1Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2Department of Human Nutrition, University of Agriculture Peshawar, Pakistan.
Corresponding author email: Yasirpcsir2006@gmail.com
Article Publishing History
Received: 18/07/2021
Accepted After Revision: 28/09/2021
As opportunistic bacterium Acinetobacter baumannii has the capability to develop resistance against different types of antibiotics. The goal of the research was to isolate and molecularly identify antibiotic resistance pathogenic bacteria from hospital and its bio-control using Coleus forskohlii ethanolic extract. For this about 78 swab samples were collected from different areas of the Prince Meshari Bin Saud General Baljarshi Hospital. The pathogenic bacteria were molecularly identified using 16s rRNA sequencing. In addition, the resistance profile of the strains was checked using different antibiotics. These includes Tetracyline (T), Chloramphenicol (C), Penicillin G (PG), Streptomycin (S), Erythromycin (E), Fusidic Acid (FC), Oxacillin (OX), Novobiocin (NO), Gentamicin (GM), Ampicillin G (AG), Sulphatriad (ST), Colistin sulphate (CS), Cotrimoxazole (CM), Cephalothin (CO), Trimethoprim (TM), Sulphametthoxazole (SMX), Clindamycin (CD), and Tetracycline (T).
Antibacterial activity was measured using the microtiter broth dilution method to determine the minimum inhibitory concentration (MIC), as well as the minimum bactericidal concentration (MBC). Approximately 112 bacterial strains were isolated from 78 swab samples. Out of these bacterial strains, eight were identified as pathogenic bacteria using 16s rRNA sequences. All of these eight strains were derived from the Genus Acinetobacter. Among them, two strains of Acinetobacter were resistant to 14 different antibiotics. These two bacteria were identified as Acinetobacter baumannii and Acinetobacter sp. These MDR strains have been used for antibacterial activity against plant extract. The results showed that the MIC of the extract against these pathogenic strains was approximately 4 mg. Therefore, it is concluded that the plant extract has the ability to kill MDR resistant Acinetobacter strains in an ecofriendly way.
Acinetobacter, Antibiotic Resistant, Bactericidal, Swab, 16s Rrna.
Alghamdi M. S. K, Fakieh M, Anwar Y, Ali H. S. H. M, Al-Matary M, Al-Maaqar S. M. S, Arif M, Al-Ahmadi T. M. Bactericidal Activity of Coleus forskohlii Extract Against Multi Drug Resistant Acinetobacter baumannii Strains Isolated from Hospital. Biosc.Biotech.Res.Comm. 2021;14(3).
Alghamdi M. S. K, Fakieh M, Anwar Y, Ali H. S. H. M, Al-Matary M, Al-Maaqar S. M. S, Arif M, Al-Ahmadi T. M. Bactericidal Activity of Coleus forskohlii Extract Against Multi Drug Resistant Acinetobacter baumannii Strains Isolated from Hospital. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/2VWvOwE“>https://bit.ly/2VWvOwE</a>
Copyright © Alghamdi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The genus Acinetobacter contains over 30 species, the majority of which live in soil and water, but some commensal strains have been isolated from human specimens (Visca et al. 2011). A. baumannii, a member of this genus, is thought to be clinically significant due to its multidrug resistance and high mortality rates in people with weakened immune systems worldwide (Peleg et al. 2008; Poulikakos et al. 2014). Several studies have supported the isolation of A. baumannii from various environmental sources.
The intensive study of clinical isolates of A. baumannii, on the other hand, does not reveal the species’ diversity. As a result, environmental isolates are critical for understanding the diverse nature of A. baummanii (Berlau et al. 1999; Huys et al. 2007; Diancourt et al. 2010; Hamouda et al. 2011; Asaad et al. 2021). Actinetobacter baummanii has emerged as a secondary infectious pathogen in critically ill patients who have been hospitalized for extended periods of time with intensive procedures prior to the use of antimicrobial drugs (Gottesman et al. 2021).
This species plays a significant role in healthcare-associated infections in institutions around the world (Perez et al. 2007; Gottesman et al. 2021). Bacterial resistance to antimicrobial drugs has prompted the United Nations to address the antimicrobial resistance problem. The emergence of multidrug resistant (MDR) A. baumannii mutants, which cause nosocomial infections, has harmed many countries in Asia, Latin America, Europe, and the Middle East (Saudi Arabia), where endemics have been reported worldwide (Tognim et al. 2004; Kim et al. 2013; Moradi et al. 2015).
MDR is most commonly found in adults in intensive care units, with cabapenem resistance being the most common phenotype (Mustasim 2018; Adel et al. 2020; Arta et al. 2019). Curing the majority of human and domestic animal diseases with natural products is primitive and goes hand in hand with human civilization. Traditional medicines are primarily derived from medicinal plants, and various active ingredients in medicinal plants are used to treat a variety of diseases (Woldeyes et al. 2012; Handique et al. 2016; Noha 2021; Fahim 2021).
Plant-based medicines account for nearly 25% of all prescribed medications worldwide (Rates 2001). Different parts of medicinal plants, such as flowers, leaves, barks, stems, fruits, and roots, are used to treat diseases caused by microorganisms because they have various pharmacological activities (Metra et al. 2020; Chen et al. 2021).
Recently, there has been an increase in concern about plant-derived drugs, which demonstrate the significance and validity of traditional claims about the value of natural products in healthcare. When compared to synthetic drugs, the researcher’s interest has been focused on the exploration of antimicrobial drugs derived from plants/microorganisms that are safe, healthy, and cost effective, with no side effects on the host (Cragg and Newman 2001; Nitha et al. 2012; Chen et al. 2021).
Pathogens are becoming increasingly resistant as a result of widespread and ineffective treatment of infectious diseases. As a result, scientists are eager to investigate plants for biologically active ingredients that are effective against infectious bacterial and other microorganisms (Shears 2000). Coleus forskohlii is a member of the Lamiaceae family, which has a vast number of members that are generally found in the Mediterranean region (lukhoba et al. 2006).
Phytochemical examination of C. forskohlii reveals the distribution of several phytochemicals such as alkaloids, reducing sugars, flavonoids, tanins, and terpenoids. Flavonoids in phenolic compounds are well-known for their anti-allergic, anti-cancer, antioxidant, and anti-inflammatory activities (Aiyelaagbe and Osamudiamen 2009; Alasbahi et al. 2010).
According to Ammon and Muller (1985), the C. forskohlii plant can be used to cure a variety of maladies including insomnia, heart disease, respiratory issues, epilepsy, angina, asthma, intestinal issues, abdominal colic, and inflammation. Different bio-active chemicals, such as terpenoids, phenolics, saponin, tanins, and alkaloids, were found to scavenge free radicals created in the body in another study (Rout et al. 2012).
GC-MS study of ethanolic extracts of C. forskohlii reveals a molecule called n-hexadecanoic acid, which has antibacterial and antifungal properties. Shanmugam and Pradeep (2019) revealed that C. forskohlii rhizome extract had antibacterial activity (Agoramoorthy et al. 2007; Shanmugam and Pradeep 2019; Khatun 2020). Our purpose was to examine MDR resistant bacteria at Prince Meshari Bin Saud General Baljarshi Hospital and employ plant extract to control pathogenic MDR bacterial strains.
MATERIAL AND METHODS
For sampling, swab samples were collected from Different area in Prince Meshari Bin Saud General Baljarshi Hospital. The bacterial Samples were directly taken to the laboratory and we kept in the refrigerator prior to further experimentation. For bacteria isolation, about 1 ml of collected samples were added to 100 ml freshly prepared nutrient broth medium and incubated at 30 °C and 180 r/min for 24 hours. The sample was serially diluted and were spreaded on the plates. After incubation the pure colonies were transfer 50 % glycerol. The samples were preserved in -80 °C until further experimentation.
Molecular identification and DNA isolation/Gene amplification was done according to the manufacturer’s instructions. DNA was extracted using the GeneJet Genomic DNA Purification Kit (Thermo Scientific). The 16F27 and 16R1525 primers were used for 16S rDNA amplification in each extracted DNA sample (Hauben et al. 1997). The polymerase chain reaction (PCR) was set for 30 cycles following amplification at the following temperatures and times: 92 °C (2 min); 42 °C (30 seconds); and 74 °C (4 min) before incubation (4 °C) at the end of the final cycle. Following amplification, the fragments were sequenced by Macrogen (Seoul, South Korea), and a phylogenetic tree was constructed using MEGA version 4 software (Tamura et al. 2007; Kubota et al. 2008; Hanan et al. 2009).
Drug susceptibility was tested using Kirby Bauer’s diffusion protocols with minor modifications. Antibiotics were used in accordance with the Clinical and Laboratory Standards Institute guidelines: Tetracyline (25 μg), Chloramphenicol (25 μg), Penicillin G (1 unit), Streptomycin (S), Erythromycin (5 μg), Fusidic Acid (10 μg), Oxacillin (5 μg), Novobiocin (5μg), Gentamicin (10 μg), Ampicillin G (1 unit), Sulphatriad (5 μg), Colistin sulphate (5 μg), Cotrimoxazole (5 μg), Cephalothin (5 μg), Trimethoprim (5 μg), Sulphametthoxazole (5 μg), Clindamycin (2 μg)), and Tetracycline (10 μg).
For the preparation of ethanolic extract, the dried plant sample was treated with absolute ethanol at a 1:20 ratio on a magnetic stirrer for 48 hours before being filtered with Whattman No. 1. The supernatant was evaporated using a Rotary Evaporator until an oily extract was obtained. The crude extract was then stored in sterile universal bottles at -20 °C. For antibacterial activity determination, agar diffusion method or agar well diffusion was used to test the antibacterial activity of various Ethanolic extracts (Daoud et al. 2015). A fresh bacterial culture (1 ml) was pipetted into the center of sterile petri dishes.
In the petri dish, molten cooled Muller Hington agar (MHA) was thoroughly mixed with the inoculum. A sterile cork borer was used to make 6 mm wells after the bacteria-containing agar plates solidified. These wells were filled with extracts (20% w/v) in 100 ml increments. Following a 24-hour incubation at 37 °C, the plates were chilled for 30 minutes to allow the extracts to better diffuse into the agar. The antibacterial activity is determined after the incubation period by measuring the zone of inhibition (including wells measurement). For the experiment, DMSO (10%) was used as a negative control (Daoud et al. 2015).
Minimum Inhibitory concentration (MIC) is the highest dilution of extracts that inhibits microorganism growth without killing the organism. The treatment of plant extracts in tested plates from the disc diffusion method that exhibit an inhibition zone was tested for MIC. The clinical laboratory standards institute (CLSI) protocols were followed when using the broth macrodilution method (Jorgensen and Turnidge 2015).
The minimal bactericidal concentrations (MBC) are the samples’ lowest concentrations at which inoculated bacteria are completely killed. The Minimal bactericidal concentration was determined by spreading 100 µl of the MIC contents that showed no bacterial growth on nutrient agar plates for 24-hour incubation at 37°C. The first well with a colony count of <5 is considered negative for growth and is reported as the MBC.
RESULTS AND DISCUSSION
Globally, the problem of Acinetobacter infections and drug resistance development is a major prevailing research issue that must be adequately addressed. Synthesizing biofilm is a key feature, particularly in bacterial diseases where its spread is aided by air and mechanics (Ming et al. 2014; Badave and Kulkarni 2015). In our study, we found a high prevalence of MDR strains among A. baumannii isolates from ICU patients. The high prevalence of MDR strains in ICUs could be attributed to the study population’s regular use of antimicrobials.
Isolation and identification: About seventy-eight swab samples were collected from different areas of the Hospital. From these swab samples about 112 bacterial strains were isolated. The list of sampling having pathogenic strain is detected is shown in Table 1.
Table 1. Samples collected from hospital area having pathogenic bacterial strains
| No. | Samples ID | Location/Room | Stools/Tables |
| 1. | 3-2 | Operations Room
|
Tool table |
| 2. | 17-3 | ER Male waiting Room | Chair handles, Door handle, Floor |
| 3. | 25-3 | ER Triage Room | Chair, Measuring device |
| 4. | 5-1 | Examination Room 2
|
Doctor Table |
| 5. | 1-2 | Examination Room 2
|
Door handle |
| 6. | 7-3 | ER Reception | Reception Table |
| 7. | 15-3 | ER Male waiting Room | Chair handles, Door Handles |
| 8. | 15-1 | ER Male waiting Room | Chair handles, Door handle, Floor |
Out of these bacterial strains, eight were identified as pathogenic bacteria using 16s rRNA sequences. All of these eight strains were derived from the Genus Acinetobacter. Among them, two strains of Acinetobacter were resistant to 15 different antibiotics. The resistant profile of all the pathogenic strains is shown in Table 2.
Table 2. Antibiotic profile of the isolates from local hospital
| S.No | ID | T | C | E | FC | OX | NO | PG | GM | CO | CS | AG | CM | ST | S | TM | SMX | T | CD |
| 1 | 3-2 | 15 | 12 | 16 | R | R | R | R | 22 | 15 | R | R | R | R | 8 | R | R | R | R |
| 2 | 17-3 | 16 | 13 | 16 | R | R | R | R | 21 | R | R | R | R | R | R | R | R | R | R |
| 2 | 25-3 | 20 | 12 | 15 | R | R | R | R | 24 | 14 | R | R | R | R | 9 | R | R | R | R |
| 4 | 5-1 | 19 | R | 15 | R | R | R | R | 20 | 14 | R | R | R | R | R | R | R | R | R |
| 5 | 1-2 | 22 | 12 | 14 | R | R | R | R | 20 | 16 | R | R | R | R | 8 | R | R | R | R |
| 6 | 7-3 | 20 | 14 | 18 | R | R | R | R | 24 | 16 | R | R | R | R | R | R | R | R | R |
| 7 | 15-3 | 18 | 15 | 16 | R | R | R | R | 21 | R | R | R | R | R | R | R | R | R | R |
| 8 | 15-1 | 22 | 20 | 18 | R | R | R | R | 20 | R | R | R | R | R | 9 | R | R | R | R |
Tetracyline (T), Chloramphenicol (C), Penicillin G (PG), Streptomycin (S), Erythromycin (E), FUSIDIC ACID (FC), Oxacillin (OX), Novobiocin (NO), Gentamicin (GM), Ampicillin G (AG), Sulphatriad (ST), Colistin sulphate (CS), Cotrimoxazole (CM), Cephalothin (CO), Trimethoprim (TM), Sulphametthoxazole (SMX), Clindamycin (CD), and Tetracycline (T)
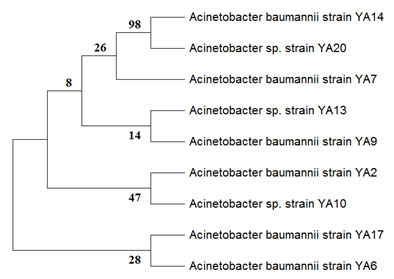
The 16s rRNA gene was amplified in order to identify the most resistant isolates molecularly. The gene product’s sequence was submitted to Genbank in order to obtain an accession number. The resistant bacteria 17.3 was identified as Acinetobacter baumannii (MT875278) and bacteria 15.3 was identified as Acinetobacter sp. (MT875271). Following the acquisition of the accession number, a phylogenetic tree was constructed using MegaX software, as shown in Figure 1. Our findings show that the 16s rRNA sequences of bacteria Acinetobacter baumannii and Acinetobacter sp. are similar to those of many other Acinetobacter strains, as shown in Figure 1.
Figure 1: Phylogenetic tree of Acinetobacter baumannii strains isolated from local hospital
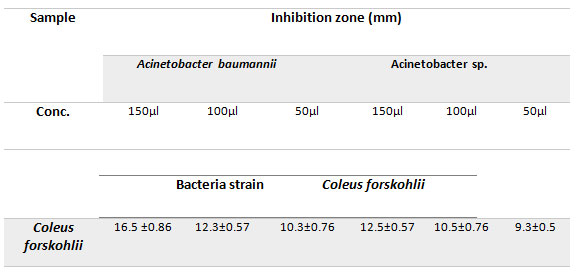
Antibacterial activity of plants extract: Plant Ethanolic extract was investigated to evaluate their antibacterial activity against the infectious bacteria isolated from local hospital. The extract’s antimicrobial activity was investigated to determine its efficacy against the two microorganisms being studied. Coleus forskohlii Ethanolic extract was quantitatively tested against two pathogenic Acinetobacter by assessing the diameter of the inhibition zones as shown in Table 3 and Figure 2. Extracts of Coleus forskohlii and had high activity against Acinetobacter. The results presented showed significant activity among (9.5±0.8 – 16.5 ±0.86) range against micro-organisms with inhibition zones.
Figure 2: Zone of inhibition of Coleus forskohlii against A). Acinetobacter baumannii B). Acinetobacter sp
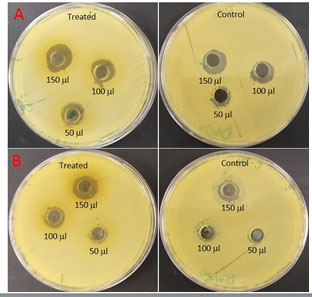
Table 3. Antibacterial activity of Coleus forskohlii against Acinetobacter strains in aerobic condition.
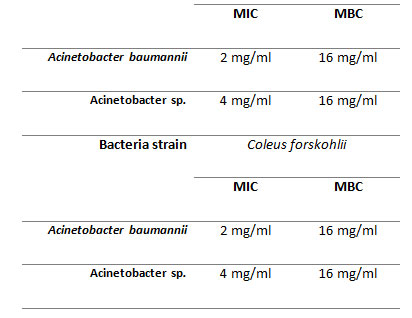
Minimum inhibitory concentration (MIC): Acinetobacter sp. and Acinetobacter baumannii were found to be most sensitive pathogens which survived up to 2 mg/ml and 4 mg/ml (Table 4), thus having an MIC of 2 mg/ml and 4 mg/ml, respectively. Acinetobacter baumannii was found to be comparatively less sensitive as they survived up to 4mg. Minimal bactericidal concentrations (MBC) results was found 16 mg/ml of Coleus forskohlii Ethanolic extract against the selected pathogenic strains.
Table 4. Minimum inhibitory concentration (MIC) of samples against Acinetobacter strains tested.
Due to its resistance to several medicines, treating Acinetobacter strains is a significant issue. So, there is a need to control Acinetobacter strains in an ecofriendly way. For this Coleus forskohlii Ethanolic plant extract was used in order to kill these multidrug resistant strains. According to our findings, this plant has the ability to kill multidrug-resistant Acinetobacter and can be utilized to control the bacteria. The two Acinetobacter strains selected were resistant to nearly fourteen antibiotics.
But results show that Coleus forskohlii plant extract has the ability to kill these strains isolated for hospital. Our findings support those of Malleswari et al. (2013) who discovered significant broad spectrum antibacterial activity against Pseudomonas fluorescence, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Streptococcus pneumonia, and Staphylococcus aureus using C. forskohlii roots, shoots, and leaves extracts (Malleswari et al. 2013; Mitra et al. 2020; Khatun 2020).
Furthermore, Lu et al. (2010) mentioned that Gallic acid have a strong antimicrobial, antioxidant and anticancer (Rice et al. 1996; Lu et al. 2010). p-hydroxybenzoic acid, has been reported to have antioxidant activity against free radicals, antimicrobial activity against pathogenic bacteria and fungi (Rice et al. 1996; Heleno et al. 2013; Mitra et al. 2020; Khatun 2020). Many researchers have identified various plant extract efficiency and active compounds as antimicrobial sources to inhibit the growth of bacteria (infectious). According to some reports, compounds found in plant extracts such as terpenes, alkaloids, and phenols affect the enzymes and proteins of the target cell membrane.
These plant compounds are responsible for cell disruption caused by proton efflux to the cell’s exterior causes cell senesces or disrupts enzymes required for amino acid biosynthesis (Burt 2004; Gill and Holley 2006). There are also some reports about the hydrophobicity of plant extracts. This property of extracts enables them to interact with microbial proteins in cell membranes and mitochondria, posing a threat to cell integrity and altering permeability (Friedman et al. 2004; Tiwari et al. 2009; Mitra et al. 2020; Khatun 2020).
CONCLUSION
The findings of the present study determines that the production of resistant strains of Acinetobacter causes hospitalizations to be prolonged, as well as high medication costs. Acinetobacter infection is significantly accelerated by factors such as improper antibiotic use, mechanical ventilation, and cross infection. There is already a high rate of resistance to common antibiotics. The Ethanolic extract of plants may play an important role in reducing Acinetobacter infections and inhibiting deadly epidemic nosocomial infections.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of King Abdulaziz University, Jeddah, Saudi Arabia.
REFERENCES
Agoramoorthy, G., Chandrasekaran, M., Venkatesalu, V. et al. (2007) Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Brazilian Journal of Microbiology, 38: 739-742.
Aiyelaagbe, O.O. and Osamudiamen, P.M. (2009) Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Journal of Plant Science and Research, 2: 11-13.
Alasbahi, R.H. and Melzig, M.F. (2010) Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology-part 2. Planta Medica, 76: 753-765.
Ammon, H.P.T. and Muller, A.B. (1985) Forskolin: From an ayurvedic remedy to a modern agent. Planta Medica, 46: 473-477.
Asaad, AM., Ansari, S., Ajlan, S.E. et al. (2021) Epidemiology of Biofilm Producing Acinetobacter baumannii Nosocomial Isolates from a Tertiary Care Hospital in Egypt: A Cross-Sectional Study. Infection and Drug Resistance, 14: 709-717.
Badave, G.K., and Kulkarni, D. (2015) Biofilm Producing Multidrug Resistant Acinetobacter baumannii: An Emerging Challenge. Journal of Clinical and Diagnostic Research, 9:8-10.
Berlau, J., Aucken, H.M., Houang, E., et al. (1999) Isolation of Acinetobacter spp including A. baumannii from vegetables: implications for hospital-acquired infections. Journal of Hospital Infection, 42, 201-204.
Burt, S. (2004) Essential oils: their antibacterial properties and potential application in foods: a review. International Journal of Food Microbiology, 94: 223–253.
Chen, J.Y. Peng, S.Y., Cheng, Y.H. et al. (2021) Effect of Forskolin on Body Weight, Glucose Metabolism and Adipocyte Size of Diet-Induced Obesity in Mice. Animals, 11, 645.
Cragg, G.M. and Newman, D.J. (2001) Natural product drug discovery in the next millennium. Pharmaceutical Biology, 39: 8-17.
Daoud, A., Malika, D., Bakari, S., et al. (2015) Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of date palm pollen (DPP) from two tunisian cultivars. Arabian Journal of Chemistry, 12: 3075-3086.
Diancourt, L., Passet, V., Nemec, A. et al. (2010) The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE, 7;5(4): e10034.
El Mekes, A., Zahlane, K., Ait Said, L., et al. (2020) The clinical and epidemiological risk factors of infections due to multi-drug resistant bacteria in an adult intensive care unit of University Hospital Center in Marrakesh-Morocco. Journal of Infection and Public Health. 13(4):637-643.
Fahim, N.A.E. (2021) Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care unit’s patients at Ain Shams University Hospitals in Egypt—a retrospective study. Journal of the Egyptian Public Health Association. doi: 10.1089/mdr.2020.0489. Epub ahead of print. PMID: 33600262.
Friedman, M., Henika, P.R., Levin, C.E., et al. (2004) Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. Journal of Agriculture and Food Chemistry, 52: 6042-6048.
Gill, A.O., and Holley, R.A. (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. International Journal of Food Microbiology, 108: 1-9.
Gottesman, T., Fedorowsky, R., Yerushalmi, R. et al. (2021) An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infection Prevention in Practice, 3(1): 100113. doi:10.1016/j.infpip.2021.100113
Hamouda, A., Findlay, J., Al Hassan, L. et al. (2011) Epidemiology of Acinetobacter baumannii of animal origin. International Journal of Antimicrobial Agents, 38, 314-318.
Hanan, I.M., Linda, M.F., and Taghleb, M.A. (2009) Mutational analysis of oil degrading genes in bacterial isolates from oil contaminated soil at the Jordanian oil refinery. World Applied Sciences Journal, 6(2): 208-220.
Handique, J.G. and Gogoi, D. (2016) Antioxidant activities of the medicinal plants used for preparation of fermentation cakes of “haanj”, the rice based alcoholic beverage of ahom community people of Assam, India. International Journal of Pharmacognosy and Phytochemical Research, 8: 217-222.
Hauben, L., Vauterin, L., Swings, J. et al. (1997) Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. International Journal of Systematic Bacteriology, 47:328-335.
Heleno, S.A., Ferreira, I.C.F.R., Esteves, A.P. et al. (2013) Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food and Chemical Toxicology, 58: 95-100
Huys, G., Bartie, K., Cnockaert, M. et al. (2007) Biodiversity of chloramphenicol-resistant mesophilic heterotrophs from Southeast Asian aquaculture environments. Research in Microbiology, 158, 228-235.
Ibrahim, M.E. (2018) High antimicrobial resistant rates among Gram-negative pathogens in intensive care units. Saudi Medical Journal, 39: 10, 1035-1043.
Jorgensen, J.H. and John, D.T. (2015) Susceptibility Test Methods: Dilution and Disk Diffusion Methods, Manual of clinical microbiology, pp.1253-1273. https://doi.org/10.1128/9781555817381.ch71.
Khatun, S. (2020) Antimicrobial activity of tuber extracts of the medicinal plant Coleus forskohlii. Plant cell biotechnology and Molecular Biology, 21(11-12), 11-17.
Kim, D.H., Choi, J.Y., Kim, H.W. et al. (2013) Spread of carbapenem-resistant Acinetobacter baumannii global clone 2 in Asia and AbaR-type resistance islands. Antimicrobial Agents and Chemotherapy, 57:5239-46.
Kubota, K., Koma, D., Matsumiya, Y, et al. (2008) Phylogenetic analysis of long-chain hydrocarbon-degrading bacteria and evaluation of their hydrocarbon-degradation by the 2,6- DCPIP assay. Biodegradation, 19:749-757.
Lin, M.F. and Lan, CY. (2014) Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World Journal of Clinical Cases, 16: 787-814.
Lu, Y., Jiang, F., Jiang, H. et al. (2010) Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. European Journal of Pharmacology, 641: 102-107.
Lukhoba, C.W., Simmonds, M.S.J. and Paton A.J. (2006) Plectranthus: A review of ethnobotanical uses. Journal of Ethnopharmacology, 103: 1-24.
Malleswari, D., Bagyanarayana, G. and Hindumathi, A. (2013) Anti-bacterial activity of Coleus forskohlii extracts against some pathogenic bacteria. Journal of Natural Product and Plant Resources, 3: 75-78.
Mitra, M., Gantait, S., and Mandal, N. (2020) Coleus forskohlii: advancements and prospects of in vitro biotechnology. Applied Microbiology and Biotechnology, 104(6):2359-2371
Mwitari, P.G., Ayeka, P.A., Ondicho, J. et al. (2013) Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS ONE, 8(6): e65619.
Nitha, B., Remashree, A.B., and Balachandran I. (2012) Antibacterial activity of some selected Indian medicinal flora. IJPSR, 3(7): 2038-2042.
Peleg, A.Y., Seifert, H., and Paterson D.L. (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clinical Microbiology Reviews, 21, 538-582.
Perez, F., Hujer, A.M., Hujer, K.M. et al. (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrobial Agents Chemotherapy, 51:3471-84.
Poulikakos, P., Tansarli, G. S., and Falagas, M. E. (2014) Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. European Journal of Clinical Microbiology & Infectious Diseases. 33, 1675-1685.
Rates, S.M. (2001) Plants as source of drugs. Toxicon, 39: 603-613.
Rice-Evans, C.A., Miller, N.J., and Paganga, G. (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine, 20: 933-956.
Rodrigues, T.S., Guimaraes, S.F., Braga, T.V., et al. (2016) Determination of content of phenolic compounds and flavonoids in leaves extracts of Plectranthus sp. (“Boldos”), potential antioxidant and antibacterial action. Academia Journal of Medicinal Plants, 4: 62-68.
Rout, O.P., Acharya, R., Mishra, S.K., et al. (2012) Pathorchur (Coleus aromaticus): A review of the medicinal evidence for its phytochemistry and pharmacology properties. International Journal of Applied Biology and Pharmaceutical Technology, 3: 348-355.
Shanmugam, S. and Pradeep B.V. (2019) Studies on Phytochemical Screening and Antibacterial Activity of Rhizome Extracts of Coleus forskohlii Briq. Journal of Pure Applied Microbiology, 13(3), 1703-1710.
Shears, P. (2000) Antimicrobial Resistance in the Tropics. Tropical Doctor, 30: 114-116.
Tamura, K., Dudley, J., Nei M, et al. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24:1596-1599.
Tiwari, B.K., Valdramidi, V.P., O’Donnell, C.P., et al. (2009) Application of natural antimicrobials for food preservation. Journal of Agriculture and Food Chemistry, 57: 5987-6000.
Tognim, M.C.B., Andrade, S.S., Silbert, S. et al. (2004) Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. International Journal of Infectious Disease, 8:284-91.
Visca, P., Seifert H., and Towner K.J. (2011) Acinetobacter infection – an emerging threat to human health. IUBMB Life, 63: 1048–1054.
Woldeyes, S., Legesse, A., Inebeb, T. et al. (2012) Evaluation of Antibacterial Activities of Compounds Isolated from Sida rhombifolia Linn. (Malvaceae). Natural Products Chemistry & Research, 1: 1-8.


