1Department of Biotechnology, Nehru Arts and Science College Coimbatore, Tamil Nadu, India.
2Department of Biotechnology, SNMV Colleges of Arts and Science College Coimbatore, Tamil Nadu, India.
Corresponding author email: anithavarshini22@gmail.com
Article Publishing History
Received: 14/10/2021
Accepted After Revision: 21/12/2021
The efficacy of antagonistic Streptomyces griseus was evaluated against tomato wilt disease incited by Fusarium oxysporum. Among the different formulations, Streptomyces griseus with chitin amended formulation showed effective increase in seed germination and seedling vigour. Further, talc-based formulations of S. griseus mixed with or without chitin was developed and tested under greenhouse conditions. Lowest disease severity of 19.1% was observed in plants treated with self fusant (SFSg 5) S. griseus suspension (root dipping – 9 x 108 cfu / mL) followed by 19.5% in treatment of chitin amended S. griseus (root dipping – 9 x 108 cfu/mL) was recorded over control. Plant growth of the treated traits were analyzed and compared with control.
The shoot length, root length, leaf area was increased significantly over the controls by the treatment of self fusant (SFSg 5) S. griseus suspension followed by nearby values were reached in chitin amended S. griseus was recorded. The chemical treatments had less effect compared with these formulations. Histochemical studies showed that cambium layers, xylem vessels per bundle, and the vessel diameter decreased in the plants inoculated with F. oxysporum over control and changes in variables were observed in infected plants treated with S. griseus. In conclusion, S. griseus can be a potential biocontrol agent against F. oxysporum for better crop production practices.
Disease Severity, S. Griseus, Seed/Root Inoculation,
Tomato Growth Promotion, Wilt.
Anitha, Saraswathi K. R, Arunkumar D. Control of Fusarium Wilt Using Streptomyces griseus with Plant Growth-Promoting Effect on Tomato. Biosc.Biotech.Res.Comm. 2021;14(4).
Anitha, Saraswathi K.R, Arunkumar D. Control of Fusarium Wilt Using Streptomyces griseus with Plant Growth-Promoting Effect on Tomato. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3EbfMjG“>https://bit.ly/3EbfMjG</a>
Copyright © Anitha et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Plants in their environment face potential deleterious organisms such as fungi, bacteria, viruses, nematodes, etc., in soil and air are responsible for losses in crop yield worldwide. Soil-borne pathogens are saprophytic in nature. They reside in the soil for brief or extended periods; enter via roots and cause disease of the roots or stem, disrupting the uptake and translocation of water and nutrients from the soil (McGovern 2015). Plant disease incidence and prevalence varies from season to season depending on the nature of the pathogen, environmental factors, and cultivars and varieties.
Acquired resistance of pests & pathogens towards the chemical fertilizers, which have detrimental effects on the environment is the major concern (Abbasi et al. 2019). The use of microorganisms to control plant pathogens is now in practice (Al-Ani and Adhab 2012, Al-Ani et al. 2012; Al-Ani and Adhab 2013; Passari et al. 2019).
The potential use of plant associated bacteria as agents for stimulating plant growth and managing soil and plant health has been well described (Compant et al. 2005). The growth-promoting bacteria colonizing the root surfaces and closely adhering soil interface (rhizosphere) and enters root interior and established as endophytic populations. The general mechanisms involved in biocontrol are to reduce the population of pathogens at their entry level and to provide strong growth promoting activity during its symbiosis (Chen et al. 2000; Naik et al. 2008).
Many plants growth promoting rhizobacteria, endophytic and actinobacteria specifically belonged to the genus Streptomyces sp., were used to improve crop fertility by enabling the plant to get nutrients from the soil (Nejad and Johnson 2000; Araujo et al. 2000; Bloemberg et al. 2001; Kanini et al. 2013; Kamal and Sharma 2014; Abbasi et al. 2019; Passari et al. 2019).
Microbial endophytic communities are currently the focal point to explain their function as plant growth promoters and their involvement in plant protection. In consideration of the above, the study was aimed to evaluate growth characteristics of tomato with respect to antagonistic Streptomyces griseus against wilt disease induced by Fusarium oxysporum under greenhouse conditions.
For the plant material, Tomato variety Co-4 susceptible to wilt disease was employed during the investigation. For the preparation of antagonist suspension inoculum, the antagonists S. griseus was grown on MS broth (with/without chitin) with constant shaking for 7 days at 30°C and at 125 shakes/min. (Remi – C 24). The bacterial cells were harvested by centrifugation at 8000 rpm for 15 min and resuspended in phosphate buffer (0.01M, pH 7.0) and used as inoculum (Bharathi et al. 2004).
To determine the efficacy of antagonists on seed germination and seedlings vigour, the mycelial suspension of the S. griseus prepared was tested for their plant growth promoting activity, which was carried out by the standard roll towel method ISTA (1996). The seeds were soaked in 10 mL of the bacterial suspension (108 cfu/mL) for 2 h and blot dried, placed in wet blotters and incubated at room temperature for 10 days. The seeds soaked in sterile water served as control. The germination percentage of seeds was recorded and the vigour index was calculated using the following formula: Vigour index = per cent Germination x seedling length (shoot length + root length).
To determine the efficacy of S. griseus against fusarium wilt under greenhouse conditions, the tomato variety Co-4 was used for in vitro and the greenhouse experiments in the entire period of investigation. All seeds were surface-disinfected with 1% sodium hypochlorite for 5 min and rinsed three times in sterile distilled water prior to sowing. Tomato seeds for glasshouse experiments were sown in trays (27 x 42 x 7 cm3) containing an autoclaved mixture of 1:1 (v:v) ratio of vermiculite and sand. Trays were maintained on benches in a glasshouse without artificial light for 4–5 weeks before transplanting. After some days, when the seedlings had 2–4 leaves, they were carefully transplanted into the pots.
To assess the effect of S. griseus on disease severity, various formulations were assessed for their efficiency in controllingF. oxysporum f. sp. lycopersici induced wilt incidence in greenhouse conditions. A pot culture study was undertaken with the following treatments by using completely randomized design (CRD) with three replications. The treatments were imposed as seed treatment and seedlings dip (Kamal and Sharma 2014). Seeds treated with formulation was sowed in the trays and 60 days old seedings were transferred to the pot and marked as T1, T2.
Tomato seeds for glasshouse experiments were sown in trays (27 x 42 x 7 cm3) containing an autoclaved mixture of 1:1(v: v) ratio of vermiculite and sand. After 60 days, the seedlings were pulled out from the trays and roots were immersed in various formulation. Various methods of treatment details include, were T1 – the plants raised from seeds treated with talk formulation of S. griseus (10 g/kg of seeds), T2 – the plants raised from seeds treated with chitin amended talk formulation of S. griseus (10 g/kg of seeds), T3 – dipped in water containing talc formulation (20 g/L) for 2 h.
The roots alone were immersed in the chitin amended suspension of S. griseus (9 x 108 cfu/mL) formulation, T4 – dipped in water containing talc formulation (20 g/L) for 2 h. The roots alone were immersed in the suspension of self fusant (SFSg 5) S. griseus (9 x 108 cfu/mL) formulation, T5 – dipped in crude chitinase enzyme prepared from S. griseus with enzyme activity of 113.3 IU/mL, T6 – dipped in partially purified endo chitinase enzyme of S. griseus with enzyme activity of 1000 IU/mL, T7 – the plants raised from carbendazim-treated seeds (2 g/ kg of seeds), served as chemical check and T8 – Healthy control. After treatment, all the treated seeding was transplanted in to the pathogen infested (substrate at 5 percent (w/w) was mixed with sterilized soil) pots at the rate single seedlings per pot. Plants in the greenhouse were watered daily.
Beginning a week after inoculation, external symptoms of Fusarium wilt (wilting and yellowing leaves) were assessed. And the disease severity was assessed as DS = (Proportion of leaves with symptoms / Total number of leaves) X 100. To assess the effect of S. griseus on growth attributes, the efficacy of the formulations on various growth parameters viz., root length, shoot length, fresh & dry root weight, fresh & dry shoot weight were recorded in all the treatments at monthly intervals. All the experiments were analyzed independently. The treatment means were compared by Duncan’s Multiple Range Test (DMRT) (Gomez and Gomez 1994).
For the microscopic sections and histological studies of tomato tissues, samples from greenhouse were used for histological studies. In all of the experiments, hypocotyls were cut into 1-cm-long segments and stored in FAA (Formalin-Acetic-Alcohol) at least a week before continuing the processing for embedding. Plant tissue was transferred to aluminum weighing dishes and filled with molten paraffin and placed in a warm oven (60oC) overnight. Segments were transversally cut into 25- to 50-μm-thick sections with a freezing microtome. The sections were affixed in the slides and stained with toluidine blue for 5 min, rinsed with sterile water, and mounted with 50% glycerin. Then viewed with microscope and photomicrographs were obtained from the microscope (Cal et al. 2000).
RESULTS AND DISCUSSION
Efficacy of antagonist on seed germination: The use of chemical fertilizers has become common which has had a harmful impact on the environment (Fravel et al. 2005). Hence, it is essential to identify endophytic microorganisms that can be used as a method for increasing plant host resistance to disease and the improvement of soil health (Barnawal et al. 2017). In this regard, a number of actinobacteria and endophytic Streptomyces sp. have been reported to stimulate plant growth, function as biocontrol agents against diverse pathogenic fungi; and in general, increase abiotic and biotic stress tolerance in plants (Kanini et al. 2013; Kamal and Sharma 2014; Passari et al. 2017; Abbasi et al. 2019; Passari et al. 2019).
In the present study, the antagonistic strain S. griseus revealed the seedlings vigour (1679) and germination percentage (92.00). The seeds, which were not treated with any PGPR strain, showed poor performance and from this screening the strain S. griseus was selected for developing formulation. The S. griseus with chitin showed 96.10% germination and the highest seedling vigour of 3954 and it was improved over control (Table 1).
Table 1. Efficacy of antagonists on seed germination and vigour index of seedling
| Treatments | Germination % | Shoot length (cm) | Root length (cm) | Vigor index |
| S. griseus | 92.00c | 7.65 d | 10.40a | 1679 |
| S. griseus with chitin | 96.10 d | 6.25bc | 14.06 d | 3954 |
| Carbendazim | 85.00ab | 5.25ab | 12.00c | 1331 |
| Control | 80.59 a | 3.95a | 9.00 a | 1044 |
Values are the mean of three replications. Means followed by a common letter are not significantly different at 5% level by DMRT. (% – percentage; Cm – Centimeter)
With an increased number of colonies, improved performance on seed germination and seedling vigour was observed. The studies on growth promotion indicated that antagonistic strain S. griseus promote plant growth directly by the production of plant growth regulators or by stimulating nutrient uptake by producing siderophores or antibiotics to protect plants from soil borne pathogens. Further significant increase in seedling vigour would have occurred by better synthesis of hormones like auxins, cytokinins, etc. This finding is consistent with the result obtained by Passari et al. (2019), were demonstrated the four bacterial isolates (BPSAC77, BPSAC101, BPSAC121, and BPSAC147) mediated enhancement in the germination of tomato seeds.
Inoculation of BPSAC147 increased the germination percentage to 100% compared to 90% in the untreated, control seeds. Similarly, Lasudee et al. (2018) who stated that S. thermocarboxydus S3 increased the germination percentage of mung bean seeds (95–98%) which was statistically higher than the control. Kanini et al. (2013) reported the emergence of the tomato plants, the in vivo experiments were the tomato seeds treated with S. rochei ACTA1551 germinated at a higher rate into F. oxysporum infected soil compared to untreated seeds (Kanini et al. 2013; Lasudee et al. 2018; Passari et al. 2019).
Green house experiments on tomato plants: Efficacy of formulations on fusarium wilt disease of tomato: Fusarium oxysporum is a soil-borne pathogen and can spread through root system and it would be better to protect the infection sites rather than alter the entire soil microbial community. Biocontrol agents can protect the infection site by producing specific nutrients and other compounds against pathogen (Passari et al. 2019). Therefore, biocontrol agent must be introduced to root systems well in advance of Fusarium oxysporum f. sp. lycopersici infestation (Lu et al. 2004; Kanini et al. 2013).
Additionally, this procedure is much easier to implement and more applicable for large scale cultivation, compared to the classic one which includes enrichment of the soil with the biocontrol agents which is time consuming one and difficult in real farming conditions (Whipps 2001; Nerey et al. 2010). Actinobacteria are identified to be existing in the rhizosphere of plants, and Streptomyces sp. are well known as endophytic in root tissues, where they can be able to function as probable biocontrol agents and furthermore modulate plant growth (Rey and Dumas 2017; Jones et al. 2019).
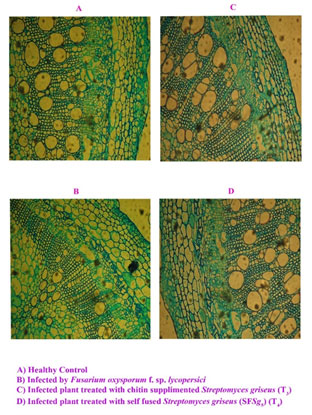
Figure 1: Cross sections of tomato stems (×100).
In the present study, lowest disease severity of 19.1% in treatment of self fusant (SFSg 5) S. griseus suspension (root dipping – 9 x 108 cfu / mL) followed by 19.5% in treatment of chitin amended S. griseus (root dipping – 9 x 108 cfu/mL) was recorded (Table 2).
Table 2. Efficacy of biocontrol agents on disease severity and yield of tomato under greenhouse conditions
| Treatments | Disease severity (%) | Yield (g/plant) |
| T1 – S. griseus (Seed treatment – 10 g / kg) | 26.0a | 485.0 |
| T2 – Chitin amended S. griseus (Seed treatment – 10 g/ kg) | 23.1a | 455.0 |
| T3 – Chitin amended S. griseus suspension (Root dipping – 4 x 108 cfu/mL) | 19.5a | 520.0 |
| T4 – self fusant (SFSg 5) S. griseus suspension (Root dipping – 4 x 108 cfu/mL) | 19.1a | 550.0 |
| T5 – foliar spray using crude chitinase enzyme (1L) of S. griseus with Apsa 80 (113.3 IU/mL) after planting | 28.9ab | 416.0 |
| T6 – carbendazim treated seeds (2 g/kg of seeds) | 31.5ab | 425.0 |
| T7 – Healthy Control | 49.3de | 425.0 |
| T8 – Inoculated Control | 61.1 h | 120.0 |
Values are the mean of three replications. In a column, means followed by common letters are not significantly different at 5% level by DMRT.
Isolate S. griseus exhibited biocontrol activity against F. oxysporum in tomato plants which may be due secondary metabolites production are in agreement with previews studies on the potential of Streptomyces sp., isolates to be used as biocontrol agents was documented in the previous studies (Passari et al. 2017). The finding is correlated with the earlier findings, were tomato seedlings treated with application of biocontrol agents mainly belongs to S. enissocaesilis IC10 and S. rochei Y28; S. flavofuscus CPP-53; S. griseorubens E44G from Saudi Arabia; S. rochei ACTA1551; S. miharaensis KPE62302H; S. psammoticus KP1404 (Kim et al. 2011) from Korea and S.
plicatus from Egypt against Fusarium wilt disease of tomato incited F. oxysporum f. sp. lycopersici showed efficient reduction in wilt disease severity and were reported as successful biocontrol agents to control tomato Fusarium wilt (Abd-Allah 2001; Kim 2012; Kanini et al. 2013; Rashad et al. 2017; Abbasi et al. 2019). In the same line, application of non-pathogenic Fusarium oxysporum and Tricoderma harzianum also showed efficient reduction in wilt incidence (Silva and Bettio 2005; Shishido et al. 2005; Yigit and Dikilitas 2007; Verma et al. 2017; Kamal and Sharma 2019).
Efficacy of S. griseus on growth attributes of tomato: Better growth may be responsible for better synthesis of auxin and cytokinin which would have helped in better partitioning efficiency which later resulted in increased economic yield. PGPR synthesize phytohormones that promote plant growth at various stages (Passari et al. 2017). In the present study, compared with control, highest shoot length of 70.60 cm, fresh weight of 23.60 g, dry weight of 6.65 g and root length of 55 cm, fresh 15.55 g, dry weight dry weight 3.08 g was observed in the treatment of self fusant (SFSg 5) S.
griseus suspension followed by nearby values were reached in treatment of chitin amended S. griseus (root dipping) was recorded (shoot and root length – 67.50 cm, 52.65 cm; Fresh & dry weight of shoot – 24.7 g; 15.75 g; Fresh & dry weight of root – 6.8 g, 2.85 g). Correspondingly, the chemical treatments had less effect compared with these formulations nevertheless showed moderate increase over control (Table 3). The possible reason might be associated with the initial increase in root growth by the application of PGRP strains, which would have helped in promoting better absorption of essential nutrients that are responsible for highly active photosynthesis as well as protein synthesis (Passari et al. 2017; Jones et al. 2019).
Table 3. Efficacy of formulations on growth attributes of tomato under greenhouse condition.
|
Treatments |
Shoot | Root | ||||
| Length (cm) | Fresh weight (g) | Dry weight (g) | Length (cm) | Fresh weight (g) | Dry weight (g) | |
| T1 – S. griseus (Seed treatment – 10 g / kg) | 63.98ef | 22.75e | 6.30 fg | 51.55gh | 14.5f | 2.48d |
| T2 – Chitin amended S. griseus (Seed treatment – 10 g/ kg) | 65.68 fg | 25.8h | 6.5gh | 52.05gh | 14.75g | 2.55de |
| T3 – S. griseus suspension (root dipping – 9 x 108 cfu/mL) | 67.50g | 24.7g | 6.8gh | 52.65gh | 15.75h | 2.85fg |
| T4 – self fusant (SFSg 5) S. griseus suspension (root dipping – 9 x 108 cfu / mL) | 70.60h | 23.60 f | 6.65h | 53.0h | 15.55b | 3.08gh |
| T5 – Crude chitinase enzyme of S. griseus suspension (root dipping – 113.3 IU / mL) | 58.35d | 21.75 de | 5.8de | 50.6gh | 13.85ef | 2.35ed |
| T6 – Partially purified chitinase enzyme of S. griseus suspension (root dipping – 1000 IU / mL) | 60.35de | 20.8ef | 5.05b | 49.85g | 13.85e | 2015ab |
| T7 – carbendazim (seed treatment – 2 g/ kg) | 54.7bc | 19.85 c | 4.5a | 22.65a | 13.35b | 1.91a |
| T8– Inoculated control | 46.85a | 15.85a | 5.08b | 39.35de | 9.8a | 1.75a |
Values are the mean of three replications. In a column, means followed by a common letter are not significantly different at 5% level by DMRT.
(g / kg – gram/kilo gram; cfu – colony forming units; IU / mL – International Units/Milli Liter; Cm – Centimeter; g – Gram)
Our study, however, is the first report on the ability of endophytic S. griseus to exhibit PGP and biocontrol activity in tomato plants. Similar results were reported in the previous studies who evidenced that endophytic S. thermocarboxydus BPSAC147; Streptomyces sp. isolate PM4 and PM5; Streptomyces sp. isolate SNL2 and S. caeruleatus isolate ZL2 significantly increased the growth against F. oxysporum in tomato plants, relatively non-inoculated control plants (Zamoum et al. 2015; Goudjal et al. 2016; Dias et al. 2017; Passari et al. 2019). Furthermore, Goudjal et al. (2014) and Kanini et al.
(2013) also reported that Streptomyces sp. CA-2 and AA-2 and Streptomyces rochei ACTA1551 significantly increased the shoot and root length of tomato plants, relative to control plants. In earlier studies, inoculation of Trichoderma sp., G. intraradices showed significant increase in growth and yield of tomato plant (Ilham et al. 2003; Akkopru and Demir 2006; Martínez-Medina et al. 2014; Ghazalibiglar et al. 2016; Lee et al. 2016; Jones et al. 2019).
Microscopic sections and histological studies: After assessing the disease severity plant tissues of specific treatment and the corresponding controls also were studied histochemically. Control plant showed normal cambium layers, xylem vessels per bundle (Plate. 1c). The cambium layers, xylem vessels per bundle, and the vessel diameter decreased in plants infected with F. oxysporum and not treated with S. griseus (Plate. 1a). Cambium is in charge of secondary growth and regression or its total differentiation has a negative effect on vascular development with inefficient water transport in the plant and subsequent stunting (Cal et al. 2000). Several authors have described the reduction in the number of xylem vessels as an effect of vascular pathogens including F. oxysporum f. sp. lycopersici (Cal 1997; Cal 2000; Abbasi et al. 2019).
The mode of application of S. griseus showed marked difference in the suppression of morphological effects caused by F. oxysporum f. sp. lycopersici in tomato plants. Changes in ciambium layers, xylem vessels per bundle and the vessel diameter under microscopy were observed (Plate. 1b) and these changes were most apparent in plants of treated with S. griseus (treatment 3). Morphological changes observed in plants treated with S. griseus in seeding before transplanting might have a role in resistance to Fusarium tomato wilt, and they remain systemic in the tomato plants (Passari et al. 2019).
The changes could be related to the growth regulators production causing cell growth, division and differentiation also the phytohormones, the major factor which controlling the plant vascular differentiation. This result was supported by the previous studies that showed tomato (Lycopersicon esculentum) plants of ‘Lorena’ were induced with a conidial suspension (107conidia/mL) of Penicillium oxalicum before inoculation with F. oxysporum f. sp. lycopersici, the wilt pathogen.
In non-induced plants, the pathogen produced almost a complete loss of cambium (75 to 100% reduction) whereas plants induced with P. oxalicum showed less disease, did not lose the cambium, had a lower number of bundles, and had less vascular colonization by F. oxysporum f. sp. lycopersici (35 to 99%). Further the suppression of disease caused by F. oxysporum f. sp. lycopersici due to application of P. oxalicum was demonstrated previously to be related to mechanisms of induced resistance in tomato plants (Cal et al. 1995; Cal et al. 1997; Cal et al. 2000; Passari et al. 2019; Abbasi et al. 2019).
Different patterns of root colonization between cultivars and F. oxysporum f. sp. pathosystems have been reported previously, such as F. oxysporum f. sp. lentis; purple passionfruit – F. oxysporum and F. solani; chickpea – F. oxysporum f. sp. ciceris; melon – Fusarium oxysporum race 1.2; bean – F. oxysporum f. sp. phaseoli (Zvirin et al. 2010; Garcia-Sanchez et al. 2010; Jimenez-Fernandez et al. 2013; Ortiz et al. 2014; Pouralibaba et al. 2016; (Abbasi et al. 2019).
CONCLUSION
The findings of the present study focused on the screening of Streptomyces griseus to control the phytopathogenic fungi Fusarium oxysporum f. sp. lycopersici. The best antifungal producer, Streptomyces griseus from the prawn cultivating soil proved to be capable of protecting tomato plants from Fusarium wilting under greenhouse conditions while confirmation that it promoted the plants growth was derived. Resistance process may functional through secondary metabolites by restricting the pathogen invasion and histological changes could be related to the growth regulators and phytohormones production in S. griseus treated plants. Hence plant growth promoting S. griseus can be used as biocontrol agent and better crop protection. Thus, the finding of present investigation holds a good promise in tomato wilt management.
Conflict of Interests: Authors declare no conflict of interests to disclose.
REFERENCES
Abbasi S, Safaie N, Sadeghi A, Shamsbakhsh M (2019). Streptomyces strains induce resistance to Fusarium oxysporum f. sp. lycopersici race 3 in tomato through different molecular mechanisms. Frontiers in Microbiology 10: 1505.
Abd-Allah E F (2001). Streptomyces plicatus as a model biocontrol agent. Folia. Microbiology 46: 309–314.
Akkopru A, and Demir S (2006). Biological control of fusarium wilt in tomato caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some Rhizobacteria. J. Phytopathology 153: 544–550.
Al-Ani RA, Adhab MA, Mahdi MH, et al. (2012). Rhizobium japonicum as a biocontrol agent of soybean root rot disease caused by Fusarium solani and Macrophomina phaseolina. Plant Protection Science 48(4): 149-155.
Al-Ani RA, and Adhab MA (2012). Protection of melon plants against Cucumber mosaic cucumovirus (CMV) infection using the biofeltilizer of Pseudomonas fluorescens. African Journal of Biotechnology 11(100): 16579-16585.
Al-Ani RA, and Adhab MA (2013). Bean yellow mosaic virus (BYMV) on broadbean: Characterization and resistance induced by Rhizobium leguminosarum. Journal of Pure and Applied Microbiology 7(1): 135-142.
Araujo JM, Silva AC, and Azevedo JL (2000). Isolation of endophytic actonomycetes from roots and leaves of maize (Zea mays L.). Biol. Technolology 43: 447-451.
Bargmann, C., and Schonbeck, F (1992). Acremonium kiliense as inducer of resistance to wilt diseases on tomatoes. J. Plant Dis. Protection 9: 266-272.
Barnawal D, Bharti N, Pandey SS, et al. (2017). Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol Plant 61: 502–514.
Bharathi R, Vivekananthan R, Harish S, et al. (2004). Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Protection 23: 835–843.
Bloemberg GV, and Lugtenberg BJ (2001). Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant biology 4: 343-350.
Cal AGD, Pascual S, and Melgarejo P (1995). Biological control of Fusarium oxysporum f. sp. lycopersici. Plant Pathology 44: 909-914.
Cal AGD, Pascual S, and Melgarejo P (1997). A rapid laboratory method for assessing the biological control of Penicillium oxalicum against Fusarium wilt of tomato. Plant Pathology 46: 699-707.
Cal AGD, Pascual S, and Melgarejo P (1997). Involvement of resistance induction by Penicillium oxalicum in the biocontrol of tomato wilt. Plant Pathology 46:72-79.
Cal AGD, Pascual S, Garcia-Lepe R, et al. (1997). Biological control of Fusarium wilt of tomato. IOBC Bull 20(4): 63-70.
Chen C, Belanger RR, Benhamou N, et al. (2000). Defense enzymes induced in cucumber roots by treatment with plant growth promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathology 56:13–23.
Compant S, Duffy B, Nowak J, et al. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology 71(9): 4951-4959.
Dias MP, Bastos MS, Xavier VB, et al. (2017). Plant growth and resistance promoted by Streptomyces sp. in tomato. Plant Physiol Biochem 118: 479–493.
Farrag AA (2011). Efficiency of different biocontol agents on both susceptible and resistant bean plants and their protein pattern consequences. Journal of American Science 7(4): 7– 14.
Fravel DR, Deahl KL, and Stomme JR (2005). Compatibility of the biocontrol fungus Fusarium oxysporum strain CS-20 with selected fungicides. Biological Control 34: 165–169.
Garcıa-Lepe R, and Melgarejo P (2000). Induced resistance by Penicillium oxalicum against of Fusarium oxysporum f. sp. lycopersici: histological studies of infected and induced tomato stems. Phytopathology 90: 260–268.
García-Sánchez MA, Martín-Rodrigues N, Ramos B, et al. (2010). Fost12, the Fusarium oxysporum homolog of the transcription factor Ste12, is upregulated during plant infection and required for virulence. Fungal Genetics and Biology 47: 216–225.
Ghazalibiglar H, Kandula DRW, and Hampton JG (2016). Biological control of fusarium wilt of tomato by Trichoderma isolates. New Zealand Plant Protection 57-63.
Gomez KA and Gomez AA (1984). Statistical procedures for agricultural research. John Wiley & Sons.
Goudjal Y, Toumatia O, Yekkour A, et al. (2014). Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiol Res 169: 59–65.
Goudjal Y, Zamoum M, Sabaou N, et al. (2016). Potential of endophytic Streptomyces spp. For biocontrol of Fusarium root rot disease and growth promotion of tomato seedlings. Biocontrol Sci Technolology 26(12):1691–1705.
Grimault V, and Prior P (1993). Bacterial wilt resistance in tomato associated with tolerance of vascular tissues to Pseudomonas solanacearum. Plant Pathology 42: 589-594.
ISTA (1996). International rules for seed testing. Proc. Int. Seed Test Association 31: 1–152.
Jiménez-Fernández D, Landa BB, Kang S, et al. (2013). Quantitative and microscopic assessment of compatible and incompatible interactions between chickpea cultivars and Fusarium oxysporum f.sp. ciceris races. PloS One 8(4): e61360.
Jones SE, Pham CA, Zambri MP, et al. (2019). Streptomyces volatile compounds influence exploration and microbial community dynamics by altering iron availability. mBio 10(2): e00171–19.
Kamal R, and Sharma A K (2014). Control of Fusarium wilt using biological agent Streptomyces sp. CPP-53 isolated from compost with plant growth promoting effect on tomato under greenhouse condition. Journal of Microbiology and Antimicrobials 6(6): 97-103.
Kanini GS, Katsifas EA, Savvides AL, et al. (2013). Greek indigenous streptomycetes as biocontrol agents against the soil-borne fungal plant pathogen Rhizoctonia solani. Journal of Applied Microbiology 114(5): 1468–1479.
Kanini GS, Katsifas EA, Savvides AL, et al. (2013). Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f. sp. lycopersici. BioMed research International 2013: 387230.
Kim JD, Han JW, Hwang IC, et al. (2012). Identification and biocontrol efficacy of Streptomyces miharaensis producing filipin III against Fusarium wilt. Journal of basic microbiology 52(2): 150-159.
Kim JD, Han JW, Lee SC, et al. (2011). Disease control effect of strevertenes produced by Streptomyces psammoticus against tomato fusarium wilt. J. Agric. Food Chemistry 59: 1893–1899.
Lasudee K, Tokuyama S, Lumyong S, et al. (2018). Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: evidence obtained from mung bean (Vigna radiata) and thai jasmine rice (Oryza sativa). Frontiers in microbiology 9: 1247.
Lee S, Yap M, Behringer G, et al. (2016). Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnology 3: 7 -12.
Lu Z, Tombolini R, Woo S, et al. (2004). In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter systems. Applied and Environmental Microbiology 70(5): 3073–3081.
Martínez-Medina A, Alguacil MDM, Pascual JA, et al. (2014). Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecolology 40: 804-815.
McGovern RJ (2015). Management of tomato diseases caused by Fusarium oxysporum. Crop Protection 73: 78-92.
Naik BS, Shashikala J, and Krishnamurthy YL (2008). Host growth characteristics influenced by seed inoculation with microorganisms. World Journal of Agricultural Sciences 4(5): 891-895.
Nejad P, and Johnson PA (2000). Endophytic bacteria induce growth promotion and wilt disease conditions. However, growth responses may change in suppression in oilseed and rape and tomato. Biol. Control 18: 208-215.
Nerey Y, Pannecoucque J, Hernandez HP, et al. (2010). Rhizoctonia spp. causing root and hypocotyl rot in Phaseolus vulgaris in Cuba. Journal of Phytopathology 158(4): 236–243.
Ortiz E, Cruz M, Melgarejo LM, et al. (2014). Histopathological features of infections caused by Fusarium oxysporum and F. solani in purple passionfruit plants (Passiflora edulis Sims). Summa Phytopathologica 40(2): 134-140.
Passari AK, Mishra VK, Singh G, et al. (2017). Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci Rep 7: 11809.
Passari AK, Upadhyaya K, Singh G, et al. (2019). Enhancement of disease resistance, growth potential, and photosynthesis in tomato (Solanum lycopersicum) by inoculation with an endophytic actinobacterium, Streptomyces thermocarboxydus strain BPSAC147. PloS one 14(7): e0219014.
Pouralibaba HR, Rubiales D, and Fondevilla S (2016). Identification of pathotypes in Fusarium oxysporum f.sp. lentis. European Journal of Plant Pathology 144(3): 539– 549.
Rafai IME, Susan MW, Omaima A et al. (2003). Biocontrol of some tomato disease using some antagonistic microorganism. Pakistan J. of Biological Science 6(4): 399 – 406.
Rashad YM, Al-Askar AA, Ghoneem KM, et al. (2017). Chitinolytic Streptomyces griseorubens E44G enhances the biocontrol efficacy against Fusarium wilt disease of tomato. Phytoparasit 45: 227.
Rey T, and Dumas B (2017). Plenty Is No Plague: Streptomyces Symbiosis with Crops. Trends in Plant Science 22(1): 30–37.
Shishido M, Miwa C, Usami T, et al. (2005). Biological control efficiency of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathology 95(9): 1072-1080.
Silva JCD, and Bettiol W (2005). Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Fitopatologia Brasileira 30(4): 409-412.
Verma NP, Kaur I, Masih H, et al. (2017). Efficacy of Trichoderma in controlling Fusarium wilt in tomato (Solanum lycopersicum L.). Research in Environment and Life Sciences 10(7): 636-639.
Whipps JM (2001). Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany 52: 487– 511.
Yigit F, and Dikilitas M (2007). Control of fusarium wilt of tomato by combination of fluorescent Pseudomonas, non-pathogen Fusarium and Trichoderma harzianum T-22 in greenhouse conditions. Plant Pathology Journal 4(2): 91 – 95.
Zamoum M, Goudjal Y, Sabaou N, et al. (2015). Biocontrol capacities and plant growth-promoting traits of endophytic actinobacteria isolated from native plants of Algerian Sahara. J Plant Dis Protect 122: 215–223.
Zvirin T, Herman R, Brotman Y, et al. (2010). Differential colonization and defence responses of resistant and susceptible melon lines infected by Fusarium oxysporum race 1.2. Plant Pathology 59: 576–585.


