1School of Biotechnology, DAVV, Takshshila campus, Indore, Madhya Pradesh, India.
2Mata Gujri College of Professional Studies, A.B. Road, Indore, Madhya Pradesh, India
3Maharaja Ranjit Singh College of Professional Sciences, Hemkunt Campus, Madhya Pradesh, India.
Corresponding Author email: nanandphd@gmail.com
Article Publishing History
Received: 19/03/2022
Accepted After Revision: 25/05/2022
Xylanase is a significant enzyme which contributes to the breakdown of xylan and is utilized in an enormous range of biotechnological applications. In the present study, alkaline xylanase was produced using Elephant Foot Yam peels (EFY) in Solid State Fermentation (SSF) by Aspergillus terreus Thom isolated from elephant dung, which was procured from Indore Zoo. The optimization of xylanase production using One Factor at a Time (OFAT) approach exhibited 121 ± 2.5 U/ml of the highest xylanase activity and obtained at 60°C, pH 8.0 in 96 h culture with inoculum size of 1x 106 spores/ml, 90% moisture and 2 mm particle size in SSF. Further, the BBD (Box-Behnken design) based on Statistical software analysis i.e., Response Surface Methodology (RSM) was employed for optimizing xylanase production which predicted 4.2% increase in value which was in concurrence with the investigational design model.
Aspergillus terreus Thom, Elephant Foot Yam Peel, Solid State Fermentation, Statistical optimization, Xylanase.
Nenava R, Nighojkar S, Patidar M. K, Kumar A, Nighojkar A. Elephant Foot Yam Peels as Substrate for The Production of Alkaline Xylanase from Aspergillus terreus Using Solid State Fermentation. Biosc.Biotech.Res.Comm. 2022;15(2).
Nenava R, Nighojkar S, Patidar M. K, Kumar A, Nighojkar A. Elephant Foot Yam Peels as Substrate for The Production of Alkaline Xylanase from Aspergillus terreus Using Solid State Fermentation. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3lL7yHn“>https://bit.ly/3lL7yHn</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Xylan represents 30-35% of the total dehydrated mass of plants and is the next most abundant carbohydrate after cellulose in plant cell walls Izidoro and Knob 2014; Marimuthu et al. 2019; Champreda et al. 2019; Xue et al. 2022). The comprehensive disintegration of xylan is embraced by exploitation of several enzymes that comprises endo β -1,4 xylanase, β-xylosidase, α-arabinofuranosidase, and acetyl xylan esterase, all of which release xylo-oligosaccharides and D-xylose as the main products Chukwuma et al. 2020; Najjarzadeh et al.
2020; Wu et al. 2020). Xylanases are extracellular enzymes produced by various bacteria like Bacillus circulans, B. subtilis, fungi like Aspergillus, Trichoderma species, yeasts and actinomycetes like Thermomonospora fusca (Hrmova et al. 1984; Ball et al. 1989; Sunna and Antranikian 1997; Liu et al. 1998, 1999; Shah and Madamwar 2005; Thomas et al. 2016; Rodrigues et al. 2017; Alves et al. 2020; Romero Victorica et al. 2020; Joshi et al. 2022).
Fungi are more efficient for enzyme production in SSF as compared to bacteria owing to their mycelial nature and requirement of lesser amount of water (Walia et al. 2017; Bhardwaj et al. 2019). Xylanase has been largely employed in animal feed, biorefinery, food, pulp and paper, and textile industries and can be rational for manufacturing numerous useful economical products such as sugar syrups, single cell proteins (SCPs), liquid and gaseous fuels. (Techapun et al. 2003; Dhiman et al. 2008; Xiao et al. 2019; Yepes-Bentacur et al. 2021; Ayubi et al. 2021). Enzyme production using SSF provides many financial benefits over Submerged Fermentation, like lower capital and operational costs, higher product yield and an effective fermentation medium (Krishna 2005; Soccol et al. 2017; Aita et al. 2019).
Several researchers effectively reported on xylanase production using an experimental design model based on Response Surface Methodology for enhancing biotechnological processes (Ezeilo et al. 2019; Azzouz et al. 2020; Prabhu et al. 2022). Many different fruit and vegetable peels viz. lemon peel, orange peel, lemon pomace, apple pomace, cassava peel, mausambi peel, pomegranate peel, banana peel have been used as solid substrates for xylanase production (Seyis and Aksoz 2005; Olanbiwoninu and Odunfa 2016; Kaur et al. 2017; Atalla and El Gamal 2020; Zehra et al. 2020).
Elephant foot yam (EFY), Amorphophallus paeoniifolius (Araceae), commonly known as Suran, is widely grown in India as a profit crop due to its high-level manufacturing potential and lucrative return value (Jogi and Lahre 2020). It supplies a satisfactory amount of protein and simple carbohydrates, and is often used as a vegetable (Singh et al. 2007). EFY has also been used for medicinal purposes such as blood purifier, piles treatment, abdominal disorders, tumours, asthma and rheumatoid arthritis (Kirtikar and Basu 1989; Mishra et al. 2001a; 2001b). The peel of suran which is nearly 20 to 25% of the corm weight and 1 to 4 cm in thickness is used as a supplement in animal feed or a waste disposed into the environment causing biological litter (Ravi et al. 2011; Kumar 2020).
EFY peels were used in present study as solid substrate for production of xylanase from Aspergillus terreus Thom isolated from elephant dung. The production was optimized using the OFAT approach and further statistically optimized using BBD based on RSM. EFY peels are an underutilized but potential bio waste that can be used as substrate for production of useful enzymes and other valuable industrial products like Bioethanol and Xylitol. However it has few anti-nutritional factors such as – it may cause itchiness due to the presence of oxalates. Hence, more investigations are needed to utilize the agro-residue elephant foot yam peels for production of useful commercial compounds.
MATERIAL AND METHODS
Birchwood xylan, xylose and xylo-oligosaccharides were procured from Sigma-Aldrich, USA and other analytical grade chemicals used were of Agricultural residues purchased from local market were f Sun-dried and used.
Samples were collected from different natural habitats like agricultural soil, dumping sites, garden area and agro-waste material from nearby locations of Indore and Mhow regions. Excreta of different animals were also collected from Indore Zoo. Samples from soil were collected after removing the upper layer of the soil upto 5 cm depth, using a spatula. The collected samples were aseptically stored in Zip lock poly bags at 4°C till further use.
Xylanase producing fungi were screened on xylan potato dextrose agar (XPDA) medium consisting of (g/l) dextrose 20, potato 200, xylan 10, agar 25 and pH 5±0.5 (Sakthiselvan et al. 2014). The XPDA plates were streaked with a loop full of 103 diluted soil samples and incubated at 30 ± 2oC for 120 h. I clear xylanolytic zone was observed around the fungal growth on XPDA plates using Congo red staining dye as per the method of Teather and Wood (1982).The pure cultures were maintained at 40C on PDA slants. Primarily, the isolate was identified by determining the physical appearance on the PDA plate followed by microscopic observations. The structural morphology and molecular characterization were employed for detection of the maximum xylanase producing fungi Aspergillus terreus Thom (Bhardwaj et al. 2019).
ITS (Internal Transcribed Spacer) region – partial sequencing was performed at the National Fungal Culture Collection of India, NFCCI, Pune. Sequence kit ABI-Big-dye Terminator v3.1 Cycle was used for PCR sequencing. DNA sequencer entitled automated ABI 3100 was employed for the attainment of raw sequence which was edited for checking irregularity on the routine basis. Publicly accessible sequences were used for the alignment of the sequence data and analysis was done to check its identity (http://www.ncbi.nlm.nih.gov/). The construction of the Phylogenetic tree was performed by employing the Maximum Likelihood tree method (Tamura et al. 2011) using MEGHA X software. The isolated fungus from elephant dung was recognized as Aspergillus terreus Thom and was applied for production of xylanases.
A 5-day-old fungal culture was used for the inoculum development as described by Nenava et al. (2021). Neubauer’s counting chamber was used to count the fungal sporesA spore suspension of 1 × 106 spores/ml was used for inoculating the
Bio-wastes wheat bran, elephant foot yam peel, orange peel, papaya peel, garadu peel, sugarcane bagasse, pea pods and apple peels were selected as solid substrates for The Sun-dried peels were pulverised using a grinder and sieved through a 0.5-3 mm mesh.
Sun-dried substrate (2 mm particle size) 10 g was moistened with 70% (v/w) sterile distilled water and autoclaved at 121oC (15 lbs pressure) for 20 min. 1 ml of inoculum (1 × 106 spores/ml) was inoculated in the flasks and incubated for 96 h at 30°C.
Xylanase enzyme extraction was carried out as described by Nenava et al. (2021) in which using 50 mM citrate buffer (pH 5±0.5). The filtrate was centrifuged at 1500 x g for 20 min at 0 to – 4º C temperature and the supernatant was used for xylanase assay.
Birchwood xylan was used as a substrate for xylanase enzyme assay. The method employed for xylanase enzyme assay and protein estimation was previously described by Nenava et al. (2021). The reducing sugar released was determined by employing 3′ 5′- dinitrosalicylic acid (DNS) method (Miller 1956; Beiley et al. 1993). One unit of endo-1, 4- β-xylanase has been as the quantity of the enzyme required to release 1 µmole of wood sugar xylose equivalent in one min under the conditions of enzyme assay (Bhalla et al. 2015). Lowry et al. (1951) method using BSA (Bovine Serum Albumin) as a standard protein was used for the estimation of protein present in the reaction mixture.
10 gm each of different sun dried agricultural residues (apple peel, banana peel, orange peel, pea pod, mausambi peel, corn husk, EFY peel, garadu peel, wheat bran, sugar cane bagasse) were spulverised and used for solid state fermentation. EFY peel gave the maximum production of xylanase when fermented by Aspergillus terreus Thom.
To determine the effect of fermentation period,1 ml of Aspergillus terreus spores (1×106 spores/ml) were inoculated to 10 g of pulverized suran (EFY) peel, and kept at 28±2°C for 10 days. Fermented substrate (0.5 g) was withdrawn after every 24 h for enzyme extraction and assay.
In order to determine the effect of moisture content 10g of EFY peel was moistened with sterile distilled water. The moisture content ranges was varied from 40% to 110% (v/w) with an interval of 10%. The flasks were autoclaved and inoculated with spore suspension of 1×106 spores of Aspergillus rerreus Thom and incubated for 96h.
The were incubated at 10oC, 20oC, 30oC, 40oC and 50oC for optimization of fermentation temperature.
The washed and dried EFY peel was milled and sieved to get 0.5, 1.0, 1.5, 2.0, 2.5- and 3-mm particle sizes. To study the effect of particle size, 10 g of different particle sized EFY peels were transferred into different 250 ml capacity Erlenmeyer flasks, and each was incubated with 1ml of Aspergillus terreus having 1 × 106 spores/ml. The final moisture content of 90% was maintained by addition of distilled water as a moistening agent. The incubation was done at 28±2oC for 96 h.
Spore count of Aspergillus terreus was varied from 1 × 103 to 1 × 109 spores/ml for optimization of inoculum size. Spore suspension of 1ml was inoculated in autoclaved 10 g EFY peel moistened with 9 ml of distilled water and incubated for 4 days at 28±2oC.
The optimization of agitation frequency was exhibited by pounding the pre-inoculated flask onto the palm surface of the hand for a minute for about one to five times a day till the end of fermentation process. For the agitation study, 10g of EFY peel with optimized conditions were utilized.
SEM analysis was performed at SEM Facility Unit, IISER, Bhopal. SEM Instrumentation used was Zeiss, Ultra Plus model, Germany. Samples were dehydrated and then, un-inoculated EFY peel (without spores) and inoculated EFY peel (with spores) were discretely placed on sample stub. Thereafter, the sample was placed into a gold sputtering system. Using a mini-gold sputter, gold was sputtered for 30 s at ~ 70 m Torr pressure. Next, a sample stub containing a sample was inserted and the SEM chamber was allowed to reach the desired vacuum pressure by turning pumps on and operating under voltage range 1-30 kV for better image analysis.
A BBD experimental model based on RSM was executed for optimizing production of xylanase. For optimization studies, elephant foot yam peels were used as solid substrate. In all the experimental assays, the pH was maintained at 8.0. In the present statistical design model, four important process parameters such as fermentation time (A), moisture content (B), particle size (C) and inoculum size (D) were finalized as self-regulating (independent) variables and xylanase activity (Y) as dependent variable response. Above mentioned self-regulating variables were deliberated at different levels, -1(low), 0 (middle), +1 (high), separately with the help of the BBD model. The trial runs (N) mandatory for the advancement of statistical model BBD is represented by the subsequent calculation:
N = 2k (k-1) + C0…………………………………(1)
k denotes the Number of Variables and Co denotes Number of Central Points.
Using the above equation mathematical correlation was developed amongst the four important variables (A-D) in consideration for xylanase enzyme production. A set of 29 experimental run was performed using 5 replicates at a central point and executed in the polynomial model implementing the succeeding equation:
Y = β0 + ∑βiXi + ∑ βiiXi2 + ∑ βijXiXj………………………….(2)
The pH and temperature of the medium were kept persistent at 8.0±1 and 28±2oC, respectively, for all the experimental runs. Experiments designed in the BBD are subjected for analysis of xylanase activity at particular time intervals. One flask was prepared as control in order to examine the activity of the enzyme, if it exists before the initiation of the SSF process. Statistical analysis of obtained experimental values was accomplished using the software Design Expert® 12. The optimum interpretations of chosen parameters of fermentation process and its cooperative outcomes were analysed exploiting the same software.
RESULTS AND DISCUSSION
Screening and isolation of fungal species for xylanase production: During primary
screening, a total of 62 isolates, were evaluated for xylanase activity. Thirteen isolates screened from 28 samples, exhibited a clear zone on xylan agar plate at pH 5.5 on staining with Congo red dye (0.2%).isolate EF1 (A. terreus) isolated from Elephant dung, Indore Zoo. gave maximum endo-1,4-β xylanase production of 94.6 ± 2.6 units/ml (Intasit et al. 2022).
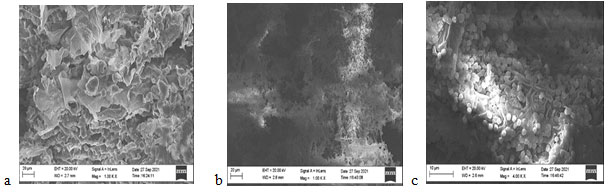
All the experimental studies were conducted using A. terreus. Its occurrence and morphological observations (Fig. 1) showed a brown colour (cinnamon); velvety appearance, and aerial hyphae with brown spots. Staining was performed using lactophenol cotton blue showed that, the conidial head was columnar while the conidial globose was slightly ellipsoid as observed through 100 X magnification (Fig. 2). Scanning electron microscopy (SEM) images of EFY peel without fungal spores and with fungal spores are represented in Fig. 3 (Intasit et al. 2022).
Figure 1: Morphological appearance of fungal isolate EF1
identified as Aspergillus terreus on PDA plate.
Figure.2 : Microscopic image of Aspergillus terreus NFCCI
4986 stained with lactophenol cotton blue (100X).
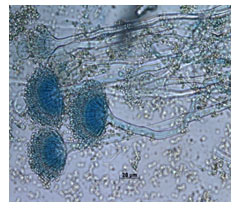
Figure 3: SEM images a. EFY Peel without fungal spores b. EFY Peel with fungal spores
c. Growth of fungal spores over substrate (EFY peel).
OFAT approach for optimization of xylanase production
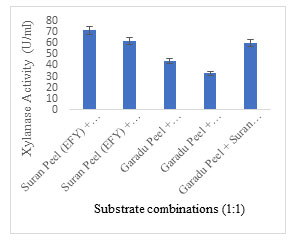
Solid substrate selection: EFY peel was projected to be an effective solid substrate for xylanase production displaying 83.10 U/ml activity followed by garadu peel 64.85 U/ml, and wheat bran 53.24 U/ml; while other solid wastes showed moderate to low xylanase activity (Fig. 4). In order to minimize the cost of xylanase production, use of low-cost substrate in the production media is recommended which also adds to the sustainability of environment (Sadaf and Khare, 2014; Gupta et al. 2015; Walia et al. 2015; Zhang and Sang, 2015; Behnam et al. 2016; Chilakamarry et al. 2022; Prabhu et al. 2022). The fermentation process is also governed by the configuration of substrate and different process parameters implemented. Different substrate combinations were also used to enhance the xylanase production potential of the medium (Fig. 5).
The combination SP +OP (Suran (EFY) peel + Orange peel) (1:1) and SP+SB (Suran (EFY) peel + Sugarcane bagasse) (1:1) showed maximum activity of 71.59 U/ml 61.91 U/ml, respectively. But the combinations used in this research investigation did not represent any prominent increase in xylanase activity, hence EFY peels were solely used as a substrate for xylanase production. Some researchers have effectively employed the combined strategy WB, WS and RB ratios in 1:1 for the production of xylanase (Rana et al. 2021; Intasit et al. 2021; Intasit et al. 2022).
In 2013, Singh et al. reported that elephant foot yam/suran is a fine source of starch, sugars, proteins and minerals. Normal nutritive profile comprises of starch (11-28%), sugar (0.7-1.7%), protein (0.8- 2.60%), fat (0.07-0.40%) and mean energy value of 236 to 566.70 KJ/100g. . According to the author’s information, this is the first report on EFY peel, a biowaste which is has been employed for the production of xylanase enzyme (Olveira et al. 2022).Fig. 4 Different agro-residues used as solid substrate showing xylanase enzyme activity
Figure 4: Different agro-residues used as solid substrate showing xylanase enzyme activity
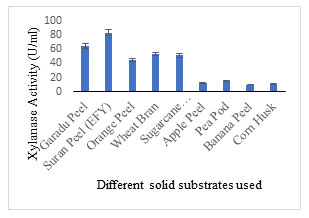
Figure 5: Different substrate combinations employed for production of xylanase showing xylanase activity.
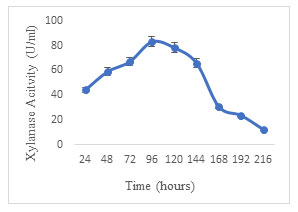
Effect of fermentation period: Xylanase production by A. terreus was studied by incubating the SSF medium for 9 days. A proportionate increase in enzyme units was observed for 96 h reaching a maximum of 83.04 U/ml. On further incubation, a regular reduction was observed in the activity of xylanase (Fig. 6). The decline in activity over the incubation period could be because of non-specific proteases in the medium. Decker (1983) emphasized the effect of nature of substrate, organism and other fermentation parameters on the optimum fermentation period. Optimum xylanase production was achieved in 4 days using Aspergillus foetidus MTCC and in 7 days using Aspergillus sp. S9 (Shah and Madamwar 2005; Sharma et al. 2015). The present results correspond with the previous studies. Similarly, the maximum xylanase activity was reported in 7 days of SSF conducted by Olveira et al. (2022).
Figure 6: Effect of fermentation period on the production of xylanase by Aspergillus terreus.
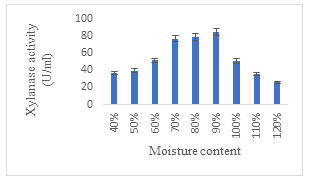
Effect of moisture content: The optimal moisture content was found to be 90% for the highest production of xylanase enzyme (Fig. 7). Amongst the different substrates, the moisture level at which free water is available varies considerably depending on their water bonding characteristics. However, Bakri, et al. (2008) reported that for solid-state fermentation process preliminary moisture content of 30–80% was critical factor because it has a substantial impact on growth, manufacture and release of different metabolites and products. Additional increase in moisture levels beyond 90%, did not elevate xylanase production. Razali et al. (2021) concluded that the moisture content along with the inoculum size triggers varied changes in the production of xylanase. On the contrary, maximum xylanase activity was reported with 60% moisture content under SSF using Lentinus strigosus from Amazon (de Oliveira et al. 2022).
Figure 7: Effect of Moisture Content on xylanase enzyme production.
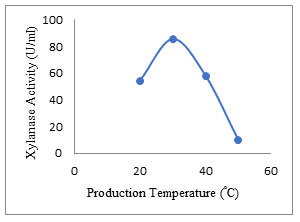
Effect of temperature: The enzyme production of 85.69 ± 2.5 U/ml was obtained at a temperature of 30°C with EFY peel. Kuhad et al. (1998) also reported optimum temperature 30°C for Fusarium sp. for xylanase production. Temperature has an important role in the advancement of biological processes as it stimulates the denaturation of protein, enzyme inhibition, and cell progress. In the present study, the enzyme activity decreased with increase of temperature up to 50°C (Fig. 8). Optimum production of endo-1,4-β xylanase was also reported to be 30 °C by Kanimozhi and Nagalakshmi (2014) and Subbulakshmi and Iyer (2014). Temperature specific xylanases (extremozymes) and their metagenomic studies were revealed by (Verma and Satyanarayana 2019; Verma and Satyanarayana 2020). Zarafeta (2020) reported thermostable xylanase belonging to GH-10 family.
Figure 8: Effect of temperature on the secretion of xylanase enzyme.
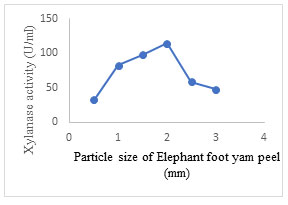
Effect of substrate particle size: The maximum xylanase production (114.6± 1.5 U/ml) was obtained at particle size of 2 mm which further decreased on changing the particle size either to higher or lower values (Fig. 9). In SSF, appropriate particle size of solid substrate plays a vital role in improving enzyme activity. Previous studies have reported that the smaller particles have a reduced amount of permeability which encourages lowering of gas diffusion, while the larger particles absorb lesser moisture and swell slightly smaller, thereby drying speedily resulting in a sub-optimal growth of the fungi (Zadrazil and Puniya, 1995). The different particle sizes of the same substrate significantly contribute to the specific degradation of substrate along with the fungal strain used for fermentation and composition of substrate Therefore, it is significant to detect the optimal particle size for xylanase production. (Lakshmi et al. 2011; Nawawi et al. 2022; Prabhu et al. 2022).
Figure 9: Effect of particle size of substrate (EFY peel) on xylanase production by A. terreus
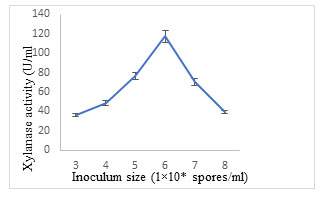
Effect of inoculum size: Maximum production of xylanase (117.23± 4.42 U/ml) was obtained using Aspergillus terreus Thom inoculum of 1×106 spores/ml (Fig. 10). An adequate concentration of inoculum must be employed to confirm good fungal growth and substrate colonization (Simotilde and Tauk-Tornisielo 2006). An inoculum size extending from 105 to 107 spores/g of substrate is mostly used for enzyme production (Silva et al. 2005, Sood et al. 2011).The higher inoculum size resulted in reduced enzyme titre because of the primary competition amongst the microbial cell population for nutrients. (Bayoumi et al. 2008; Razali et al. 2021; Prabhu et al. 2022).
Figure 10: Effect of inoculum size on xylanase secretion by Aspergillus terreus.
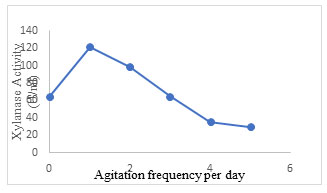
Effect of agitation frequency: In SSF, agitation study is important and plays a crucial role in the enhancement of production of target enzymes. Inoculated flasks when tapped gently over the palm of the hand one time a day, gave maximum xylanase production of 121.3±1.5 U/ml (Fig. 11). The enzyme activity declined on increasing agitation frequency. Ibrahim et al. (2012) presented that, dissimilarities in optimum level of agitation rate may be correlated to the category of microorganism used, the level at which evolution of heat occurs, the dissipation of the quantity of carbon dioxide and other volatile metabolites, the thickness of substrate bed height, and also the dimensions of pore space in the substrate. Syarifah et al.
(2014) noticed that mixing was an essential phenomenon for few substrates used in SSF where it can induce the fungal progression and yield of enzymes. Ooijkas et al. (2000) conveyed that the activity of microorganism is adversely affected by mixing on some solid supports whereas in others no adverse effect is observed. However, agitation is not recommended in static reactors in several aerobic SSF processes like tray fermenters (Lonsane et al.1992; Viesturs et al.1987; Wang et al. 2021; Prabhu et al. 2022).
Figure 11: Effect of agitation frequency on xylanase secretion by Aspergillus terreus
3.2.8 RSM for Statistical Optimization: BBD was employed for the evaluation of xylanase activity at optimum level of each parameter varied along with their interactions. In Table 2, several amalgamations of process parameters such as fermentation period (A), moisture content (B), particle size (C) and inoculum size (D) are represented. The tentative results of response generated were tailored using the second order polynomial equation (1) (Mitri et al. 2022). Thus, the second order polynomial equation can be represented as follows:
R2 (Xylanase units)
= 121.30 – 2.20 A + 1.53 B + 0.448 C + 1.88 D – 0.525AB + 5.66 AC + 4.91 AD + 6.71 BC –7.98 BD + 1.31CD – 26.58 A2 –26.78 B2 – 25.23 C2 – 26.32 D2…………………………….(Eq. 3)
Detailed range of all the chosen variables executed in the optimization process are given in Table 1 along with the design of coded and uncoded levels. The statistical implication of the second order polynomial equation (Eq. 3) was confirmed by Fisher distribution (F- test) and the outcomes are revealed in Table 2. The statistical model BBD represents the F-value of 94.06. The remarkable model terms are A, D, AC, AD, BC, BD, A², B², C², D² which show a P-value less than 0.05. Values greater than 0.1 indicate that model terms are not important. The lack of Fit F-value of 1.16 indicates that it is possibly due to error with 48.29% chance that could have occurred owing to noise. Significance of model is exhibited by the coefficient of determination (R2) of 0.98 value.
The Predicted R² of 0.950 is in rational arrangement with the Adjusted R² of 0.979; i.e., the difference is less than 0.2. The motion to noise ratio is measured by adequate precision and comparability of the range of the predicted values at the design points to the average prediction error. A ratio of more than 4 is desirable. In the existing study, the motion to noise ratio was 29.366 which specified that the system contained adequate signal. Navigation of the design space can be implemented by this statistical model. Additionally, the coefficient of variation (CV= 4.0 %) was low, indicative of an improved accuracy and evenness of the experimental runs (Mitri et al. 2022).
Table 1. Coded levels and Actual response generated by Aspergillus terreus in SSF
| Run | A | B | C | D | ||
| Fermentation time | Moisture Content | Particle size | Inoculum size | Response R1 | Predicted R2 | |
| 1 | 0 | 0 | 0 | 0 | 124.3 | 123.1 |
| 2 | 0 | -1 | 0 | -1 | 58.6 | 56.8 |
| 3 | -1 | 1 | 0 | 0 | 69.1 | 72.1 |
| 4 | 0 | 0 | 0 | 0 | 118.7 | 120.8 |
| 5 | 0 | 0 | -1 | 1 | 71.04 | 69.8 |
| 6 | 1 | 0 | 0 | 1 | 71.5 | 72.98 |
| 7 | 1 | 0 | 1 | 0 | 76.06 | 75.9 |
| 8 | 1 | 0 | -1 | 0 | 59.1 | 61.18 |
| 9 | 0 | 0 | 0 | 0 | 121.4 | 124.5 |
| 10 | -1 | 0 | 1 | 0 | 70.4 | 66.4 |
| 11 | 0 | 0 | 0 | 0 | 126.4 | 128.1 |
| 12 | -1 | 0 | 0 | 1 | 69.1 | 67.57 |
| 13 | 0 | -1 | 1 | 0 | 60.9 | 61.49 |
| 14 | 0 | 1 | 0 | 1 | 63.67 | 63.62 |
| 15 | 0 | 1 | -1 | 0 | 64.85 | 65.2 |
| 16 | 1 | -1 | 0 | 0 | 65.4 | 64.7 |
| 17 | -1 | 0 | 0 | -1 | 75.7 | 73.6 |
| 18 | 0 | 0 | -1 | -1 | 66.8 | 68.7 |
| 19 | 1 | 0 | 0 | -1 | 58.47 | 59.4 |
| 20 | -1 | -1 | 0 | 0 | 64.5 | 68.09 |
| 21 | 0 | 0 | 1 | -1 | 63.4 | 67.0 |
| 22 | 0 | -1 | 0 | 1 | 75.8 | 76.53 |
| 23 | -1 | 0 | -1 | 0 | 76.06 | 76.81 |
| 24 | 0 | 0 | 1 | 1 | 72.87 | 74.02 |
| 25 | 0 | -1 | -1 | 0 | 76.5 | 73.38 |
| 26 | 0 | 1 | 0 | -1 | 78.4 | 74.02 |
| 27 | 1 | 1 | 0 | 0 | 67.9 | 75.8 |
| 28 | 0 | 0 | 0 | 0 | 118.6 | 121.9 |
| 29 | 0 | 1 | 1 | 0 | 76.1 | 77.9 |
Table 2. Analysis of Variance of Xylanase
| Source | Sum of Squares | df | Mean Square | F-value | p-value | Level |
| Model | 12196.63 | 14 | 871.19 | 94.17 | < 0.0001 | Significant |
| A-A | 58.21 | 1 | 58.21 | 6.29 | 0.0251 | |
| B-B | 27.97 | 1 | 27.97 | 3.02 | 0.1040 | |
| C-C | 2.41 | 1 | 2.41 | 0.2607 | 0.6176 | |
| D-D | 42.60 | 1 | 42.60 | 4.61 | 0.0499 | |
| AB | 1.10 | 1 | 1.10 | 0.1192 | 0.7351 | |
| AC | 127.92 | 1 | 127.92 | 13.83 | 0.0023 | |
| AD | 96.33 | 1 | 96.33 | 10.41 | 0.0061 | |
| BC | 180.23 | 1 | 180.23 | 19.48 | 0.0006 | |
| BD | 254.88 | 1 | 254.88 | 27.55 | 0.0001 | |
| CD | 6.84 | 1 | 6.84 | 0.7392 | 0.4044 | |
| A² | 4583.11 | 1 | 4583.11 | 495.43 | < 0.0001 | |
| B² | 4651.03 | 1 | 4651.03 | 502.77 | < 0.0001 | |
| C² | 4129.81 | 1 | 4129.81 | 446.43 | < 0.0001 | |
| D² | 4494.74 | 1 | 4494.74 | 485.88 | < 0.0001 | |
| Residual | 129.51 | 14 | 9.25 | |||
| Lack of Fit | 101.61 | 10 | 10.16 | 1.46 | 0.3825 | not significant |
| Pure Error | 27.90 | 4 | 6.98 | |||
| Cor Total | 12326.14 | 28 |
Std. Dev.- 3.13, C.V.% – 4.0, R- Squared – 0.9895, Adj. R-Squared- 0.9790, Pred. R- Squared- 0.9508
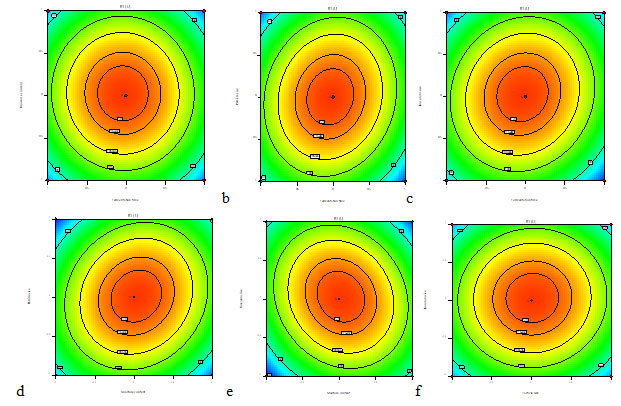
The response fitted for the above model was strategized for the duo grouping of the utmost collaborative variables (Fig. 12 A-F). The maximum xylanase production was acquired at 96 h of fermentation period, 90% of Moisture content, inoculum size of 1×106 spores/ml and particle size of 2 mm. Highest probable response was intended to be 126.4 U/ml for production of xylanase enzyme. The Fig. 12 (A, B) indicated production of xylanase enzyme upto 89% of moisture content, higher or lower values resulted in reduction of enzyme yield. The xylanase production decreased with higher moisture level, probably because of the reduction in the exchange of gases and air, degradation of the substrate structure, inhibition of fungal growth due to increase in adhesiveness (Shah and Dutta 2005; Azzuoz et al. 2020).
On adjusting the fermentation time to superior (120 h) or inferior (72 h) values the yield of xylanase production decreased (Fig. 12 A-C). The maximum xylanase enzyme was produced using fermentation time of 96 h. The xylanase production reduced below and above the optimized fermentation time may be because of minimized fungal growth and accumulation of secondary metabolites promoting inhibition of fungal growth followed by enzyme liberation. (Ajijolakewu et al. 2017; Azzouz et al. 2020; Mitri et al. 2022).
The Fig. 12 (D-F) showed that xylanase production is increased due to the particle size variable. The maximum production of xylanase occurred at 2 mm particle size. By employing suitable particle size of the solid substrate, the flow of air and mycelial growth was distributed in a space of the medium used (Manan and Webb 2017; Manan and Webb 2020).
Xylanase production was influenced by high and low values of inoculum size. An elevated xylanase secretion was predicted at 1×106 spores/ml. Inoculum density confirms to be a substantial factor in production of enzymes (Krishna 2005). A low concentration of inoculum may cause underprovided biomass followed by less product formation whereas high concentration of inoculum may cause excessive supply to biomass which triggers reduction in the availability of essential nutrients to the substrate for product development (Kavya and Padmavathi 2009; Naidu et al. 2020).
Recently, Razali et al. (2021) inferred that the temperature, moisture content and inoculum size affect the production of xylanase. Temperature and moisture content also affected the enzyme activity (Razali et al. 2021).
Figure 12: Contour plots showing the effect of (a) Fermentation period and moisture content (b) Fermentation Period and Particle size (c) Fermentation period and Inoculum Size (d) Moisture content and Particle size (e) Moisture content and Inoculum size (f) Particle size and Inoculum size
Thus, the investigative plan may conclude that the statistical model employed for analysis of variance was satisfactory. Also, it reflects the accuracy and suitability of RSM in optimization of the process parameters employed for xylanase production (Ajijolakewu et al. 2017; Razali et al. 2021).
Figure 13
Validation of RSM model: During analysis the result obtained after selecting important parameters such as fermentation time, moisture content, inoculum size and particle size for augmented medium model. The response surface model was also validated in triplicate. Experiments designed in BBD are subjected for analysis of xylanase activity at optimum time interval to determine the precision of the model and to authenticate the optimized results. Under optimized conditions, 128.1 ± 1.2 U/ml of xylanase production was attained. This value matches precisely to the predicted value of 126.4 U/ml, by the model which is 4.2% more than the OFAT optimized value 121.3 U/ml.
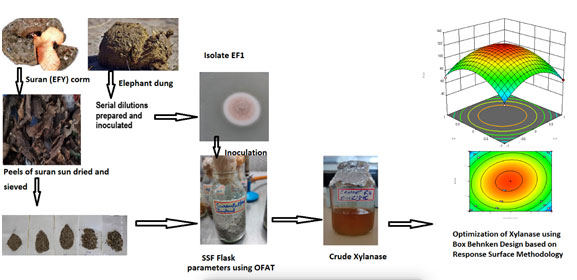
Azzouz et al. (2020) reported that xylanase activity increased phenomenally from 8475.87 to 14766.28 U/mL from Trichoderma afroharzianum isolate AZ 12 representing that RSM experimental design has established itself to be a successful tool in governing the optimum parameters for production of enzyme. Ezeilo et al. (2019) inferred xylanase production from a novel Trichoderma asperellum UC1 along with other enzymes with maximum xylanase activity of 255.5 U/g. However, the production of xylanase-pectinase cocktail using RSM based on CCD was reported to be as an effective tool used for enhancing production of enzymes using Bacillus amyloliquefaciens ADI2 conveyed by Nawawi et al. (2022). A brief pictorial representation of present work in shown in graphical abstract (Fig.13) (Nawawi et al. 2022).
Figure 14: Graphical Abstract
CONCLUSION
The findings of the present study has shown that the elephant dung isolated Aspergillus terreus Thom. was found to produce xylanase maximally in statistically optimised SSF on EFY peels. Alkali and thermo-tolerant Xylanase produced can be of effective use in industries such as pulp and paper industry, recycling by deinking in paper industry, bioethanol production, bakery, fruit juice clarification and many more. The utilization of biowaste for production of industrially useful enzymes can be an effective strategy for Bio sustainability.
Abbreviations: SSF- Solid State fermentation, SEM- Scanning Electron Microscopy, EFY-Elephant foot yam, OFAT- one factor at a time, RSM- Response surface methodology, BBD- Box Behenken Design, XPDA- Xylan potato dextrose agar. DNS (3,4- dinitrosalicylic acid) method, BSA- Bovine serum albumin,
ACKNOWLEDGEMENTS
The authors acknowledge the facilities provided by the School of Biotechnology, Devi Ahilya University, Indore and Department of Biosciences, Maharaja Ranjit Singh College of Professional Sciences, Indore. SEM analysis by Mr. Azhar Ansari (SEM FACILITY), Central Instrumentation Facility-12, IISER Bhopal, Madhya Pradesh is acknowlwdged. At last, would like to acknowledge Dr. Uttam Yadav, Director of Indore Zoo for granting permission for obtaining samples and Zookeepers for helping in collecting some herbivores dung samples for present research investigation.
Role of Funding Sources: This research did not receive any specific grant from the funding agencies in the public, commercial, or not for profit sectors.
Conflict of Interest: Authors declare no conflict of interest to disclose.
Data Availability Statement: All data generated /results / information is available with the authors and can be shared on a reasonable request made to the corresponding author when required.
REFERENCES
Aita, B.C., Spannemberg, S.S., Schmaltz, S., et al. (2019). Production of cell-wall degrading enzymes by solid-state fermentation using agroindustrial residues as substrates. Journal of Environmental Chemical Engineering, 7(3), 103193.
Ajijolakewu, A.K., Leh, C.P., Abdullah, W.N.W., et al. (2017). Optimization of production conditions for xylanase production by newly isolated strain Aspergillus niger through solid state fermentation of oil palm empty fruit bunches. Biocatalysis and Agricultural Biotechnology, 11, 239-247.
Alves K. J., da Silva M. C. P. and Cotta S. R. (2020). Mangrove soil as a source for novel xylanase and amylase as determined by cultivation-dependent and cultivation-independent methods. Brazilian Journal of Microbiology, 51, 217–228. 10.1007/s42770-019-00162-7
Atalla, S.M. and El Gamal, N.G. (2020). Production and characterization of xylanase from pomegranate peel by Chaetomium globosum and its application on bean under greenhouse condition. Bulletin of the National Research Centre, 44(1), 1-11.
Ayubi, M. M., Werner, A., Steudler, S., et al. (2021). Enhanced xylan conversion to xylitol in a bio- and chemo-catalytic one-pot process. Catalysis, 367,137–144. doi: 10.1016/j.cattod.2020.06.009
Azzouz, Z., Bettache, A., Boucherba, N., et al. (2020). Optimization of xylanase production by newly isolated strain Trichoderma afroharzianum isolate az 12 in solid state fermentation using response surface methodology. Cellulose Chemistry and Technology, 54, 451-462.
Bakri, Y., Jahwar, M. and Arabi, M.I.E. (2008). Improvement of xylanase production by Cochliobolus sativus in submerged culture. Food Technology and Biotechnology, 46(1), 116-118.
Ball, A.S. and Mccarthy, A.J. (1988). Saccharification of straw by actinomycete enzymes. Microbiology, 134(8), 2139-2147.
Bayoumi, R.A., Yassin, H.M., Swelim, M.A., et al. (2008). Production of bacterial pectinase (s) from agro-industrial wastes under solid state fermentation conditions. Journal of Applied Science and Research, 4(12), 1708-1721.
Behnam, S., Karimi, K., Khanahmadi, M., et al. (2016). Optimization of xylanase production by Mucor indicus, Mucor hiemalis, and Rhizopus oryzae through solid state fermentation. Biological Journal of Microorganism, 4(16), 1-10.
Beily, P., Coughlan, M.P. and Hazlewood, G.P. (1993). Biochemical aspects of the production of microbial hemicellulose. Hemicellulose and hemicellulases. Portland Press, London, 29-51.
Bhalla, A., Bischoff, K.M. and Sani, R.K. (2015). Highly thermostable xylanase production from a thermophilic Geobacillus sp. strain WSUCF1 utilizing lignocellulosic biomass. Frontiers in Bioengineering and Biotechnology, 3, 84.
Champreda, V., Mhuantong, W., Lekakarn, H., et al. (2019). Designing cellulolytic enzyme systems for biorefinery: from nature to application. Journal of Bioscience and Bioengineering, 128(6), 637-654.
Chilakamarry, C.R., Sakinah, A.M., Zularisam, A.W., et al. (2022). Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresource technology, 343, 126-165.
Chukwuma, O.B., Rafatullah, M., Tajarudin, H.A., et al. (2020). Lignocellulolytic enzymes in biotechnological and industrial processes: a review. Sustainability, 12(18), 72-82.
De Oliveira Júnior, S.D., dos Santos Gouvêa, P.R., de Aguiar, L.V.B., et al. (2022). Production of Lignocellulolytic Enzymes and Phenolic Compounds by Lentinus strigosus from the Amazon Using Solid-State Fermentation (SSF) of Guarana (Paullinia cupana) Residue. Applied Biochemistry and Biotechnology, 1-19.
Decker, C., Visser, J. and Schreier, P.J.A.M. (2001). β-Glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Applied Microbiology and Biotechnology, 55(2), 157-163.
Dhiman, S.S., Sharma, J. and Battan, B. (2008). Pretreatment processing of fabrics by alkalothermophilic xylanase from Bacillus stearothermophilus SDX. Enzyme and Microbial Technology, 43(3), 262-269.
Ezeilo, U.R., Lee, C.T., Huyop, F., et al. (2019). Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. Journal of Environmental Management, 243, 206-217.
Ghoshal, G., Banerjee, U.C. and Shivhare, U.S. (2016). Utilization of agrowaste and xylanase production in solid state fermentation. Journal of Biochemical Technology, 6(3), 1013-1024.
Gupta, V., Garg, S., Capalash, N., et al. (2015). Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of kraft pulp and deinking of waste paper. Bioprocess and Biosystems Engineering, 38(5), 947-956.
Hrmová, M., Biely, P., Vršanská, M., et al. (1984). Induction of cellulose-and xylan-degrading enzyme complex in the yeast Trichosporon cutaneum. Archives of Microbiology,138(4), 371-376.
Ibrahim, D., Puspitaloka, H., Rahim, R.A., et al. (2012). Characterization of solid-state fermentation culture conditions for growth and mannanase production by Aspergillus niger USM F4 on rice husk in tray system. Biotechnology Journal International, 133-145.
Intasit, R., Cheirsilp, B., Suyotha, W., et al. (2021). Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid state-submerged fermentation and co-cultivation of different filamentous fungi. Biochemical Engineering Journal, 173,108086.
Intasit, R., Cheirsilp, B., Suyotha, W., et al. (2022). Purification and characterization of a highly-stable fungal xylanase from Aspergillus tubingensis cultivated on palm wastes through combined solid-state and submerged fermentation. Preparative Biochemistry & Biotechnology, 52(3), 311-317.
Izidoro, S.C. and Knob, A. (2014). Production of xylanases by an Aspergillus niger strain in wastes grain. Acta Scientiarum. Biological Sciences, 36(3), 313-319.
Jogi, P. and Lahre, N. (2020). Impact of front-line demonstration on yield and economics of elephant foot yam (Amorphophallus paeoniifolius) in Mungeli District of Chhattisgarh. Journal of Pharmacognosy and Phytochemistry, 9(5), 3205-3208.
Joshi, J.B., Priyadharshini, R. and Uthandi, S. (2022). Glycosyl hydrolase 11 (xynA) gene with xylanase activity from thermophilic bacteria isolated from thermal springs. Microbial cell factories, 21(1), 1-13.
Kanimozhi, K. and Nagalakshmi, P.K. (2014). Xylanase production from Aspergillus niger by solid state fermentation using agricultural waste as substrate. International Journal of Current Microbiology and Applied Sciences, 3(3), 437-446.
Kaur, A., Singh, A., Dua, A., et al. (2017). Cost-effective and concurrent production of industrially valuable xylano-pectinolytic enzymes by a bacterial isolate Bacillus pumilus AJK. Preparative Biochemistry and Biotechnology, 47(1), 8-18.
Kavya, V. and Padmavathi, T. (2009). Optimization of growth conditions for xylanase production by Aspergillus niger in solid state fermentation. Polish Journal of Microbiology, 58(2), 125-130.
Kirtikar, K.R. and Basu, B.D. (1989). Indian medicinal plants Eds. E Blatter, Caius JF, Lalit Mohan Basu, Allahabad, 2, 2389.
Krishna, C. (2005). Solid-state fermentation systems – an overview. Critical Reviews in Biotechnology, 25(1-2), 1-30.
Kuhad, R.C., Manchanda, M. and Singh, A. (1998). Optimization of xylanase production by a hyperxylanolytic mutant strain of Fusarium oxysporum. Process Biochemistry, 33(6), 641-647.
Kumar, D., Kumar, S.S., Kumar, J., et al. (2017). Xylanases and their industrial applications: a review. Biochemical Cellular Archives, 17(1), 353-360.
Kumar, H., Bhardwaj, K., Sharma, R., et al. (2020). Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules, 25(12), 2812.
Lakshmi, G.S., Bhargavi, P.L. and Prakasham, R.S., (2011). Sustainable bioprocess evaluation for xylanase production by isolated Aspergillus terreus and Aspergillus fumigatus under solid-state fermentation using oil palm empty fruit bunch fiber. Current Trends in Biotechnology and Pharmacy, 5(4),1434-1444.
Liu, W., Lu, Y. and Ma, G. (1999). Induction and glucose repression of endo-β-xylanase in the yeast Trichosporon cutaneum SL409. Process Biochemistry, 34(1), 67-72.
Liu, W., Zhu, W., Lu, Y., et al. (1998). Production, partial purification and characterization of xylanase from Trichosporon cutaneum SL409. Process Biochemistry, 33(3), 331-336.
Lonsane, B.K., Saucedo-Castaneda, G., Raimbault, M., et al. (1992). Scale-up strategies for solid state fermentation systems. Process Biochemistry, 27(5), 259-273.
Manan, M.A. and Webb, C. (2017). Design aspects of solid-state fermentation as applied to microbial bioprocessing. Journal of Applied Biotechnology and Bioengineering, 4(1), 91.
Manan, M.A. and Webb, C. (2020). Newly designed multi-stacked circular tray solid state bioreactor analysis of a distributed parameter gas balance during solid-state fermentation with influence of variable initial moisture content arrangements. Bioresources and Bioprocessing, 7, 16. https://doi.org/10.1186/s40643-020-00307-9
Marimuthu, M., Sorimuthu, A. and Muruganantham, S. (2019). Production and Optimization of Xylanase Enzyme from Bacillus subtilis using Agricultural Wastes by Solid State Fermentation. International Journal of Pharmaceutical Investigation, 9(4), 169-173.
Miller, G.L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426-428.
Misra, R.S. and Sriram, S. (2002). Medicinal value and export potential of tropical tuber crops. Crop improvement, production technology, trade and commerce, 373-386.
Misra, R.S., Shivlingaswamy, T.M. and Maheshwari, S.K. (2001). Improved production technology for commercial and seed crops of elephant foot yam. Journal of Root Crops, 27(1), 197-201.
Mitri, S., Salameh, S.J., Khelfa, A., et al. (2022). Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation, 8(2), 50.
Naidu, Y., Siddiqui, Y. and Idris, A.S. (2020). Comprehensive studies on optimization of ligno-hemicellulolytic enzymes by indigenous white rot hymenomycetes under solid-state cultivation using agro-industrial wastes. Journal of environmental management, 259, 110056.
Najjarzadeh, N., Matsakas, L., Rova, U., et al. (2020). Effect of oligosaccharide degree of polymerization on the induction of xylan-degrading enzymes by Fusarium oxysporum f. sp. Lycopersici. Molecules, 25(24), 5849.
Nawawi, M.H., Ismail, K.I., Sa’ad, N., et al. (2022). Optimisation of Xylanase–Pectinase Cocktail Production with Bacillus amyloliquefaciens ADI2 Using a Low-Cost Substrate via Statistical Strategy. Fermentation, 8(3), 119.
Nenava, R., Nighojkar, S., Patidar, M., et al. (2021). Purification and Characterization of Thermostable Alkaline Xylanase by Aspergillus terreus from Elephant dung. Journal of Advances in Biology and Biotechnology, 24(12), 1-12. DOI: 10.9734/jabb/2021/v24i1230254
Olanbiwoninu, A.A. and Odunfa, S.A. (2016). Production of cellulase and xylanase by Aspergillus terreus KJ829487 using cassava peels as subtrates. Advances in Microbiology, 6(07), 502.
Ooijkaas, L.P., Weber, F.J., Buitelaar, R.M., et al. (2000). Defined media and inert supports: their potential as solid-state fermentation production systems. Trends in Biotechnology,18(8), 356-360.
Pérez-Rodríguez, N., Moreira, C.D., Agrasar, A.T., et al. (2016). Feruloyl esterase production by Aspergillus terreus CECT 2808 and subsequent application to enzymatic hydrolysis. Enzyme and Microbial Technology, 91, 52-58.
Pietikäinen, J., Pettersson, M. and Bååth, E. (2005). Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiology Ecology, 52(1), 49-58.
Prabhu, G., Bhat, D., Bhat, R.M., et al. (2022). A Critical Look at Bioproducts Co-cultured Under Solid State Fermentation and Their Challenges and Industrial Applications. Waste and Biomass Valorization, 1-17.
Rana, P., Inbaraj, B.S., Gurumayum, S. et al. (2021). Sustainable Production of Lignocellulolytic Enzymes in Solid-State Fermentation of Agro-Industrial Waste: Application in Pumpkin (Cucurbita maxima) Juice Clarification. Agronomy, 11(12), 2379.
Razali, S.A., Rasit, N. and Ooi, C.K. (2021). Statistical analysis of xylanase production from solid state fermentation of rice husk associated fungus Aspergillus niger. Materials Today: Proceedings, 39, 1082-1087.
Victorica, M.R., Soria, M. A., Batista-García, R. A., et al. (2020). Neotropical termite microbiomes as sources of novel plant cell wall degrading enzymes. Scientific Reports. 10, 38-64.
Sadaf, A. and Khare, S.K. (2014). Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosachharide synthesis. Bioresource Technology, 153,126-130.
Sakthiselvan, P., Naveena, B. and Partha, N. (2014). Molecular characterization of a Xylanase-producing fungus isolated from fouled soil. Brazilian Journal of Microbiology, 45, 1293-1302.
Seyis, I. and Aksoz, N. (2005). Xylanase production from Trichoderma harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technology and Biotechnology, 43(1), 37-40
Shah, A.R. and Madamwar, D. (2005a). Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochemistry, 40(5),1763-1771.
Shah, A.R. and Madamwar, D., (2005b). Xylanase production under solid-state fermentation and its characterization by an isolated strain of Aspergillus foetidus in India. World Journal of Microbiology and Biotechnology, 21(3), 233-243.
Sharma, S., Vaid, S. and Bajaj, B.J. (2015). Screening of thermo-alkali stable fungal xylanases for potential industrial applications. Current Research in Microbiology and Biotechnology, 3(1), 536-541.
Silva, L.D.O., Terrasan, C.R.F. and Carmona, E.C. (2015). Purification and characterization of xylanases from Trichoderma inhamatum. Electronic Journal of Biotechnology,18(4), 307-313.
Simotilde, M.L.G. and Tauk-Tornisielo, S.M. (2006). Optimization of xylanase biosynthesis by Aspergillus japonicus isolated from a Caatinga area in the Brazilian state of Bahia. African Journal of Biotechnology, 5(11),1135-1141.
Singh, A., Srivastava, K.C., Banerjee, A., et al. (2013). Phytochemical analysis of peel of Amorphophallus paeoniifolius. International Journal of Pharma and Bio Sciences, 4(3), 810 -815.
Singh, R.K., Singh, A. and Sureja, A.K. (2007). Sustainable use of ethnobotanical resources. Indian Journal of Traditional Knowledge, 6(3), 521-530.
Soccol, C.R., da Costa, E.S.F., Letti, L.A.J., et al. (2017). Recent developments and innovations in solid state fermentation. Biotechnology Research and Innovation, 1(1), 52-71.
Sood, S., Singhal, R., Bhat S, et al. (2011). In book: Comprehensive Biotechnology Edition 2nd: 2.13 – Inoculum Preparation. Elsevier, 151-164.
Subbulakshmi, S. and Iyer, P.R. (2014). Production and purification of enzyme xylanase by Aspergillus niger. International Journal of Current Microbiology and Applied Sciences, 3, 664-668.
Sunna, A. and Antranikian, G. (1997). Xylanolytic enzymes from fungi and bacteria. Critical reviews in biotechnology, 17(1), 39-67.
Syarifah, A.R., Darah, I. and Nyoman, I. (2014). A New Latent Lovastatin Producer viz. Fusarium pseudocircinatum IBRL B3-4, Produced in Laboratory Tray System. Pertanika Journal of Tropical Agricultural Science, 37(4).
Tamura, K., Peterson, D., Peterson, N., et al. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10), 2731-2739.
Teather, R.M. and Wood, P.J. (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology, 43(4), 777-780.
Techapun, C., Poosaran, N., Watanabe, M., et al. (2003). Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochemistry, 38(9), 1327-1340.
Thomas, L., Parameswaran, B. and Pandey, A. (2016). Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renewable Energy, 98, 9-15.
Verma, D., and Satyanarayana, T. (2020). Xylanolytic extremozymes retrieved from environmental metagenomes: characteristics, genetic engineering, and applications. Frontiers in Microbiology, 11, 551109. doi: 10.3389/fmicb.2020.551109
Viesturs, U.E., Strikauska, S.V., Leite, M.P., et al. (1987). Combined submerged and solid substrate fermentation for the bioconversion of lignocellulose. Biotechnology and Bioengineering, 30(2), 282-288.
Walia, A., Guleria, S., Mehta, P., et al. (2017). Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech, 7(1), 1-12.
Walia, A., Mehta, P., Guleria, S., et al. (2015). Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech, 5(6), 1053-1066.
Wu, X., Zhang, Q., Zhang, L., et al. (2020). Insights into the role of exposed surface charged residues in the alkali-tolerance of GH-11 xylanase. Frontiers in Microbiology, 11, 872. doi: 10.3389/fmicb.2020.00872
Xiao, W., Li, H., Xia, W., et al. (2019). Co-expression of cellulase and xylanase genes in Sacchromyces cerevisiae toward enhanced bioethanol production from corn stover. Bioengineered, 10, 513–521. doi: 10.1080/21655979.2019.1682213
Xue, S., Pattathil, S., da Costa Sousa, L., et al. (2022). Understanding the structure and composition of recalcitrant oligosaccharides in hydrolysate using high-throughput biotin-based glycome profiling and mass spectrometry. Scientific reports, 12(1), 1-13.
Yepes-Betancur, D.P., Márquez-Cardozo, C.J., Cadena-Chamorro, E.M., et al. (2021). Solid-state fermentation–assisted extraction of bioactive compounds from hass avocado seeds. Food and Bioproducts Processing, 126, 155-163.
Zadrazil, F. and Puniya, A.K. (1995). Studies on the effect of particle size on solid-state fermentation of sugarcane bagasse into animal feed using white-rot fungi. Bioresource Technology, 54(1), 85-87.
Zarafeta, D., Galanopoulou, A. P., Leni, M. E., et al. (2020). XynDZ5: a new thermostable GH-10 xylanase. Frontiers in Microbiology. 11:545. doi: 10.3389/fmicb.2020.00545
Zehra, M., Syed, M.N. and Sohail, M. (2020). Banana peels: a promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Polish Journal of Microbiology, 69(1), 19-26.
Zhang, H. and Sang, Q. (2015). Production and extraction optimization of xylanase and β-mannanase by Penicillium chrysogenum QML-2 and primary application in saccharification of corn cob. Biochemical Engineering Journal, 97, 101-110.


