Department of Biology, Faculty of Science, King Abdulaziz
University, Jeddah 21589, Saudi Arabia
Corresponding author email: majdahaburas@kau.edu.sa
Article Publishing History
Received: 10/07/2021
Accepted After Revision: 22/09/2021
The unregulated, discriminate and increasing applications of pesticides to enhance plant production and reduce vector-borne diseases caused environmental pollution and human health problems. Problems of contamination by pesticide must be alleviated by developing physical, chemical and/or biological methods to detoxify these compounds. Biodegradation is a promising and economic method to remove these compounds by breakdown to small inert end products. Different bacterial genera were active under their favorable environmental conditions due to production of some degradative enzymes. In biological processes, biodegradation of the different pesticides by naturally occurring microorganisms enhance soil fertility and quality, improve human health and preserve life on our earth.
Using bacteria for biodegradation is one of the most environmentally important processes because it is cost effective and quick processes which remove pesticide contamination from different environments effectively, easily and quickly. Genetically modified bacteria, immobilization of hydrolytic enzymes and application of the best conditions increased the degradation process. The use of the bacterial cells or their active enzymes with high capacity for pesticide degradation effectively hydrolyzes the toxic materials into less toxic and simple compounds. Diazinon from organophosphate pesticide was used mainly for control of red palm weevil. This review is an interesting attempt to approach bioremediation strategies of Organophosphate pesticide specially Diazinon in order to prevent the increasing earth pollution and contamination by dangerous compounds.
Biodegradation, Diazinon, Enzyme Organophosphate, Pesticide, Red Palm Weevil.
Aburas M. Experimental Insights into Biochemical Degradation of the Organophosphate Pesticide, Diazinon by Soil Microorganisms and Mechanisms of Actions. Biosc.Biotech.Res.Comm. 2021;14(3).
Aburas M. Experimental Insights into Biochemical Degradation of the Organophosphate Pesticide, Diazinon by Soil Microorganisms and Mechanisms of Actions. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/2WnCqEf“>https://bit.ly/2WnCqEf</a>
Copyright © Aburas This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The removal of wide range of pesticide groups from the environment requires knowledge of its concentration. In the environment, they can be degraded mainly by the action of indigenous bacteria or fungi through process called biodegradation which required further exploration in relation to total microbial population and their biochemical activities, environmental limiting factors, physiology and genetics of the degrading organisms (Singh and Walker, 2006, Bhattet al., 2019, Kaushal et al., 2021). Bioremediation technology was used to remove toxic materials from the environment. Studying physiologic, biochemical and genetic characters of bacteria may improve pesticide degradation process.
Genes encoding for the active enzyme were identified for several pesticides that provide better understanding and inputs to develop a super strain to achieve the desired effect of bioremediation. Organophosphate pesticides constitute a group of widely used, very heterogeneous compounds that share a phosphoric acid derivative chemical structure. The wide use of organophosphate pesticide has created numerous problems, including the pollution of the environment (Singh, 2008, Aly et al., 2017a, b, Sarkar et al., 2021).
The red palm weevil biocontrol: The red palm weevil is a member of Coleoptera which were belonging to family Curculionidae. The adult weevil is reddish brown beetle, capable of undertaking long flights due to strong wings. It was 3 cm long and had characteristic long curved rostrum. It is a serious pest of coconuts, originating in southern Asia and since the mid-1980s, it had advancing westwards very rapidly. It had reached the eastern region of the Kingdom of Saudi Arabia in 1985 and afterwards reached many other areas in the Kingdom (Abozuhairahet al., 1996). It was first recorded and spread in the United Arab Emirates since 1985 (El-Ezaby, 1998). The high rate of spread of this pest is by transporting infested young or adult date palm trees and offshoots from contaminated to uninfected areas where this insect infects mainly Phoenix dactylifera (Barranco et al., 2000).
Mature females put more than 200 eggs at the base of young date leaves. After infection, palm damage is produced mainly by the larvae which are visible as the first symptoms of the attack result in the death of the tree. No safe techniques for early detection of the pest have been reported. Intensive chemical treatments have been applied to protect or cure the Phoenix trees. Despite the difficulty in operating in the public garden’s environment, foliage spraying has been conducted with various insecticides.
Organophosphate pesticides, especially Diazinon were used mainly to control palm tree pests. Diazinon and preventive treatment of all the palms, even healthy ones, has been repeated once a month outside the tourist season (Gomez and Ferry, 1998). Kaushal et al. (2021) reported that biodegradation of Diazinon by bacterial cells is very important for faster clean of soil and water environments. Now due to harmful effects, many agents recommitted the no use of Diazinon to control palm bests and applied biological biocontrol methods.
Organophosphate pesticides: Due to rapidly growing population, it is necessary to increase food production which leads to increase use of chemical pesticides or xenobiotics to control pests (Ding et al., 2014, Pang et al., 2020). About 50% of annual food production was lost due to the pest attack (Odukkathil and Vasudevan 2013). The term pesticides include algaecides, antimicrobial agents, pheromones, avicides, biopesticides defoliants, desiccants, fumigants, fungicides, herbicides, insect growth regulators, insecticides, mitticide/acaricides, molluscicides, nematicide, ovicides, pesticides, predacides, repellents and rodenticides (Gavrilescu, 2005).
More than two million tons/year of pesticides were used. The highest pesticide-consuming countries were Italy, Turkey, Colombia, India and Japan (Verma et al., 2014) whiles USA exports each year big quantities of insecticides to the developing countries (WHO, 2017). According to Garrigouet al. (2019), about 90% of the used fungicides, 60% of herbicides and 30% of insecticides are reported as potent toxics and carcinogens materials (Table 1). Moreover, they decrease soil fertility and useful flora, increase soil acidity, nitrate leaching, floral and faunal resistance and cause groundwater pollution (Kumar et al, 2018, Sarkar et al., 2021).
The most used insecticides were organochlorides, organophosphates, carbamates, and pyrethroids (Aktar et al., 2009). It is clear that to decrease pollution, a single compound with numerous properties may be used like hexachlorophene, methiocarb, coumaphos and Diazinon. Farmers usually used pesticides without looking at their harmful worldwide effects and environmental problems. Now commercially, the degradable organophosphate pesticides are the most used group all over the world and have many worldwide applications (Kaushal et al., 2021).
Organophosphate insecticides are like chlorpyrifos, malathion, acephate, phosmet, dicrotophos and Diazinon. They are esters, amides, or thiols forms from derivatives of phosphoric, phosphonic, phosphinic or thiophosphoric acids which were coupled with two organic groups and a side chain consisting of cyanide, thiocyanate, or phenoxy groups (Balali-Mood and Abdollahi, 2014).
They are generally regarded as safe for use on crops and animals due to their relatively fast degradation rates. They are soluble in water with low persistence on foliage and are susceptible to degradation in the environment (Dhas and Srivastava, 2010, Ding and Tian, 2014). Due to the increased demand and consumption of pesticides, it is necessary to protect soil, water and air. In this sense, biological or chemical degradation processes have been intensively studied (Pang et al., 2020).
Table 1. Types of pesticides and their bad effects on human health (Mesnage and Séralini, 2018).
| Pesticide | Class | Health effect |
|
Insecticides |
Organophosphates | Neuropathy, myopathy, tremors, irritability, convulsions, inhibiting the enzyme acetylcholine esterase, paralysis |
| Carbamates | Inhibition of acetylcholine esterase enzyme, paralysis | |
| Organochlorines (dichlorodiphenyl methane and cyclodienes) | Stimulation of the nervous system by disrupting the sodium/potassium balance of the nerve fiber, tremors, irritability, convulsions, hyperexcitable state of the brain, cardiac arrhythmias and reproductive problems | |
| Herbicides | Phenoxy, benzoic acids, riazines, ureas and chloroacetanilides | Dermal toxicity, carcinogenic effect, damage to liver, thyroid, nervous system, bones, kidneys, blood and immune system
|
| Fungicides | Substituted benzenes, thiocarbamates, thiophthalimides, organomercury compounds etc. | Damage to liver, thyroid, nervous system, bones, kidneys, blood and immune system, carcinogenic property also |
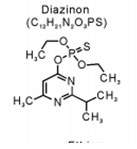
The insecticide Diazinon from the Organophosphate group: The insecticide Diazinon (Figure 1), O, O-diethyl O- [6-methyl-2-(1- methylethyl-4-pyrimidinyl)] ester is highly soluble in water (60 mg/l) and its half-life in soil was about 40 days while it requires 138 days for complete hydrolysis (Di-Bartolomeis et al., 2019). It is generally to control pests and reduce their harmful effects in agriculture sectors.
Diazinon was used for inhibition of a wide variety of insects like cockroaches, ants, and fleas in homes, big gardens or farms (Grube et al., 2011). It was found in many formulas as dusts, granules, seed dressings, powders or emulsion. WHO (2009) classified it as hazardous material which showed a moderate toxicity (class II) with IC50 ranged from 26 to300 mg/kg for oral administration and 379 mg/kg for dermal toxicity.
Figure 1: The molecular structure of the organophosphate pesticide Diazinon
Organophosphate pesticides -Degrading Microorganisms: To mitigate the problem of contamination by pesticide, treatments have been developed to detoxify and/or degrade these pesticides through physical, chemical and/or biological processes (Bhatt et al., 2020a,b,c). In biological processes, biological systems (whole cells or isolated enzymes) are used to catalyze chemical reactions that transform the pesticide into simpler and less toxic compounds or mineralize them into molecules. The search for microorganisms with high capacity for pesticide degradation is a very interesting attempt to approach bioremediation strategies and prevent contamination. To evaluate the potential of agricultural soils and solid organic waste cultures, important strategies for microbial isolation and screening of the potential insecticide degradation organisms must be applied (Singh, 2006, Mesnage and Séralini, 2018; Sarkar et al., 2021).
There is a significant role of metabolic activity of bacteria (including rhizosphere bacteria), fungi, actinomycetes and plants in Organophosphate pesticide degradation process. Bacteria are the potential degraders of complex molecules and most used pesticides for their own metabolism and growth (Hussain et al., 2009 and Sinha et al., 2009). Therefore, under laboratory conditions, the ability of microbes to decrease contamination has been studied and biodegradation techniques were faster than chemical or physical methods (Abraham et al., 2013).
Under environmental limiting nutrient conditions of carbon and/or phosphorus, most microorganisms degrade organophosphates compounds through oxidation, hydrolysis, and alkylation or dealkylation (Singh and Walker, 2006). Hydrolysis using enzymes like microbial hydrolase, phosphotriesterase, phosphatase and Carboxylesterases of P–O alkyl and aryl bonds were reported for organophosphate compounds biodegradation (Gao et al., 2014; Zuo et al., 2015). Phosphotriesterase degrades the triester bond of organophosphorus compounds and phosphodiesterase and monoesterase are essential to make the phosphorus atom available for uptake as a source of inorganic phosphorus (Cui, 2001, Lu et al., 2013, Sarkar et al., 2021).
Subsequently, many bacteria, fungi, algae, and cyanobacteria are active in biodegradation process of different pesticides, insecticides and herbicides. Flavobacterium sp. was isolated and was highly degraders of organophosphate compounds (Singh and Walker, 2006). Fungi such as Aspergillus niger, A. sydowii, A. fumigatus, Cladosporium cladosporioides and Penicillium raistrickii have been isolated from various contaminated sites and confirmed to be capable of degrading different organophosphate pesticides (Liu et al., 2001, Gao et al., 2014; Pandey et al., 2014, Alvarenga et al., 2014). Also, the algal genera Scenedesmus, Stichococcus and Chlorella (Megharaj et al., 1987; Caceres et al., 2009) and, the cyanobacterial genera, Nostoc, Anabaena and Oscillatoria have been established as organophosphates biodegraders (Ibrahim et al., 2014, Salman and Abdul-Adel, 2015, Sarkar et al., 2021).
Degradation of Diazinon by Bacteria: Diazinon is one of the most commonly and widely used commercial insecticides. It is a type of worldwide used organophosphate pesticide. The reported half-life of Diazinon in soil varies from 10-40 days (Aly et al., 2017a,b). Extensive use of Diazinon contaminates air, ground water, rivers and lakes. If the pesticide is not degraded or detoxified rapidly enough, the risk of its offsite migration may pose a health risk to humans. Catabolism and detoxification metabolism occurred when a soil microorganism uses the pesticide as a carbon and energy source. The biodegradation of Diazinon by soil bacteria has been reported by many workers where they use Diazinon as carbon and energy sources. Different bacterial species apparently showed different sensitivity to pesticides and can degrade pesticides with different ability (Hussain et al., 2009, Sinha et al., 2009, Feng et al., 2020b).
In some cases, bio- stimulation is required for in situ remediation. In spite of limitations, naturally occurring or native bacteria can be used for bioremediation process in a certain area while the use of genetically modified bacteria for bioremediation has high impact and long-term effects on the degradation processes. The rhizosphere of date palm trees contained many bacterial isolates that degradation Diazinon to CO2 and clean the environment and soil (Al- aidaroos, 2017). She added that mixed cultures could be relatively more effective in bioremediation of Diazinon from contaminated soil and water compared to bacterial mono-cultures. Proper optimization of treatment like duration and culture volume of bacteria mono- and mixed-cultures, prior to bioremediation process could yield better results. The results of biodegradation by both Pseudomonas and Bacillus suggested that no toxic intermediates during the degradation of Diazinon was detected and thus could be effectively utilized for the bioremediation process in contaminated soil and water (Kumar, 2016, Feng et al., 2020b).
Arthrobacter sp. was recorded as diazinon degrader by Ohshiro et al. (1996) and it can also use other organophosphate compounds like ethoprophos, fenitrothion, chlorpyrifos, isofenphos and parathion. From petroleum contaminated field, two species of genera Arthrobacter and Mycobacterium were isolated by Seo et al. (2007) and can efficiently degrade Diazinon. Biodegradation of Diazinon was also studied by Cycon et al. (2009) using Serratia liquefaciens, S. marcescens, and Pseudomonas sp. isolated from contaminated agricultural soil of Poland. Similarly, Cho et al. (2009) and Zhang et al. (2014) reported that Lactobacillus brevis, L. plantarum, and L. sakei can efficiently use the pesticide Diazinon as carbon and energy sources. From agricultural soil of Saudi Arabia and using enrichment technique, rod-shaped, Gram-negative Serratia marcescens was obtained on minimal salt medium.
It can completely degrade Diazinon (50 mg/ l) in 11 days and it can use successfully other organophosphate compounds like as chlorpyrifos, coumaphos, parathion and isazofos (Abo-Amer, 2011). Previous results (Table 2) have been reported that several bacterial species such as Pseudomonas sp. (Ramanathan, 1999), Agrobacterium sp., Flavobacterium sp. and Serratia marcescenscan utilize Diazinon as a source of carbon (Ohshiro, 1996, Ghassempour, 2002, Yasouri, 2006, Feng et al., 2020b, Kaushal et al., 2021).
It is well known that Pseudomonas species, known as a very metabolically bacterial species may contribute in biotransformation of other organophosphorus insecticides (Cycon, 2009, Ortiz-Hernandez, 2010). Serratia sp. seems to be an active bacterium that may contribute in complete degradation of Diazinon but there is little information concerning the pathways of utilization. Nevertheless, some studies reported the ability of Serratia to complete the degradation of other organ phosphorus insecticides, chlorpyrifos, fenitrothion, and parathion at 50 mg/l in 14 days in three different soils (Cycon et al., 2009, 2013).
Unfortunately, S. marcescens is a facultative anaerobe bacterium and is an opportunistic pathogen. Moreover, from sludge chlorpyrifos manufacturing plant in China, Stenotrophomonas sp. was isolated and showed excellent capacity in Diazinon and other organophosphate pesticides removal (Deng et al., 2015). Moreover, endophytic bacteria may hypothetically helpful for biocontrol pesticides in contaminated areas (Barman et al., 2014, Kaushal et al., 2021).
Table 2. Degradation of Diazinon by different microorganisms a and their sources of isolation
| Organo-phosphate pesticides | Microorganisms used for Degradation | Source of isolation (country) | Reference |
|
Diazinon
|
Arthrobacter sp. Mycobacterium sp. | Petroleum-contaminated soil (Hilo, Hawaii, USA) | Seo et al., 2007
|
| Trichoderma atroviride | Not mentioned
|
Tang et al., 2009 | |
| Leuconostoc mesenteroides
L. brevis L. plantarum L. sakei |
Kimchi during fermentation (Korea) | Cho et al., 2009 | |
| Serratia liquefaciens
S. marcescens Pseudomonas sp. |
Agricultural soil (Poland) | Cycon et al., 2009 | |
| Serratia marcescens | Agricultural soil (Saudi Arabia) | Abo-Amer, 2011
|
|
| Lactobacillus brevis | Zhang et al., 2014 | ||
| Stenotrophomonas sp. | Industrial sludge (China) | Deng et al., 2015
|
|
| Bacillus Sefensis 7 | Rhizosphere of Date palm tree | Aly et al., 2017b |
Diazinon degrading bacteria from date palm soils: The isolation of native bacterial species associated with date palm root soil was performed using different nonspecific media, in order to select a wide range of bacterial genera but presence of the synthetic pestside Diazinon in soil prevents the proliferation of bacteria. To determine the active bacteria, able to use Diazinon, bacterial isolation from different Diazinon contaminated soils must be carried out (Yasouri, 2006, Cycon 2009, Ortiz-Hernandez, 2010).
Many attempts to isolate Diazinon degrading bacteria from the rhizosphere of date palm plant have been carried out (Aly et al., 2017a,b). A wide diversity was detected into date palm rhizosphere bacterial community and total of 440 isolates were retrieved from seven analyzed stations of Date palm. To manage such a large set of isolates, 16S rRNA gene were amplified and used for molecular identification which represents a useful molecular tool for the discrimination of bacterial isolates up to the subspecies level (Ferjani et al., 2015, Sarkar et al., 2021).
Significant differences were observed in the degradation ability of the bacterial communities in the rhizosphere of the analyzed area. Presence of bacterial cell obtained from date palm soil enhanced the degradation of Diazinon (60 mg /l) compared to control where it takes 40 days for complete degradation but it was found that it takes 11 days using the isolate BMRF3, 9 days using isolate BMNF7 and 17 days using isolate BMTF8 (Alidros, 2017). Improvement of culture conditions like addition of glucose and yeast extracts, pH, temperature, incubation period and inoculums size increased degradation process. Furthermore, four isolates able to use Diazinon as of carbon and energy sources were obtained from soil samples collected from different sites by using an enrichment culture technique. All four isolates were Gram-negative, rod shaped, oxidase negative bacteria (Alipour et al., 2017, Sarkar et al., 2021).
Factors affecting biodegradation process: It is very difficult to precisely determine the part of particular microorganisms in pesticide destruction, since there are many factors that have an effect on this process. Moreover, microorganism activity may be specific to chemical structure, chemical binding, or a group of selected substances. In soil combination of chemical and biological process was noticed during decomposition synthesized pesticide. Biological degradation is faster and eco-friendly method of converting pesticides by microbial enzymes into simple non-toxic.
The success and the failure of biological treatment depends on factors that affect the degradation such as the competitive ability of the suitable microorganisms, moisture level, pH, temperature, salinity, nutrient and water contents, light intensity and oxygen concentration in addition to pesticide structure, molecular weight, functional groups, concentration and solubility in water (Goldstein et al., 1985, Geer and Shelton, 1992, Alexander, 2000, Aly et al., 2017a, Matsuda et al., 2020).
Organic matter content: The organic matter content of the soils had no apparent effect on fenamiphos or chlorpyrifos degradation while a previous report indicated that high organic matter reduced degradation (Karpouzas and Walker, 2000b). High organic matter leads to reduced bioavailability of substrate to the degrading microorganisms. However, many degrading microorganisms produce surfactants or other emulsifiers that desorb chemical compounds from soil and make them bioavailable (Weber and Huang, 1996, Aly et al., 2017b).
Presence of glucose enhanced organophosphate pesticide degradation rates. The degradation rate of Diazinon in medium supplemented with succinate or glucose by strain DI101 was increased to 15 days with degradation rates of 29.3/day compared to 11 days and degradation rates of 40.7/ day for control. The degradation pattern of the strain was greatly affected by the presence of other carbon sources. There was almost no degradation of Diazinon through the first 6 days in the presence of succinate or glucose. However, after 7 days, Diazinon was degraded rapidly in these two modified media.
The relative degradation rates of Diazinon in medium without addition of carbon source were significantly different as compared with medium supplemented with carbon sources. Maximum organophosphate compounds hydrolysis of about 84.5% was observed by bacterial isolate in presence of glucose as compared to 73.3% in absence of glucose while fungal isolate had 76% hydrolysis in presence of glucose and only 58% in absence of glucose. The two isolates were resistant to chlorpyrifos at 10 ppm therefore; these isolated could be potential candidates for microbe mediated bioremediation of chlorpyrifos contaminated soils (Hindumathy, 2013, Aly et al., 2017b).
On contrast, the addition of glucose to the soil samples significantly reduced the initial degradation rate but this lag phase was followed by a log phase. This result contrasts with previous findings of Karpouzas and Walker (2000a, b), where addition of glucose stimulated the degradation rate of ethoprophos. However, initial inhibition of pesticide degradation in the presence of glucose can be attributed to the environmental adaptation of bacterial isolates where easily available and rich carbon sources are preferentially utilized. Once the readily available carbon source is depleted, the bacteria begin to utilize the pesticides. This approach gives the bacterial isolates a competitive advantage since they are able to utilize both readily available and less available carbon sources. However, the addition of other carbon sources such succinate or glucose stopped the degradation of Diazinon. When these carbon sources were exhausted, it then degraded Diazinon as a source of carbon. Environmental adaptation of bacteria in natural environment for the competition for carbon sources is massive and the utilization of Diazinon as an energy source by bacteria increased with a significant competitive.
Phosphorus concentration: Degradation rates of Diazinon in MSM supplemented with phosphorus and MSM without phosphorus source were not significant (p<0.005). Diazinon in each medium was completely degraded by the strain DI101 on day 11, with degradation rates of 0.241/day for MSM with phosphorus and 0.221 /day for MSM without phosphorus. Therefore, the degradation of Diazinon was quite similar in both media and was not affected by the absence or presence of phosphorus source. Moreover, enzymatic assays of the strain DI101 gave positive results for both phosphodiesterase and alkaline phosphatase activities. The utilization of organophosphorus insecticides as a source of phosphorus by DI101 is a significant observation. There are few reports in which an organophosphorus compound was used as a source of carbon and phosphorus by a single species such as Enterobacter strain B-14, which could use organophosphorus chlorpyrifos as a source of carbon and phosphorus (Eissaet al., 2014). However, Flavobacterium species could use parathion as a source of phosphorus but not carbon and diazinon as a carbon source. Nevertheless, a Flavobacterium strain was not able to utilize organic phosphorus as a source of phosphorus (Sethunathan, 1973). Moreover, a variety of bacteria that could utilize phosphorothionate or phosphorodithionate compounds as a source of phosphorus were unable to use these compounds as a carbon source (Rosenberg, 1979 Matsuda et al., 2020).
Concentration of pesticide and inoculum density: Concentration of pesticide had no apparent effect on the degradation rate except the longer initial lag phase which may be due to the need for greater numbers of bacteria to initiate rapid degradation of the pesticides. Also, Time is required for propagation of a small population of pesticide-degrading microorganisms to reach the essential level for efficient degradation of pesticide (Karpouzas and Walker, 2000b). Inoculum density had a noticeable effect on degradation of fenamiphos and chlorpyrifos. No degradation of fenamiphos was observed in soils inoculated with o104cfu/g. Similarly, Enterobacter sp. was not capable to degrade chlorpyrifos under an inoculum density of 103 cells/g. Similar results were cited by Ramadan et al., (1990) who found that a Pseudomonas sp. did not use p-nitrophenol at a density less than 104 cells/ml.
Lower inoculum densities mean the small number of bacteria which was not able to survive the initial competition and population decline. Similar results were obtained by Comeau et al. (1993) who reported degradation by Pseudomonas cepacia. The bioavailability of the pollutant is one of the most important factors. There was no effect of ageing for 60 days on subsequent fenamiphos degradation. In contrast, chlorpyrifos was degraded rapidly in all inoculated soil samples, at the end of the 10 days incubation. Similar results were obtained by Cullington and Walker (1999) and Karpouzas and Walker (2000b). Previous studies with a range of pollutants have demonstrated that increased soil residence time of the compound leads to a reduction in bioavailability (Blair et al., 1990; Chung and Alexander, 1998; Alexander, 2000, Matsuda et al., 2020).
The above findings may explain why bioremediation by microorganisms often does not result in total elimination of target contaminants. The effect of the inoculum density on the degradation of Diazinon is illustrated by many authors (Abo-Amer, 2011, Aly et al., 2017b). The data indicated that there were significant differences in the relative degradation rates of Diazinon between inoculum densities. At the high inoculum density (106 CFU/ml), Diazinon was degraded completely on day 11, with a relative degradation rate of 36.37/day. At moderate inoculum densities of 104 and 102cfu/ml, degradation of Diazinon was complete on days 13 and 16, respectively, having relative degradation rates of 30.10and 29.15 mg/day, respectively. However, the relative degradation rate (4.364 /day) was slower at lower density (50 cfu/ml); that is only 37% of Diazinon degradation on day 16 was detected. No apparent Diazinon removal was detected in the control cultures. Abo-Amer (2011) noticed that Diazinon was hydrolyzed in liquid medium within 11 days by phosphotriesterase of cells inoculated at inoculum density of 106/ ml.
They added that 106 CFU/g of was used to inoculate soils, and this inoculum density appeared to be able to degrade Diazinon completely (As revealed in other reports, inoculum density is a significant factor determining the effective biodegradation of applied pesticides. In other study, lower inoculation densities of strain Sphingomonas chlorophenolica RA2 (104 – 106 cfu/g soil) resulted in strongly decrease mineralization rate of pentachlorophenol and 104 cfu/g soil was not significantly differing from the control (Miethling, 1996). The introduction of 104 cfu/g soil of Chelatobacter heintzii resulted in a 3-fold increase of atrazine mineralization capacity (Rousseaux, 2003). The addition of Enterobacter asburiae B-14 (106 cfu/ g) to soil with a low indigenous population of chlorpyrifos-degrading bacteria supplemented with 35 mg/ kg of chlorpyrifos resulted in a higher degradation rate than that was observed in non-inoculated soils (Singh et al., 2003, 2004).
In addition, Ramadan et al. (1990) reported that when lower inoculum densities (less than 104 cfu/g soil) were added only a small numbered of bacteria can survive due to competition with other bacteria, thus low numbers contribute in pesticide degradation. At low inoculum density of 103cfu/g soil of Enterobacter sp., no degradation of chlorpyrifos was noticed in soil (Singh et al., 2004). A higher initial inoculum density can compensate for the initial population decline, and the survivors can multiply and degrade pollutants. Moreover, these results supported the view that particular species of soil microorganisms fluctuate in their general activity responding to degradation of pesticides.
Some physical factors: The effect of temperature on Diazinon degradation by strain DI101 was significant and the most rapid degradation was recorded at 20˚C, 25˚C, and 30˚C, with relative degradation rates of 28.08, 32.99, and 36.57/day, respectively. On the other hand, the slowest degradation was determined at the two extreme temperatures (10 and 40˚C) with relative degradation rates of 0.724 and 1.624/day, respectively. Complete Diazinon removal was observed on day 11 when incubated at 25˚C and 30˚C, but on day 13 at 20˚C. These results were expected, since most members of the family Enterobacteriaceae grow well at 37˚C (Abo-Amer, 2011). Similar results reported that Serratia marcescens growth and hexachlorobutadiene degradation were at 25 to 30˚C (Li et al., 2008) while they were 20-40ᵒC and pH 7.5 for Burkholderia sp. FDS-1 (Hong et al., 2007, Matsuda et al., 2020).
Moisture content is important for availability of chemical materials and for movement and proliferation of microorganisms. Degradation was the slowest at low moisture contents (Singh et al., 2004). Also, soil pH had a marked influence on pesticide degradation by the bacterial isolates used for inoculation. Degradation rate for ethorprophos by Pseudomonas putida was similar at neutral and slightly alkaline pH (Karpouzas and Walker, 2000b) while Vidali (2001) reported that pH 5.5-8.8 is required for most soil bacteria with optimum pH between 6.5 and 8.
The changes in relative degradation rates of bacteria in the presence of different pHs were significant. Diazinon was completely degraded by strain DI101 with relative degradation rates of 32.6, 31.8 and 30.8/day when pH values were 7, 7.5, and 8, respectively. However, the relative degradation rates of Diazinon were low at pH 6 and pH 9 (Abo-Amer, 2011). They added that degradation of Diazinon was negligible at pH 5 and pH 10 as well as in the control. Diazinon biodegradation was approximately inhibited in relatively acidic (pH 5) and alkaline conditions (Aly et al., 2017b, Matsuda et al., 2020).
Immobilization methods used for biodegradation process: Immobilization of bacteria on different supports increased degradation. Entrapment of enzymes and/or cells in alginate is one of the simplest methods of immobilization. Alginates are available commercially as water-soluble sodium alginates, and have been used for more than 65 years in the food and pharmaceutical industries as thickening, emulsifying, film forming, and gelling agents. Entrapment in insoluble calcium alginate gel is recognized as a rapid, nontoxic, inexpensive, and versatile method for immobilization of enzymes and cells (Datta et al., 2013). The procedure of immobilization in alginate beads is not only inexpensive but also easy to carry out and provides extremely mild conditions, so there is a higher potential for industrial application (Rios et al., 2019, Feng et al., 2020b).
The immobilization strategies were used for some enzymes to facilitate the degradation of organophosphate pesticides. Enterobacter aerogenes contained golycero-phosphodiesterase enzyme which was immobilized on a nano-materials and results revealed that the immobilized system worked actively for numerous cycles, thus it may be used commercially for purification of water contaminated with organophosphate pesticides (Daumann et al., 2014). The covalently immobilized enzyme A (Opd A) on porous and nonwoven polyester fabric showed high ability for degradation of organophosphate pesticides (Zhang and Qiao, 2002, Gaoa et al., 2014, Feng et al., 2020b) and immobilization process increased the Km, stability and pH range of the enzyme for methyl parathion. The immobilized enzymes removed 50 μM/cycle and we active for more than two months.
Molecular Biology of Pesticides Degradation: To protect crops from harmful insects and to increase their productivity and yields, pesticides are frequently used and due to the excessive use of pesticides the activity of some microbial enzymes in soil which are indicators of soil health are of great significance. In soil, microbial enzymes had significant roles in degradation of natural and synthetic organic compounds. Enzyme activity is closely correlated to microbial activity. To evaluate the effects of pesticides, molecular techniques were used to detect the microbial community changes in structures and functions (Parween et al., 2016).
For large-scale and effective bioremediation, molecular tools help to identify related genes of certain enzymes ineffective bacteria or fungi. In pesticide contamination sites, biodegradation of pesticides by some degrading genes of soil microorganisms were studied using recombinant DNA technology (Parween et al., 2016). The microorganisms degrading ability of toxic pollutants from the environment depend on their genetic content of the cells (Matsuda et al., 2020, Feng et al., 2020b). Deoxyribose nucleic acid coding genes of chromosome or plasmid were activated to form of mRNA which was translated to specific protein act as degradative and detoxifying enzymes (Guo et al., 2020).
Many enzymes in recombinant bacteria were promising for detoxification and biodegradation of organophosphorus pesticides. Methyl parathion hydrolysis occurred 25-fold faster than in wild type Escherichia coli while 100% paraoxon and 80% of Malathion were degraded after 45 days by Escherichia coli, Pseudomonas putida or Moraxella sp. Ice nucleation protein (Hussain et al. (2009). Bacteria may have many copy numbers of degradative enzymes like as esterase, monooxygenases, glutathione S-transferase, and P450s which removed insecticides and toxins by increasing mRNA levels of the important enzymes (Li et al., 2007).
Cytochrome P450 is well known in oxidation, hydroxylation or degradation of many industrial toxins (Scott et al., 2008). The gene (ophB) encoding a protein from Pseudomonas sp. BF1–3, specific for the organophosphate, Chlorpyrifos degradation was cloned in E. coli which degrade 95% of the organophosphate pesticide in 9 days. The previous enzyme showed excellent activity at 35◦C at pH of 8 (Barman et al., 2014, Matsuda et al., 2020).
CONCLUSION
With the knowledge that microbes can degrade xenobiotics such as pesticides, researchers are now focusing on microbial diversity, particularly at contaminated sites. Among all the microbes, bacterial degradation has been extensively studied worldwide. This review summarizes the modes and pathways for microbial degradation of organophosphate pesticides and bacteria degradation of the most commonly used organophosphate pesticides.
The conditions under which the bacteria are isolated are crucial not only with the desired degradative enzyme systems, but also with specific regulation mechanisms for the degradation pathways. However, the utilization of Diazinon as a source of phosphorus attributed to the presence of phosphodiesterase and phosphomonoesterase activities in the bacterial strains.
REFERENCES
Abo-Amer AE. (2011). Biodegradation of diazinon by Serratia marcescens DI101 and its use in bioremediation of contaminated environment. J Microbiol Biotechnol. 21: 71–80.
Abo-Amer AE. (2012). Characterization of a strain of Pseudomonas putida isolated from agricultural soil that degrades cadusafos (an organophosphorus pesticide). World J Microbiol Biotechnol. 28: 805–814.
Abozuhairah RA, Vidyasagar P.S and Abraham V.A. (1996). Integrated management of red palm weevil, Rhynchophorus ferrugineus in date palm plantations of the Kingdom of Saudi Arabia. Proceedings of the International Congress of Entomology. Firenze, Italy, August 1996: 541.
Abraham J, Silambarasan S. (2013). Biodegradation of chlorpyrifos and its hydrolyzing metabolite 3,5,6-trichloro-2-pyridinol by Sphingobacterium sp. JAS3. Process Biochem., 48: 1559– 1564.
Aktar MW, Sengupta D, Chowdhury A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology, 2(1):1–12.
Al- aidaroos B. (2017). Biodegradation of the Organophosphorus Insecticide Diazinon by bacteria isolated from rhizosphere of Date palm tree. PhD thesis, Faculty of Science, KAU, Jeddah, Saudi Arabia.
Alexander M. (2000). Aging, bioavailability and overestimation of risk from environmental pollutants. Environmental Science and Technology 34, 4259–4265.
Alipour A, Alizadeh A, khodaygan P (2017). Isolation and identification of Diazinon degrading bacteria and residue concentrations of Diazinon by HPLC. Metal matrix composites, 11: 12129.
Alvarenga N, Birolli W G, Seleghim M H R, Porto A L M. (2014). Biodegradation of methyl parathion by whole cells of marine-derived fungi Aspergillus sydowii and Penicillium decaturense. Chemosphere. 117: 47–52.
Aly MM, Al-aidaroos B. A. and Alfassi FA. (2017a). Pesticides Characters, Importance and Microbial Degradation IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) Vol. 12(2):20-28.
Aly MM, Al-aidaroos B. A. and Alfassi FA. (2017b). Factors Affecting Biodegradation of the Organophosphorus Insecticide Diazinon by Bacterial Mono-Culture of Bacillus sefensis 7, Isolated from the Rhizosphere of Date Palm Tree. IOSR Journal of Pharmacy and Biological Sciences, Vol. 12(3):18-26.
Balali-Mood M and Abdollahi M. (2014). Basic and Clinical Toxicology of Organophosphorus Compounds. New York, NY; Heidelberg; Dordrecht; London: Springer-Verlag.
Barman D N, Haque A , Islam A , Yun H D, Kim M K. (2014). Cloning and expression of ophB gene encoding organophosphorus hydrolase from endophytic Pseudomonas sp. BF1-3 degrades organophosphorus pesticide chlorpyrifos. Ecotoxicol. Environ. Saf., 108, pp. 135-141.
Barranco, P., De La Peña J., Martin M.M. and Cabello T. (2000). Rango de hospedantes de Rhynchophorus ferrugineus (Olivier,1790) y diámetro de la palmera hospedante. (Coloptera, Curculionidae). Boletin de Sanidad Vegetal Plagas 26: 73–78.
Bhatt P, Bhatt K, HuangY, Lin Z and Chen S. (2020a). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 244:125507.
Bhatt P, Huang Y, ReneER, Kumar AJ and Chen S. (2020b). Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 305:123074.
Bhatt P, Huang Y, Zhan, H and Chen S. (2019). Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 10:1778.
Bhatt P., Zhang, W., Lin, Z., Pang, S., Huang, Y., and Chen, S. (2020c). Biodegradation of allethrin by a novel fungus Fusarium proliferatum strain CF2, isolated from contaminated soils. Microorganisms 8:593.
Caceres T P, Megharaj M, Malik S, Beer M, Naidu R. (2009). Hydrolysis of fenamiphos and its toxic oxidation products by Microbacterium sp. in pure culture and groundwater. Bioresour Technol. 100: 2732–2736.
Cho K M, Math R K, Islam S M A, Lim W J, Hong S Y, Kim J M, Yun M G, Cho J J, Yun H D. (2009). Biodegradation of chlorpyrifos by lactic acid bacteria during kimchi fermentation. J Agric Food Chem. 57: 1882–1889.
Cui Z L, Li S P, Fu G P. (2001). Isolation of methyl parathiondegrading strain M6 and cloning of the methyl parathion hydrolase gene. Appl Environ Microbiol. 67: 4922–4925.
Cycon M, Zmijowska A, W´ojcik M, Piotrowska-Seget Z. (2013). ˙ Biodegradation and bioremediation potential of diazinondegrading Serratia marcescens to remove other organophosphorus pesticides from soils. J Environ Manage. 117: 7–16.
Cycon, M., Wojcik, M. and Piotrowska-Seget, Z. (2009). Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere, 76, 494-501.
Datta S, Christena LR, Rajaram YR. (2013). Enzyme immobilization: an overview on techniques and support materials. 3 Biotech.; 3(1):1-9.
Daumann L J., Larrabee JA, Ollis D, Schenk G, Gahana L R. (2014). Immobilization of the enzyme GpdQ on magnetite nanoparticles for organophosphate pesticide bioremediation. J. Inorg. Biochem., 131, pp. 1-7.
Dhas S and Srivastava M. (2010). An Assessment of carbaryl residues on brinjal crop in an agricultural field in Bikaner, Rajasthan (India). Asian Journal of Agricultural Science, 2: 15–17.
Di-Bartolomeis M, Kegley S, Mineau P, Radford R, Klein K. (2019). An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. Tosi S, editor. PLOS ONE, 14: e0220029.
Ding G and Tian Y., 2014. Organophosphate pesticide exposure and child health in China. Environ Sci Pollut Res Int. 21(1):759-61.
Eissa FI, Mahmoud HA, Massoud ON, Ghanem KM and Gomaa I M (2014). Biodegradation of Chlorpyrifos by Microbial Strains Isolated from Agricultural Wastewater. J Am Sci., 10(3):98-108.
El Ezaby FA., Khalifa O. and El Assal A. (1998). Integrated pest management for the control of red palm weevil Rhynchophorus ferrugineus Oliv. In The United Arab Emirates, Eastern Region, Al Ain. In Rahman-Al Afifi, M. A. & Al-Sherif Albadawy, A. (Eds) Proceedings of the First International Conference on Date Palms. Al-Ain, UAE, 8-10 March. Faculty of Agricultural Sciences, UAE University: 269–281.
Feng Y, Huang, Y, ZhanH, Bhatt H and Chen S (2020). An overview of strobilurin fungicide degradation: current status and future perspective. Front. Microbiol., 10:389.
Ferjani R, Marasco R, Rolli E, Cherif H, Cherif A, Gtari M, Boudabous A, Daffonchio D and Ouzari H. (20150. The Date Palm Tree Rhizosphere Is a Niche for Plant Growth Promoting Bacteria in the Oasis Ecosystem. BioMed Research International Vol. 2015, Article ID 153851, 10 pages.
Gao Y, Truong YB, Cacioli P, Butler P, Kyratzis IL. (20140. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzyme Microb Technol 54:38–44
Gaoa Y, Truonga Y B, Cacioli P, Butlerb P, Kyratzisa I, (2014). Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym. Microb. Technol., 54: pp. 38-44.
Garrigou A, Laurent C, Berthet A, Colosio C et al. (2019). Critical Review of the Role of PPE in the Prevention of Risks Related to Agricultural Pesticide Use’, Safety Science 123(104527).
Gavrilescu M (2005). Fate of pesticides in environment and its bioremediation. Eng Life Sci 5 (6):497–526.
Geer, L.E., Shelton, D.R. (1992). Effect of inoculant strain and organic matter content on kinetics of 2,4-dichlorophenoxyacetic acid degradation in soil. Applied Environmental Microbiology 58, 1459–1465.
Ghassempour A, Mohammadkhah A, Najafi F and Rajabzadeh M. (2002). Monitoring of the pesticide diazinon in soil, stem and surface water of rice fields. Anal. Sci., 18, 779-783.
Goldstein, R.M., Mallory, L.M., Alexander, M (1985). Reason for possible failure of inoculation to enhance biodegradation. Applied Environmental Microbiology 50, 977–983.
Gomez Vives S and Ferry M. (1999). Attempts at biological control of date-palm pests recently found in Spain. In: Canard M. & Beyssatarnaouty V. (Eds) Proceedings of the First Regional Symposium for Applied Biological Control in Mediterranean Countries. Cairo, 25–29 October 1998. Imprimerie Sacco, Toulouse, France: 121–125.
Grube A, Donaldson D, Kiely T, Wu L (2011). Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates. Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency, Washington, D.C.
Guo, L., Dai, Z., Guo, J., Yang, W., Ge, F., and Dai, Y. (2020). Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water. AMB Express 10:7.
Huang X J, Lee L S, Nakatsu C (2000). Impact of animal waste lagoon effluents on chlorpyrifos degradation in soils. Environ Toxicol Chem. 19: 2864–2870.
Hussain S, Siddique T, Arshad M, Saleem M. (2009). Bioremediation and phytoremediation of pesticides: recent advances. Crit Rev Environ Sci Technol 39(10):843–907
Ibrahim W M, Karam M A, El-Shahat R M, Adway A A. (2014). Biodegradation and utilization of organophosphorus pesticide malathion by Cyanobacteria. BioMed Res Int. 2014: 392682.
Karpouzas, D.G., Walker, A (2000b). Factors influencing the ability of Pseudomonas putida epI to degrade ethoprophos in soil. Soil Biology & Biochemistry 32, 1753–1762
Karpouzas, D.G., Walker, A. (2000a). Factors influencing the ability of Pseudomonas putida strains epI and II to degrade the organophosphate ethoprophos. Journal of Applied Microbiology 89, 40–48.
Kaushal J., Khatri, M. and Arya, S.K. (2021). A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicology and Environmental Safety, Volume 207, 111483,
Kumar S, Kaushik G, Dar M A, Nimesh S, López-Chuken U, Villarreal-Chiu J F. (2018). Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere,Volume 28, Issue 2, Pages 190-208.
Kumar S, Kaushik G, Villarreal-Chiu J F (2016). Scenario of organophosphate pollution and toxicity in India: A review. Environ Sci Pollut Res. 23: 9480–9491.
Li X H, Jiang J D, Gu L F, Ali S W, He J, Li S P (2008). Diversity of chlorpyrifos-degrading bacteria isolated from chlorpyrifos contaminated samples. Int Biodeterior Biodegrad. 62: 331– 335.
Li, X., Schuler, M. A., and Berenbaum, M. R. (2007). Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253.
Liu Y H, Chung Y C, Xiong Y. (2001). Purification and characterization of a dimethoate-degrading enzyme of Aspergillus niger ZHY256, isolated from sewage. Appl Environ Microbiol. 67: 3746–3749.
Lu P, Li Q F, Liu H M, Feng Z Z, Yan X, Hong Q, Li S P (2013). Biodegradation of chlorpyrifos and 3,5,6-trichloro-2- pyridinol by Cupriavidus sp. DT-1. Bioresour Technol. 127: 337–342.
Matsuda, K., Ihara, M., and Sattelle, D. B. (2020). Neonicotinoid insecticides: molecular targets, resistance, and toxicity. Ann. Rev. Pharmacol. Toxicol. 60, 241–255.
Megharaj M, Venkateswarlu K, Rao A S., (1987). Metabolism of monocrotophos and quinalphos by algae isolated from soil. Bull Environ Contam Toxicol. 39: 251–256.
Mesnage R and Séralini G (2018). Editorial: Toxicity of Pesticides on Health and Environment. Front. Public Health doi: 10.3389/fpubh.2018.00268.
Odukkathil G, Vasudevan N (2013). Toxicity and bioremediation of pesticides in agricultural soil. Rev Environ Sci Bio/Technol 12:421–444.
Ohshiro K, Ono T, Hoshino T, Uchiyama T. (1997). Characterization of isofenphos hydrolases from Arthrobacter sp. strain B-5. J Ferment Bioeng. 83: 238–245.
Ortiz-Hernandez, M.L. and Sanchez-Salinas, E. (2010). Biodegradation of the organophosphate pesticide tetrachlorvinphos by bacteria isolated from agricultural soils in Mexico. Rev. Int. Contam. Ambient, 26, 27-38.
Pandey B, Baghel P S, Shrivastava S. (2014). To study the bioremediation of monocrotophos and to analyze the kinetics effect of Tween 80 on fungal growth. Indo Amer J Pharm Res. 4: 925–930.
Pang S, Lin Z, Zhang W, Mishra S, Bhatt P and Chen S (2020). Insights Into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 11:868.
Parween T, Jan S, Mahmooduzzafar S, Fatma T And Siddiqui Z H (2016). Selective Effect of Pesticides on Plant: A Review. Critical Reviews in Food Science and Nutrition, 56:160–179.
Ramadan, M.A., EL-Tayeb, O.M., Alexander, M., 1990. Inoculum size as a factor limiting success of inoculation for biodegradation. Applied and Environmental Microbiology 56, 1392–1396.
Ramanathan M.P. and Lalithakumari D., 1999. Complete mineralization of methylparathion by Pseudomonas sp. A3. Appl. Biochem. Biotechnol., 80, 1-12.
Rios, N. S., Arana-Peña, S., Mendez-Sanchez, C., Lokha, Y., Cortes-Corberan, V., Gonçalves, L. R. B., et al., 2019. Increasing the enzyme loading capacity of porous supports by a layer-by-layer immobilization strategy using PEI as glue. Catalysts 9:576.
Rosenblatt DH, MillerTA, Dacre JC, Muul I & Cogley DR., 975. Problem Definition Studies on Potential Environmental Pollutants. II. Physical, Chemical, Toxicological, and Biological Properties of 16 Substances,Tech rpt 7509; AD A030428.
Salman J M, Abdul-Adel E., (2015). Potential use of cyanophyta species Oscillatoria limnetica in bioremediation of organophosphorus herbicide glyphosate. Mesop Environ J. 1: 15–26.
Sarkar S, Dias J, Bernardes Gil, Möhring N, Jansen K., (2021). The use of pesticides in developing countries and their impact on health and the right to food. European Parliament’s Committee on Development, PE 653.622 – January 2021.
Scott, C., Pandey, G., Hartley, C. J., Jackson, C. J., Cheesman, M. J., Taylor, M. C., et al., (2008). The enzymatic basis for pesticide bioremediation. Indian J. Microbiol. 48, 65–79.
Seo J S, Keum Y S, Harada R M, Li Q X. (2007). Isolation and characterization of bacteria capable of degrading polycyclic aromatic hydrocarbons (PAHs) and organophosphorus pesticides from PAH-contaminated soil in Hilo, Hawaii. J Agric Food Chem. 55: 5383–5389.
Sethunathan N and Yoshida (1973). A Flavobacterium that degrades diazinon and parathion. Can J Microbiol., 127:2123-2129.
Singh H. (2006). Mycoremediation: Fungal bioremediation. John Wiley & Sons Inc, New Jersey.
Singh B K, Walker A. (2006). Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev., 30:428–471.
Singh BK, Walker A, Morgan JA, Wright DJ. (2003). Effects of soil pH on the biodegradation of chlorpyrifos and isolation of a chlorpyrifos-degrading bacterium. Appl Environ Microbiol., 69:5198–5206.
Singh BK, Walker A, Morgan JAW, Wright DJ. (2004). Biodegradation of Chlorpyrifos by Enterobacter Strain B-14 and its use in Bioremediation of Contaminated Soils. Appl Environ Microbiol., 70:4855–4863.
Singh DK. (2008). Biodradation and bioremediation of pesticide in soil: Concept, method and recent developments. Indian J Microbiol.,48:35–40.
Sinha S, Chattopadhyay P, Pan I, Chatterjee S, Chanda P, Bandyopadhyay D, Das K, Sen SK. (2009). Microbial transformation of xenobiotics for environmental bioremediation. Afr J Biotechnol., 8(22):6016–6027
Verma JP, Jaiswal DK, Sagar R. (2014). Pesticide Relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Bio/Tech., 13:429–466.
Vidali M. (2001). Bioremediation: An overview. Pure Applied Chemistry 73, 1163–1172.
Weber W.J, Huang W. (1996). A distributed reactivity model for sorption by soil and sediments. Intraparticle heterogeneity and phase-distribution relationships under nonequilibrium conditions. Environmental Science and Technology 30, 881–888.
World Health Organization, WHO (2009). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. WHO, Geneva.
World Health Organization. WHO (2017). Don’t Pollute My Future! The Impact of the Environment on Children’s Health, Licence: CC BY-NC-SA 3.0 IGO, p. 7.
Yasouri, F.N., 2006. Plasmid mediated degradation of diazinon by three bacterial strains, Pseudomonas sp., Flavobacterium sp. and Agrobacterium sp. Asian. J. Chem.,18, 2437-2444.
Zhang Y H, Xu D, Liu J Q, Zhao X H (2014). Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem. 164: 173–178.


