities. These threats severely affecting the bird’s population and their existence. Various factors like grazing by domestic animals, disturbance by stray dogs, and excessive fishing activities through wetland patches forces the birds species to choose another area.
To mitigate these issues, there is an urgent need of patrolling in the more sensitive areas, particularly during the breeding season. For the long-term management of this water body, there is a urgent need to spread awareness among the local peoples about these issues. So that they can also contribute for the conservation and management activities in collaboration with Government authorities, local administration and various NGOs. The present study provides the baseline data of Avian diversity at Upper Wardha Reservoir Morshi and this data will help for various conservation and management activities in near future.
Plate 1: Significant Bird Sighting At the Upper Wardha Reservoir, Morshi, Amravati.

CONCLUSION
In the present study, 151 bird’s species (84 species from wetland and 67 species from wetland associated) were recorded. The observation shows that this water body supports a large number of birds species. This water body has been a main source of water for recharging the surrounding wells, bore wells and agriculture fields and also supply water to industries of MIDC, Amravati and also to the people of Amravati area. Large number of cattle graze and inhabit at the mudflats of the dam, due to this number of eggs and nests of the birds get destroyed. These threats along with some others, severely affected bird’s population. Mitigation of various threats is urgently needed and for this combined efforts from the concerned departments of the government, local people, volunteers and NGOs are needed. The present study has documented the avian fauna of Upper Wardha reservoir which will help in further bird diversity research, management and conservation.
ACKNOWLEDGEMENTS
The Authors sincerely acknowledge the Government authorities, for providing all the needed information regarding the study area. We are also grateful to the principal of Shri Shivaji Science College Dr. G.V. Korpe for their valuable support and encouragements.
REFERENCES
Ali, S. and Ripley, S. D. (1987). The compact handbook of the birds of India and Pakistan together with those of Bangladesh, Nepal, Bhutan and Sri Lanka. 2nd edition. Delhi: Oxford University Press
Anon, (2009). Checklist of Birds Vidarbha region of Maharashtra. VNHS Center, Nagpur. Bibby, C., B.N. Burges and D.A. Hill (1992): Birds census techniques. Academic press, London.
Birdlife, I. Streptopelia Turtur. The IUCN Red List of Threatened Species. (2019). Available online: https://www.iucnredlist.org. (accessed on 12 August 2020).
Fule, P.M. (2017). Bird Diversity In Simbhora Reservoir, Morshi Di – Amravati (M.S.) India. International Journal of Researches in Biosciences and Agriculture Technology. 10.29369/ijrbat.2017.05.II.0062.
Grimmett, R., Inskipp, C., Inskipp, T., (2000). “Pocket Guide of the Birds of the Indian subcontinent” Oxford University Press. Mumbai.
Johnson, A. E. M., Sillett, T. S. Luther, D., Herrmann, V., . Akre, T. A. and William, J. M. (2019). Effects of grassland management on overwintering bird communities. The Journal of Wildlife Management 83(7):1515–1526.
Kasambe. R. (2016). Standard Marathi names of Birds found in Maharashtra. Bombay Natural History Society and Maharashtra Pakshimitra Sanghatana PP. 24
Manakandan, R. (2014). The grassland birds of Rollapadu Wildlife Sanctuary, Andrapradesh, India with special reference to the impact of grazing-free enclosures. Journal of the Bombay Natural History society, 111(2): 81-89.
Praveen J, Rajah Jayapal & Aasheesh Pittie. (2022). Taxonomic updates to the checklists of birds of India, and the South Asian region—2022.Indian Bir ds Vol. 16 No. 1 (Publ. 2022).
Puri, S. D. (2020). “Updated status of waterbirds from Bodalkasa, Chorkhamara and Khairbandha lakes of Gondia district, Maharashtra State (India).” Vidyabharati International Interdisciplinary Research Journal 11, no. 1: 146-151.
Rasmussen, P.C. and Anderton, J.C. (2012). Birds of South Asia. The Ripley Guide. Vols. 1 and 2, 2nd edition. National Museum of Natural History – Smithsonian institution, Michigan State University and Lynx Edicions, Washington, D.C. Michigan and Barcelona.
Sangha H.S. (2021). Waders of Indian Subcontinent, Jaipur. PpXVI-520 India.
Wadatkar, J.S., Kasambe, R., Wagh G., Abhang, N. & Morey, K. (2016). Checklist of Birds of Amravati District, Maharashtra WECS Amravati. PP.1-22
Wagh, G. A. (2015). Waders diversity of Wetlands in Amravati region, Maharashtra. National Conference proceeding, M.D. College, Parel Mumbai.
Wagh G. A. (2019): Wetlands and Water birds of Amravati District., A field Guide; First edition, Wildlife and Environment Conservation Society.
Wagh, G.A. & Tiwari, P. (2020). On the Diversity and Abundance of Avian Species from Grassland and Wetland Areas of an Industrial Zone of Tropical Maharashtra. Bioscience Biotechnology Research Communications. 13. 770-780.
West, A. S., Keyser, Patrick P. D., Lituma, C. M., Beuchler, D. A. and Applegate R. D. (2016). Grassland Bird occupancy of native Warm-season grass, The Journal of Wildlife Management 80(6): 1081-1090.


