1School of Studies in Microbiology Vikram University, Ujjain, Madhya Pradesh, India.
2Department of Botany, Government Kalidas Girls P.G. College, Ujjain, Madhya Pradesh, India.
3Soil Testing Lab, Ujjain, Madhya Pradesh, India.
4School of Studies in Microbiology, Vikram University, Ujjain, Madhya Pradesh, India.
Corresponding author email: uzmaanwar403@gmail.com
Article Publishing History
Received: 22/04/2021
Accepted After Revision: 25/06/2021
In most of the agricultural soils phosphorus is present in considerable amounts but its availability to plants is limited because it is fixed in soil as insoluble phosphate. The over application of chemical phosphorus fertilizers does not increase the bioavailability of phosphorus but the leads to adverse effects on soil fertility. Phosphate solubilizing microorganisms (PSMs) can hydrolyse phosphorus compound into soluble form and make them available to plants. There is need of identifying such microbes which can be used as bioinoculants in agricultural soils to increase plant growth and productivity. In this study 64 endophytic fungi were isolated from maize plants by inoculating surface sterilized parts on potato dextrose agar medium. The phosphate solubilization index of fungal cultures was determined by measuring the hole zone formed around cultures on Pikovskaya’s agar medium and the morphological identification of cultures was based on study of colony morphology and microscopic characters. It was seen that about 41% of the cultures had phosphate solubilization potential.
The morphological characterization of cultures revealed that 23 cultures were of Aspergillus niger, 2 cultures were of Penicillium oxalicum and 1 culture was of Curvularia sp. aff. C. Verruculosa Tandon & Bilgrami ex M. B. Ellis. Penicillium oxalicum (E 137) and two culture of Aspergillus niger (E 204 and E 215) having high PSI were identified using molecular techniques and the effect of addition of spore suspension of these cultures on seed germination, seedling growth and plant growth was determined. The results of the study indicate that the addition of fungal spores significantly increases the seedling growth and plant growth in pot culture experiments suggesting that they may serve as potential biofertilizer/ bioinoculants.
Endophytic Fungi, Phosphate Solubilizing Microorganisms, Plant Growth, Seed Germination, Zea Mays
Choudhary U, Vyas H, Patidar C.P, Vyas A. Molecular Characterization of Phosphate Solubilizing Endophytic Fungi and its Effect on Growth of the Maize, Zea mays. Biosc.Biotech.Res.Comm. 2021;14(2).
Choudhary U, Vyas H, Patidar C.P, Vyas A. Molecular Characterization of Phosphate Solubilizing Endophytic Fungi and its Effect on Growth of the Maize, Zea mays. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3xBqqx7“>https://bit.ly/3xBqqx7</a>
Copyright © Choudhary et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Agriculture is the utmost important area of Indian economy and it accounts for 18% of GDP (Gross domestic product). About 50% of Indian population depends upon agriculture for their livelihood and increasing agricultural production is crucial for feeding current and future population of humans (Madhusudhan 2015). Phosphate is one of the major limiting factors for proper plant growth due to its low availability in agricultural fields.
Phosphorus forms an essential constituent of many cellular molecules including nucleic acids, phospholipids, ATP’s and enzymes. It is needed in almost every aspect of plant growth like growth of roots, shoots, formation of flowers, seeds, energy production, nitrogen fixation, etc. and it accounts for 0.2-0.8% of dry weights of plants (El-Hamshary et al. 2019; Kalayu 2019).
Most of the soils contain considerable amount of phosphate but its availability becomes limited because a large portion of phosphorus is fixed as insoluble phosphate of iron, aluminium and calcium. Organic matter present in soil is a major source of immobilized phosphate and 20-80% of phosphate present in soil is in this form.
The soluble phosphate is highly reactive with other elements and in acidic soils phosphate is complexed with iron and aluminium compounds while in calcareous soils calcium phosphate is predominately formed (Rashmi et al. 2018).The acidic soils found in tropical and subtropical regions are often phosphorus deficient because they have high phosphate fixation capacity (Rashmi et al. 2018). Most of the former use chemical overcome phosphorus deficiency (Chouyia et al. 2020; Mazrou et al. 2020).
The addition of chemical fertilizers to overcome the phosphate availability in soil results not only in increase in agricultural cost but also causes adverse effect on soil health. The addition of phosphorus fertilizers in soils does not increase its bioavailability as 75-90% of phosphate fertilizers are precipitated by formation of metal ion complexes and result in fixation in soil and only 5% of added phosphorus is available to plants (Pande et al. 2017). The repeated non judicial application of phosphate containing chemical fertilizers result in loss of soil fertility by decreasing the microbial diversity.
It has been suggested that accumulated phosphate in agricultural soils can sustain crop yields over 100 years (Gizaw et al. 2017). Phosphate solubilizing microorganisms are a group of microorganisms which help in solubilizing the phosphate present in soil and make it available for plant growth (Rashmi et al. 2018). The addition of PSMs to soil is an eco-friendly alternative which can make the phosphorus available to plant and can decrease the agricultural cost and toxic effect of indiscriminate use of chemical fertilizers (Qarni et al. 2021; Raymond et al. 2021).
The knowledge about phosphate solubilizing microorganisms has gradually increased over the last few years and a large number of bacteria and fungi have been identified which have high phosphate solubilizing activity. Fungi can travel long distances and are more important and they form about 0.1-0.5% of the total fungal soil population in nature (Mahadevamurthy et al. 2016; Zhu et al. 2017; Mazrou et al. 2020).
Bioprospection of phosphate solubilizing endophytic microbes has become extremely relevant as they can colonize plants without inducing any apparent disease symptoms and benefit the host plant by providing protection against stress and pathogens (Zheng et al. 2016; Mazrou et al. 2020). They produce various secondary metabolites and help in different process of plant including nitrogen fixation, phosphate solubilization etc.
(Santoyo et al. 2016). Phosphate solubilizing endophytic fungi are very competitive and aggressive colonizers. The common phosphate solubilizing endophytic fungal genera are Aspergillus, Penicillium, Curvularia and Piriformospora, whereas the common phosphate solubilizing endophytic bacterial genera are Bacillus, Pseudomonas and Rhizobium (Matos et al. 2017; Singh et al. 2020; Abawari et al. 2021; Fouda et al. 2021).
An important constraint in use of biological organisms in agricultural fields is their inability to grow and adapt to different habitats. Hence, it is necessary that indigenous microorganisms may be isolated and identified which can be used for inoculation in agricultural fields. The indigenous microorganisms are native to the particular environment as they are adapted for growth at particular climatic condition.
The application of indigenous microorganisms as biofertilizers/ bioinoculants/ biocontrol agent is crucial for sustainable agriculture (Kumar and Gopal 2015; Jan et al. 2020; Fouda et al. 2021). In view of above it was of interest to isolate and identify indigenous fungal genera having high phosphate solubilization potential, so they may be used in agricultural fields as bioinoculants for promoting sustainable agriculture in local fields.
In the present study we have isolated and identified 26 endophytic fungi having phosphate solubilizing activity from maize (Zea mays) plants growing agricultural fields of Ratadiya Village near local fields. Three cultures showing high phosphate solubilizing index, namely E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E137 (Penicillium oxalicum) were characterized at molecular level and their effects as bioinoculants on seedling growth and plant growth were studied.
MATERIAL AND METHODS
Plant material was collected in July, 2017 from the agricultural fields of Ratadiya village near Ujjain (M.P.). Randomly growing sixteen completely healthy and mature maize plants along with roots were uprooted, packed in sterilized polythene bags and brought to the laboratory. The plant samples were stored at room temperature and processed within 24 hours. Different parts of plant i.e., roots and leaves were used for isolation of endophytic fungi by following the modified method of Han et al. (2013).
The plant material was thoroughly washed with tap water and cut into 1-1.5 cm pieces and surface sterilization was done using 70% ethanol and 0.1% HgCl2 for 2 minutes and then washed twice with sterilized distilled water. The plant pieces were placed on potato dextrose agar (PDA) medium containing chloramphenicol (40µg/ml) and incubated at 28 ± 2ºC for 5 to 7 days. The hyphal tips which emerged from the plant material were aseptically cut and transferred to fresh PDA medium slants and incubated at 28 ± 2ºC to obtained fungal cultures. Sub culturing of cultures was done to obtain pure cultures of endophytic fungi.
Morphological identification of fungal cultures was done based on study of colony characteristics and arrangement of spores using fungal keys. A small amount of culture was used for preparing wet mount on sterile glass slide. The culture was stained with lactophenol cotton blue (LCB) and the slide was examined under light microscope at different magnifications (10X, 45X and 100X). The identification was confirmed by National Fungal Culture Collection of India (NFCCI), Pune, India. The fungal cultures were preserved in PDA slants and stored at -20ºC (Visagie et al. 2014; Nyongsa et al. 2015).
Molecular characterization of three cultures was done. Genomic DNA was extracted by using 5 to 7 days old fungal cultures grown on PDA medium. The DNA was amplified using primers ITS 4 (R-5’TCCTCCGCTTATTGATATGC3′) and ITS 5(F-5’GGAAGTAAAAGTCGTAACAAGG3′) and the PCR product were purified and sequenced. The resulting sequencing was matched in BLAST analysis software at NCBI (https://blast.ncbi.nlm.nih.gov) and phylogenetic tree were constructed using MEGA X software.
Fungal cultures were deposited at NFCCI (National Fungal Culture Collection of India), Pune, India, for obtaining accession numbers (Singh et al. 2020). The phosphate solubilizing potential of endophytic fungal cultures was determined by using modified method of Mazrou et al. (2020). The cultures were grown in Petri dishes containing Pikovskaya’s agar medium at 28 ± 2ºC for 5 days and the halo zone formed around the fungal colony was measured. The phosphate solubilization index (PSI) was calculated by using the following formula:
PSI = Colony diameter + Halo zone diameter
Colony diameter
The spore suspension of fungal cultures was prepared in sterile distilled water. The fungal cultures of Aspergillus niger (E 204 and E 215) and Penicillium oxalicum (E 137) were grown in 250 ml conical flask containing 50 ml PDA medium for 10 days at 28 ± 2ºC and the spores were harvested using 50 ml sterile distilled water. Concentration of spores per ml was determined using Haemocytometer and a final spore suspension 105-106 spores per ml was made. The spore suspension was used for bioinoculation on same day on which it was prepared (Mahadevamurthy et al. 2016).
The seeds of Zea mays variety MRM 3777 were purchased from local market and stored in air tight containers at room temperature. The maize seeds were surface sterilized using 70% ethanol and 0.1% HgCl2 and washed twice with sterile distilled water. The seeds were soaked in spore suspension for 24 hours at room temperature and washed once with sterile distilled water. Three seeds were placed in sterilized Petri dishes containing sterilized cotton layer. The Petri dishes were incubated at 28 ± 2ºC for 5 days.
The experiments were done in triplicate and seeds treated with distilled water were used as control. After 5 days of growth the germination percentage and the length of root and shoot was measured. The plant parts were kept at 80ºC for 2 hours after which their dry weights were determined. The vigor index was calculated using the mathematical expression described below (Mahadevamurthy et al. 2016).
Vigor index = Seed germination (%) x [Mean Root Length + Mean Shoot Length]
Soil from agricultural field of Ratadiya village was used for performing pot culture experiments. The air dried and sieved soil samples were used for determining pH, available potash, available phosphorus, available nitrogen and organic carbon (Wagh et al. 2013; Das et al. 2017). The soil used in experiments was sterilized three times with an interval of 24 hours between each sterilization cycle.
This was done to remove microbes present in soil so that only the effect of inoculated fungal cultures could be observed. The experiments were performed in 1L poly propylene autoclavable beakers having capacity of containing 1kg soil. In each pot (beaker) 6 seeds were placed 1 cm below the top layer and 5 ml spore suspension (105-106 spores per ml) was added on the top of each seed then the seeds were covered with soil layer.
The pots were kept at room temperature and exposed to natural conditions throughout the day. The soil in pots was kept moist by adding 50-100 ml water every day according to weather conditions. After 7 days the plants were removed from the soil and the plant height, root length and area of largest leaf were determined. The plants parts were wrapped in aluminum foil and kept at 80ºC for 24 hours and dry weights were determined to measure the biomass formed.
The pot culture experiments were performed in the month of October and November (Singh et al. 2018). All the experiments were performed in triplicates and statistical analysis was done using one-way analysis of variance (ANOVA) followed by Tukey’s HSD test. The mean values of samples and standard deviation were calculated. The values at P ≤ 0.01 and P ≤ 0.05 were considered as significant.
RESULTS AND DISCUSSION
Isolation and identification of phosphate solubilizing endophytic fungi: In the present study 64 endophytic fungal cultures were isolated from different parts of maize. Out of these cultures 20 (31.25%) were isolated from roots and 44 (68.75%) were isolated from leaves (Table 1). The phosphate solubilizing potential of fungal cultures was determined by measuring the halo zone formed around the cultures.
It was seen that 26 (40.62%) fungal cultures had phosphate solubilizing activity (Table 1). The morphological identification of these cultures showed that 23 (35.93%) cultures belonged to Aspergillus niger, two (3.12%) cultures belonged to Penicillium oxalicum and one (1.56%) belonged to Curvularia sp. aff. C. Verruculosa Tandon & Bilgrami ex M. B. Ellis (Table 1).
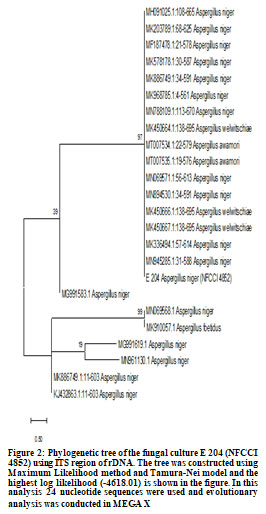
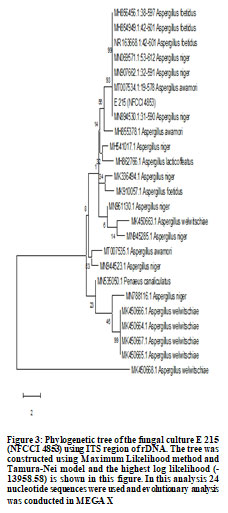
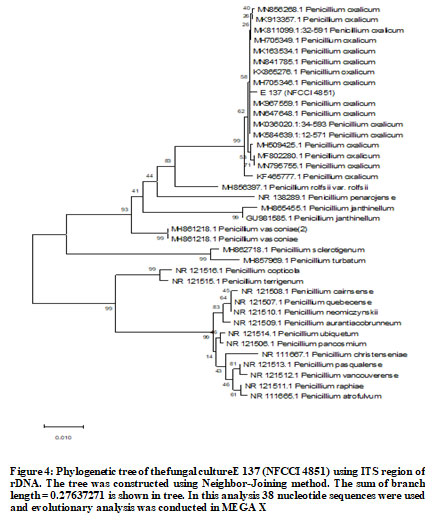
E137 (Penicillium oxalicum) showed highest phosphate solubilization index followed by E 215 (Aspergillus niger) and E 204 (Aspergillus niger) (Figure 1 and Table 1). Molecular characterization two cultures of Aspergillus niger (E 204, E 215) and one culture of Penicillium oxalicum (E 137) was done using 18S rDNA sequencing and phylogenetic trees constructed using MEGA X software are shown in Figure 2, Figure 3 and Figure 4.
The accession numbers of cultures obtained from NFCCI, Pune are NFCCI 4851 (E 137: Penicillium oxalicum), NFCCI 4852 (E 204: Aspergillus niger) and NFCCI 4853 (E 215: Aspergillus niger). Earlier study of rhizospheric soil samples from different plants in Jimma town and Manna district farmlands in Ethiopia revealed that Aspergillus spp., Penicillium spp. and Fusarium spp. had phosphate solubilization activities (Elias et al. 2016). Cultures of Aspergillus niger having high phosphate solubilization potential were also isolated from rhizospheric soil of Cucumis, Ocimum and Eruca plants in Jeddah, Saudi Arabia (El-Hamshary et al. 2019).
Phosphate solubilizing endophytic fungi belonging to Penicillium oxalicum, P. citrinums and Aspergillus sp. were also reported from seaweeds collected from Rameswarem coastal regions in India (Noorjahan et al. 2019). Culturable endophytes belonging to species of Aspergillus niger and Penicillium oxalicum having phosphate solubilizing potential have been reported from T. wallichiana (Adhikari and Pandey 2018).
In the present study also phosphate solubilizing potential was seen in endophytic fungal cultures belonging to Aspergillus niger, Penicillium oxalicum and Curvularia sp. aff. C. verruculosa Tandon & Bilgrami ex M. B. Ellis. Thus, the results of present study corroborate with the findings of earlier worker and strengthen the fact that cultures of Aspergillus spp. and Penicillium spp. have high phosphate solubilizing potential.
It is suggested that the application of these fungi as bioinoculants in agricultural fields can act as alternatives of chemical fertilizers and help in promoting sustainable agriculture practices (Baron et al. 2018; Noorjahan et al. 2019). The potential of phosphate solubilizing microorganisms as biofertilizers / bioinoculants was also been suggested by other workers (Mazrou et al. 2020; Abawari et al. 2021).
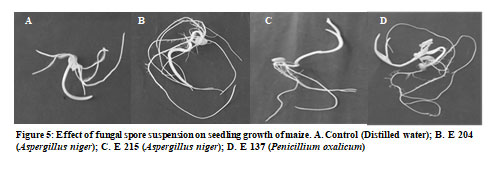
Figure 1: Morphological characterization and phosphate solubilization potential of fungal cultures. A. Halo zone around colony of Aspergillus niger (E 204); B. Conidiospores of Aspergillus niger (E 204); C. Halo zone around colony of Aspergillus niger (E 215); D. Conidiospores of Aspergillus niger (E 215); E. Halo zone around colony of Penicillium
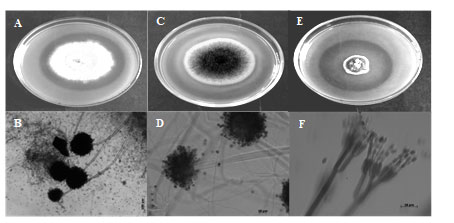
Table 1. Phosphate solubilization index of endophytic fungi isolated from different parts of maize
| S. No. | Culture ID | Plant part used for isolation | Name of fungi | Phosphate Solubilization Index (PSI) on
5th Day |
| 1 | E 198 | Root | Aspergillus niger | 1.32 ± 0.02 |
| 2 | E 204 | Root | Aspergillus niger | 1.42 ± 0.06 |
| 3 | E 205 | Root | Aspergillus niger | 1.35 ± 0.01 |
| 4 | E 209 | Root | Aspergillus niger | 1.14 ± 0.02 |
| 5 | E 210 | Root | Aspergillus niger | 1.13 ± 0.02 |
| 6 | E 215 | Root | Aspergillus niger | 1.67 ± 0.08 |
| 7 | E 216 | Root | Aspergillus niger | 1.08 ± 0.03 |
| 8 | E 218 | Root | Penicillium oxalicum | 1.06 ± 0.03 |
| 9 | E 224 | Root | Aspergillus niger | 1.08 ± 0.03 |
| 10 | E 102 | Leaf | Aspergillus niger | 1.17 ± 0.02 |
| 11 | E 105 | Leaf | Aspergillus niger | 1.07 ± 0.03 |
| 12 | E 114 | Leaf | Aspergillus niger | 1.10 ± 0.02 |
| 13 | E 115 | Leaf | Curvularia sp. aff. C. verruculosa Tandon & Bilgrami ex M. B. Ellis | 1.02 ± 0.02 |
| 14 | E 118 | Leaf | Aspergillus niger | 1.04 ± 0.03 |
| 15 | E 122 | Leaf | Aspergillus niger | 1.27 ± 0.03 |
| 16 | E 123 | Leaf | Aspergillus niger | 1.23 ± 0.04 |
| 17 | E 124 | Leaf | Aspergillus niger | 1.10 ± 0.02 |
| 18 | E 125 | Leaf | Aspergillus niger | 1.37 ± 0.04 |
| 19 | E 128 | Leaf | Aspergillus niger | 1.12 ± 0.04 |
| 20 | E 135 | Leaf | Aspergillus niger | 1.10 ± 0.02 |
| 21 | E 137 | Leaf | Penicillium oxalicum | 2.93 ± 0.05 |
| 22 | E 139 | Leaf | Aspergillus niger | 1.04 ± 0.03 |
| 23 | E 142 | Leaf | Aspergillus niger | 1.35 ± 0.01 |
| 24 | E 147 | Leaf | Aspergillus niger | 1.08 ± 0.03 |
| 25 | E 158 | Leaf | Aspergillus niger | 1.08 ± 0.03 |
| 26 | E 164 | Leaf | Aspergillus niger | 1.16 ± 0.04 |
Figure 2
Effect of fungal treatment on seed germination, seedling growth and plant growth: The effect of E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E 137 (Penicillium oxalicum), as bioinoculants on seed germination and seedling growth was studied in Petri dishes (Figure 5). It was seen that when spore suspension containing 105-106 spores per ml was used then percentage of germination in control (seeds treated with sterile distilled water) and seeds treated with fungal spores remained 100% indicating that these fungi do not have negative effect on germination of seeds when added in this concentration.
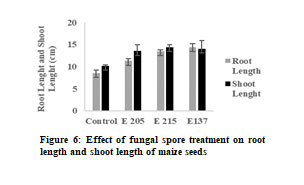
However, the application of spore suspension above this concentration resulted in damage to seeds. The effect of fungal treatment on root length and shoot length is shown in Figure 6. The analysis of results reveal that these fungal cultures significantly enhance the root length and shoot length. The mean dry weights of roots in control seeds were 0.087 ± 0.003 mg and the mean dry weights of roots of seeds treated with E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E 137 (Penicillium oxalicum) was 0.147 ± 0.004 mg, 0.183 ± 0.003 mg and 0.135 ± 0.003 mg respectively.
Similarly, the mean dry weights of shoots in control seeds were 0.673 ± 0.004 mg and the mean dry weights of shoots of seeds treated with E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E 137 (Penicillium oxalicum) was 0.762 ± 0.003 mg, 0.883 ± 0.004 mg and 0.889 ± 0.004 mg respectively. The seedling vigor also increased as a result of fungal treatment.
The vigor index of control seeds was 1886 and the vigor index increased on treatment with fungal spores. The highest vigor index was seen by treatment with E 204 (3603) followed by E 137 (2856) and E 215 (2759). These results indicate that treatment of maize seeds with fungal spores significantly improves seedling growth and vigor of seedlings.
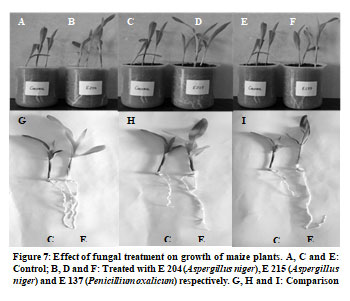
The effect of fungal treatment on plant growth was studied in pot culture experiments (Figure 7). The maize plants were grown in sterilized soil having pH 7.61, organic carbon 0.86%, available nitrogen 291 kg/ha, available phosphorus 36.33 kg/ha and available potassium 723.33 kg/ha. This indicates that the agricultural field soil contains high level of phosphorus and other nutrients which could be due to continuous addition of chemical fertilizers in the agricultural fields. The plant height, root length, area of largest leaf and dry weights of roots and shoots were compared in control plants and in plant grown in soil supplemented with fungal spores of E 204, E 215 and E 137 (Yin et al. 2015).
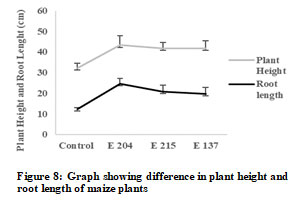
It was seen that root length and shoot length significantly increased in all the fungal treatments in comparison to control (Figure 7 and Figure 8). E 204 (Aspergillus niger) culture showed best results and there was 1.34 folds increase in plant height and 1.58 folds increase in root length when treated with E 204 (Aspergillus niger). There was 1.29 folds increase in plant height when treated with E 215 (Aspergillus niger) and 1.29 folds increase in plant height when treated with E137 (Penicillium oxalicum).
The root length increased 1.66 folds when treated with E 215 (Aspergillus niger) and 1.97 folds when treated with E137 (Penicillium oxalicum). Further, the mean dry weight of control plants was 0.117 ± 0.004 mg and the mean dry weight plants grown in soil containing E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E137 (Penicillium oxalicum) was found to 0.123 ± 0.005 mg, 0.132 ± 0.004 mg and 0.123 ± 0.005 mg respectively (Jan et al. 2020).
Mean dry weight of roots of control plants was 0.122 ± 0.005 mg while the mean dry weight roots grown in soil containing E 204 (Aspergillus niger), E 215 (Aspergillus niger) and E137 (Penicillium oxalicum) was seen to be 0.130 ± 0.004 mg, 0.140 ± 0.004 mg and 0.172 ± 0.004 mg respectively. The leaf area of the longest leaf of control plant was 81.9 cm2 and it increased significantly when treated with fungal cultures.
The largest leaf area was found by treatment with E 137 (170.0 cm2) followed by E 215 (116.9 cm2) and E 204 (109.2 cm2). These results indicate that addition of fungal spores in soil positively influence the growth of maize plants which could be due to phosphate solubilizing ability of these fungal cultures or due to other plant growth promoting activities of these endophytic fungi (Yin et al. 2015).
Over application of chemical fertilizers for increasing the agricultural productivity adversely affects agricultural sustainability and causes harmful effects on environment. Hence, steps are needed to develop alternative strategies for improving plant nutrition and increasing agricultural production (Yin et al. 2015). There is a global trend for bioprospecting indigenous microorganisms which may be used for promoting sustainable agriculture practices and help in decreasing the use of chemical fertilizers in agricultural fields.
It has been suggested that bioformulations of indigenous microbes having phosphate solubilizing activities may be used for promoting plant growth in view of food security scenario. The application of indigenous microbes is an ecofriendly, environmentally safe and healthy practice having potential to create better crops. It has been observed that endophytic microbes have several plant growths promoting activities including phosphate solubilization, siderophore production, IAA production, etc (Kumar and Gopal 2015; Ahkami et al. 2017; Jan et al. 2020).
The relationship of host plant with endophytic microorganisms is very special and significantly influence the formation of different metabolites in plant which provide various benefits to plants (Jia et al. 2016). In this study it has been demonstrated that bioinoculation of endophytic fungi belonging to Aspergillus niger and Penicillium oxalicum significantly improves growth of maize plants.
Earlier studies have also demonstrated that inoculation of endophytic bacteria and fungi have positive influence on plant growth (Matos et al. 2017; Adhikari and Pandey 2018). It has been reported that endophytic fungi Cladosporium cladosporiodes positively influenced the shoot growth while Aspergillus amstelodami positively influenced the root length in the rice seedlings (Lalngaihawmi et al. 2018).
Study by Yin et al. (2015) reported that Penicillium oxalicum is capable of promoting maize growth in calcareous soils and studies in Egypt have demonstrated that inoculation of Penicillium chrysogenum and Penicillium crustosum significantly increase root length in maize (Hassan et al. 2017). The plant growth promotion activities of various bacteria and fungi have also been reported by other (Banu et al. 2019; Aliyat et al. 2020, Chouyia et al. 2020; Turbat et al. 2020). Hence, our results are in accordance to earlier studies. Moreover, the fungal cultures isolated in this study are indigenous to this region and it appears that they may be used as bioinoculants for improving soil health and increasing plant growth. However, further studies are needed to examine the effects of these cultures in unsterilized soil and in field conditions (Turbat et al. 2020).
CONCLUSION
In this study 26 phosphate solubilizing endophytic fungi have been isolated and identified. Three fungal cultures having high PSI have been identified as Aspergillus niger (E 204 and E 215) and Penicillium oxalicum (E 137) using 18S rDNA sequencing. These three fungi positively influence the seedling growth and growth of maize plants in pot culture experiments when used as bioinoculants. The findings suggest that these fungi may be used as bioinoculants, biofertilizers for promoting sustainable agriculture. However, further studies are needed to strengthen these findings and examine the effect of these cultures in field conditions in unsterilized soils before they may be used as bioinoculants in field conditions.
ACKNOWLEDGEMENTS
We thank National Fungal Culture Collection of India (NFCCI), Pune (India) for help in identification of cultures.
Conflict of Interests: The authors declare no conflict among their interests while completing this research.
REFERENCES
Abawari RA Tuji FA and Yadete DM (2021) Multi trait of phosphate solubilizing bacterial and fungal isolates and evaluation of their potential as biofertilizer agent for coffee production Vol 7 No 1 Pages 1-15.
Adhikari P and Pandey A (2018) Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots Rhizosphere Vol 9 Pages 2-9.
Ahkami HA White RA Handakumbura PP and Janson C (2017) Rhizosphere engineering: enhancing sustainable plant ecosystem productivity Rhizosphere Pages 233-243.
Aliyat FZ Maldani M Guilli ME Nassiri L and Ibijbijen J (2020) Isolation and characterization of phosphate solubilizing bacteria from phosphate solid sludge of the Moroccan phosphate mines The Open Agricultural J Vol 14 Pages 16-24.
Bano N Mahadevamuurthy M and Nagaraj A (2020) Plant growth-promoting fungi (PGPF) instigate plant growth and induce disease resistance in Capsicum annum L. upon infection with Colltotrichum capsica (Syd.) Butler & Bisby Biomolecules Vol 10 No 41 Pages 1-18.
Baron NC Costa NTA Mochi DA and Rigobelo EC (2018) First report of Aspergillus sydowii and Aspergillus brasiliensis as phosphorus solubilizers in maize Ann Microbiol Vol 68 Pages 863-870.
Chouyia FE Romano I Fechtail T Fagnano M Fiorentino N Visconti D Idbella M Ventorino V and Pepe O (2020) P-Solubilizing Streptomyces roseocunereus MS1B15 with multiple plant growth-promoting traits enhance barley development and regulate rhizosphere microbial population Front. Plant Sci Vol 11No 1137 Pages 1-10.
Das SK Avasthe RK Sharma K Singh M and Sharma P (2017) Soil fertility assessment in different villages of East Sikkim District Ind J Hill Farming Vol 30 No 1 Pages 14-16.
El-Hamshary OIM Bohkari FM Al-Aklouk LA Noor SO and Najjar AA (2019) Molecular characterization of some phosphate solubilizing microorganisms Pharmacophore Vol 10 No 1 Pages 37-71.
Elias F Woyessa D and Muleta D (2016) Phosphate solubilization potential of rhizosphere fungi isolated from plants in Jimma zone, Southwest Ethiopia Int J Microbiol Vol 2016 Pages 1-11.
Fouda A Eid AM Elsaied A El-Belely EF Barghoth MG Azab E Gobouri AA and Hassan SE-D (2021) Plant growth promoting endophytic bacterial community inhabiliting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions Plants Vol 10 No 76 Pages 1-22.
Gizaw B Tsegay Z Tefera G Aynalem E Wassie M and Abatneh E (2017) Phosphate solubilizing fungi isolated and characterized from teff rhizosphere soil collected from North Showa and Gojam, Ethiopia J Fertil Pestic Vol 8 No 2 Pages 1-9.
Han LR Wang ZH Zhang HJ Xue LS Feng JT and Zhang X (2013) Isolation of endophytic fungi from Tripterygium wilfordii and their insecticidal activities. African J Microbio Res Vol 7 No 9 Pages 771-776.
Hassan SE-D (2017) Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv Res Vol 8 Pages 687-695.
Jan U Feiwen R Masood J and Chun SC (2020) Characterization of soil microorganisms from humus and indigenous microorganism amendments Microbiol Pages 1-7.
Jia M Chen L Xin HL Zheng CJ Rahman K Han T and Qin LP (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review Front Microbiol Vol 7 No 906 Pages 9-14.
Kalayu G (2019) Phosphate solubilizing microorganisms: Promising approach as biofertilizers Int J Agron Vol 2019 Pages 1-7.
Kumar BL and Gopal DVR (2015) Effective role of indigenous microorganisms for sustainable environment 3 Biotech Vol 5 Pages 867-876.
Lalngaihawmi Banik S Chakruno P and Khatemenla (2018) Effect of rice fungal endophytes on seed germination and seedling growth of rice Int J Curr Microbiol App Sci Vol 7 No 4 Pages 3653-3663.
Madhusadhan L (2015) Agriculture role on Indian economy Bus Eco J Vol 6 No 4.
Mahadevamurthy M Channappa TM Sidappa M Raghupathi MS and Nagaraj AK (2016) Isolation of phosphate solubilizing fungi from rhizosphere soil and its effect on seed growth parameters of different crop plants J App Biol Biotech Vol 4 No 06 Pages 022-026.
Matos ADM Gomes ICP Nietsche S Xavier AA Gomes WS Neto JADS and Pereira MCT (2017) Phosphate solubilization by endophytic bacteria isolated from banana trees An Acad Bras Cienc Pages 1-10.
Mazrou YS Makhlouf AH Elbealy ER Salem MA Farid MA Awad MF Hassan MM and Ismail M (2020) Molecular characterization of phosphate solubilizing fungi Aspergillus niger and its correlation to sustainable agriculture J Environ Biol Vol 41 Pages 592-599.
Noorjahan A Aiyamperumal B and Anantharaman P (2019) Isolation and characterization of seaweed endophytic fungi as an efficient phosphate solubilizers Biosci Biotech Res Asia Vol 16 No 1 Pages 33-39.
Nyongesa BW Okoth S and Ayugi V (2015) Identification key for Aspergillus species isolated from maize and soil of Nandi County Kenya Adv Microbiol Vol 5 Pages 205-229.
Pande A Pandey P Mehra S Singh M and Kaushik S (2017) Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize JGEB Vol 15 Pages 379-391.
Qarni A Billah M Hussain K Shsh SH Ahmed W Alam A Sheikh AA Jafri L Munir A Malik KM and Khan N (2021) Isolation and characterization of phosphate solubilizing microbes from rock phosphate mines and their effect for sustainable agriculture Sustainability Vol 13 No 2151 Pages 1-14.
Rashmi I Mina BL Kala S, Meena HR Singh RK and Kartika KS (2018) Important phosphate solubilizing microbes for agriculture HaritDhara Vol 1 No 1 Pages 13-14.
Raymond NS Gómez-Muñoz B van der Bom FJT Nybrose O Jensen LS Müller-Stöver D Oberson A and Richardson AE (2021) Phosphate- solubilizing microorganisms for improved crop productivity: a critical assessment New Phytologist Vol 229 Pages 1268-1277.
Santoyo G Hagelseib MG and Mosqueda OCM (2016) Plant growth promoting bacterial endophytes Microbiol Res Vol 183 Pages 92-99.
Singh P Singh KG and Singh JP (2018) Indirect method for measurement of leaf area and leaf area index of soilless cucumber crop Adv Plants Agric Res Vol 8 No 2 Pages 188-191.
Singh P Sharma A Bordoloi M and Nandi SP (2020) Molecular identification of endophytic fungi isolated from medicinal plant Biointerface Res Appl Chem Vol 10 No 5 Pages 6436-6443.
Turbat A Rakk D Vigneshwari A Kocsubé S Thu h Szepesi Á Bakacsy L Škrbic BD Jigjiddorj E-A Vágvölgyi C and Szekeres A (2020) Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens Microorganisms Vol 8 Pages 1-15.
Visagie CM Houbraken J Frisvad JC Hong SB Klaassen CHW Perrone G Seifert KA Varga J Yaguchi T and Samson RA (2014) Identification and nomenclature of the genus Penicillium Studies in Mycology Vol 78 Pages 343-371.
Wagh GS, Chavhan DM and Sayyed MRG (2013) Physiochemical analysis of soils from Eastern part of Pune city Universal J Environ Res Tech Vol 3 No 1 pp 93-99.
Yin Z Shi F Jiang H Roberts DP Chen S and Fan B (2015) Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil Can J Microbiol Vol 61 Pages 913-923.
Zhu J Li M and Whelan M (2017) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review Sci total Environ Vol 612 No 2018 Pages 522-537.
Zheng YK Qiao XG Miao CP Liu K Chen YW Xu LH and Zhao LX (2016) Diversity, distribution and biotechnological potential of endophytic fungi Ann Microbiol Vol 66 Pages 529-542.


