Department of Biotechnology, K. L.E. Technological University, Hubballi, Karnataka, India.
Corresponding author email: anilrshet@gmail.com
Article Publishing History
Received: 05/12/2020
Accepted After Revision: 28/03/2021
Biodiesel is an environmental friendly, renewable and biodegradable fuel and a potential substitute to conventional diesel, and can be produced using vegetable oil with short chains of alcohol. Due to the high cost of produced biodiesel, it is required to explore inexpensive feedstock with high value-added by products. Rice bran is a by-product of rice milling which is mainly used as animal feed and oil produced is used for industrial applications. Rice bran oil (RBO) can be used as a low-cost feedstock for biodiesel production as compared to traditional oils derived from cereal or seed sources. In this study, rice bran was taken as feedstock and oil was extracted from it which was further converted to Biodiesel. Optimization of oil extraction process from rice bran was done taking into account the affecting factors like solvent used for extraction, solvent to solid ratio and extraction time. Biodiesel was produced from extracted rice bran oil using lipase as catalyst. Process variables like molar ratio of methanol to oil and catalyst concentration were studied for maximum ester yield. The optimized conditions for oil yield were found to be hexane as best solvent, 10:1 solvent to solid ratio and 8 h extraction time. Further, the produced RBO was utilized for lipase enzyme catalyzed biodiesel production. The process parameters affecting ester yield were optimized and were found to be 6:1 methanol to oil molar ratio and 4% (w/woil) lipase enzyme concentration. The RBO biodiesel was characterized and found to fulfill the requirements of ASTM and DIN international standards for biodiesel.
Biodiesel; Rice Bran; Optimization; Oil Extraction; Lipase Enzyme.
Shet A.R, Patil L.R, Hombalimath V. S, Sharanappa A. Parametric Optimization of Oil Extraction and Lipase Catalyzed Biodiesel Production from Rice Bran. Biosc.Biotech.Res.Comm. 2021;14(1).
Shet A.R, Patil L.R, Hombalimath V. S, Sharanappa A. Parametric Optimization of Oil Extraction and Lipase Catalyzed Biodiesel Production from Rice Bran. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/30ikDgx“>https://bit.ly/30ikDgx</a>
Copyright © Shet et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The increase in industrialization and population worldwide have created a huge demand for fuels and at the same time increased air pollution and decrease in fossil fuels deposits. Hence, there is a requirement of developing new forms of renewable and eco-friendly fuels (Venkanna and Venkataramana, 2009; Dayang et al., 2019; Febrian et al., 2020). One of the approaches is waste-to-energy technologies, where waste matter is converted into renewable energy. This technology can solve both problems: waste and energy. Waste can be treated and reused to be converted into the various forms of fuel which can be used for energy generation. The conversion of waste into biofuel has become more attractive due to it being low-carbon, locally available, safe, and sustainable for the economy (Rengasamy et al., 2018; Goga et al., 2019
Energy generation from biofuel in various approaches has been explored in order to generate a suitable quality of bioethanol, biodiesel, biogas, and biohydrogen. Biofuel can be produced from three main sources: vegetable oil and animal fat, nonfood crops and algae (Dayang et al., 2019; Anh et al., 2020). There are various types of reaction that can be applied to the production of biofuels, such as fermentation, transesterification, and pyrolysis of biomass and industrial and domestic waste. Transesterification is a simple and efficient method for biofuel production. Transesterification involves catalytic (acid, base and enzymes) reaction between alcohol and triglycerides of fatty acids to form esters and glycerol. Enzymatic transesterification is better than chemical transesterification, due to low energy requirements, easy glycerol recovery and transesterification of high free fatty acid content glycerides (Xiulian et al., 2012; Bani et al., 2018; Goga et al., 2019, Dharmaraja et al., 2019; Febrian et al., 2020).
Biodiesel is a biofuel that is produced from the transesterification reaction of vegetable oils, animal fats, or grease. Biodiesel can be used as a substitute for petro diesel because it can be used in any diesel engine without modification (Dayang et al., 2019). Biodiesel is nontoxic, biodegradable, has a high flash point, and reduces emissions of unburned matter and particulate matter (Dayang et al., 2019; Anh et al., 2020). Initially the production of biodiesel was focused on edible oils such as vegetable oil (soybean oil, sunflower oil, and cottonseed oil) and animal fat. Agricultural industries are the main contributor of raw material in producing biodiesel (Nguyen et al., 2019).
India being the largest producer of rice (Oryza sativa) has a capability to manufacture about 1 million tonnes of RBO per year. Rice bran containing 15-23% oil is a byproduct of rice milling (Syed et al., 2016; Bani et al., 2018). Rice bran oil contains naturally occurring bioactive and antioxidant compounds. De-oiled rice bran is used as poultry and cattle feed due to its high protein content and vitamins (Fajriyati et al., 2019; Anh et al., 2020). Presently, the industry is utilizing rice bran of around 3.5 million tonnes to produce around 0.65 million tonnes RBO. This unused non-edible oil can be used as an alternative energy source to reduce the biodiesel production cost. The objective of this study was to extract oil from rice bran and produce biodiesel from it. The effect of different process parameters like solvent type, solvent to solid ratio, reaction time affecting the oil yield and variables like alcohol to oil molar ratio, concentration of catalyst affecting the biodiesel yield were studied. Characterization of the produced biodiesel was also done in this study.
MATERIAL AND METHODS
For the materials, rice bran was obtained from local rice mill, Hubballi. Laboratory grade hexane, petroleum ether and methanol were procured from SRL Pvt Ltd. Lipase enzyme was procured from Sigma Aldrich Company. For the extraction of oil from rice bran, twenty grams of powdered rice bran husk was taken in a Soxhlet extractor. 200 ml of hexane / petroleum ether was used in the extraction process. Soxhlet extractor was operated for a reaction time of 8 h and the extract was subjected to filtration using Whatman filter paper to remove the suspended particles. Then it was subjected to rotary evaporator for oil solvent separation. The percentage of oil yield was determined by the ratio of amount of oil extracted by the amount of rice bran husk taken multiply by 100.
For the process parameter optimization for rice bran oil extraction, the effect of three main parameters on oil extraction was studied. The solvent type, extraction time and solvent to solid ratio were studied and optimized the operating conditions for maximum oil yield. Twenty grams of meal (rice bran husk) was subjected to two different solvents namely petroleum ether and hexane, solvent to solid ratio between 6:1 to 10:1 v/w and reaction time between 1 h to 8 h. The optimum conditions were determined by varying one factor at a time. For the process parameter optimization for RBO biodiesel production, transesterification involves catalyzed reaction between triglycerides and an alcohol yielding esters and glycerol. To optimize the operating conditions for RBO biodiesel production, one factor at a time (OFAT) was used. The effect of lipase concentration (1%, 2%, 3%, 4% w/woil), and molar ratio of methanol to oil (2:1, 4:1, and 6:1) on biodiesel production was studied. Methanol and lipase catalyst were mixed and then added to RBO in the conical flask. The conical flask was plugged to avoid evaporation of methanol and then placed on a shaker for 24 h at 150 rpm at room temperature.
The mixture was kept in the separating funnel overnight where glycerol was separated by gravity separation. The biodiesel produced was washed with warm water several times to remove catalyst, glycerol residuals and methanol. The crude biodiesel was then heated at 100 oC in an open pan to remove all the water particles. The % biodiesel yield was found by the equation: [weight of Biodiesel produced/ weight of oil taken] * 100. For the produced biodiesel physical properties such as carbon residue, density, flash point and viscosity were found. For the characterization of rice bran oil biodiesel, the characterization of RBO biodiesel was done using standard test procedures. Density was determined by ASTM D1298, Flash point by ASTM D93, Kinematic viscosity by ASTM D445 and Carbon residue by ASTM D4530.
RESULTS AND DISCUSSION
Rice bran oil extraction
Figure 1: Powdered Rice bran husk and Rice bran oil

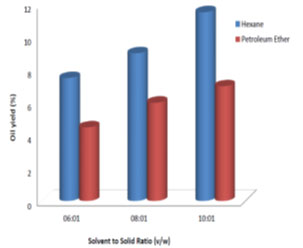
Process parameter optimization for RBO extraction: Effect of solvent type on oil yield: Two different solvents petroleum ether and hexane were used to determine their influence on oil yield. Fig. 2 shows the oil yields by the solvent’s petroleum ether and hexane. The oil yield with hexane (6.25%) was observed to be more than that of petroleum ether (4.16%) by 2.09% as evaluated at the end of all experiments conducted, by varying the other two parameters. Therefore, hexane was considered best solvent for RBO extraction. It is similar to the results obtained by (Tamilarasan and Sahadevan, 2012; Syed et al., 2016; Shukla and Pratap, 2017; Majid et al., 2019).
Effect of solvent to solid ratio on oil extraction: Fig. 3, 4, 5 and 6 show the percent of oil extracted by hexane and petroleum ether at different solvent to solid ratios (6:1, 8:1 and 10:1 v/w) and different processing time (1, 3, 6 and 8 h). By increasing the ratio from 6:1 to 10:1 and processing time from 1 h to 8 h, the oil yield using hexane increased from 1.5% to 11.5% and for petroleum ether it increased from 1% to 7%. For both solvents used, the oil yield was observed to increase with the increase in solvent to solid ratio and processing time, which indicated good mass transfer due to the concentration difference between the solid and the liquid phase (Balaji et al., 2012; Majid et al., 2019). Therefore, solvent to solid ratio of 10:1 and processing time of 8 h showed maximum oil yield. Similar results were observed by (Oliveira et al., 2012; Syed et al., 2016; Shukla and Pratap, 2017; Pandey and Shrivastava, 2018).
Figure 2: Effect of different solvents on oil yield
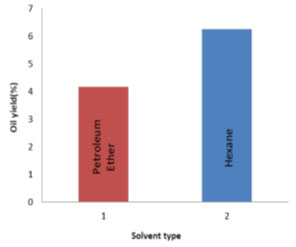
Figure 3: Effect of solvent to solid ratio on oil yield for a reaction time of 1 h.
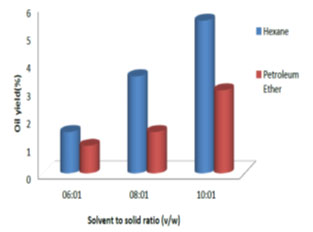
Figure 4: Effect of solvent to solid ratio on oil yield for a reaction time of 3 h.
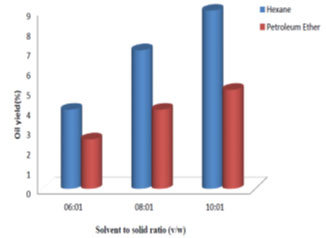
Figure 5: Effect of solvent to solid ratio on oil yield for a reaction time of 6 h.
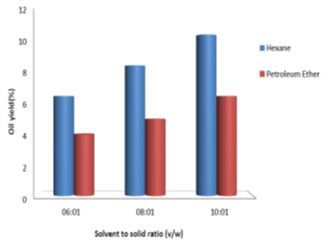
Figure 6: Effect of solvent to solid ratio on oil yield for a reaction time of 8 h.
Effect of alcohol to oil molar ratio on the biodiesel yield: Different molar ratio of methanol to oil ranging from 2:1 to 6:1 was used to determine the effect on ester yield. The transesterification reaction was conducted with varying concentrations of the catalyst. From the Fig. 7, it can be observed that with the increase in the methanol to oil molar ratio the ester yields increased. The maximum ester yield of 99.25% was observed for molar ratio of 6:1 and catalyst concentration of 4%. The similar trend in the results were obtained by other researchers (Sanjay et al., 2011; Anil et al., 2012a; Anil et al., 2012b; Joshua, 2013; Jayaprabakar et al., 2019; Febrian et al., 2020).
Figure 7: Effect of molar ratio of methanol to oil on biodiesel yield for a catalyst concentration of 4%
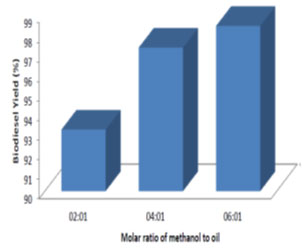
Figure 8: Effect of catalyst concentration on biodiesel yield for 2:1 molar ratio of methanol to oil
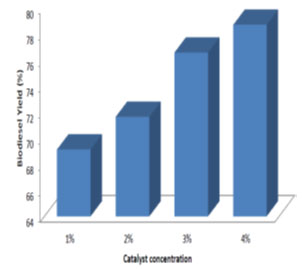
Figure 9: Effect of catalyst concentration on biodiesel yield for 4:1 molar ratio of methanol to oil
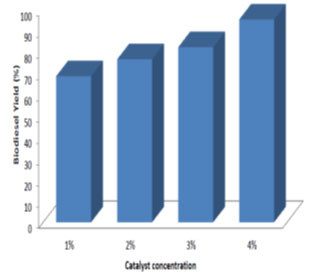
Figure 10: Effect of catalyst concentration on biodiesel yield for 6:1 molar ratio of methanol to oil
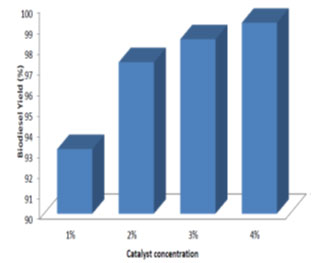
Effect of catalyst concentration on the biodiesel yield: Different catalyst concentration ranging from 1% to 4% (w/woil) was used in the study. The processing time and temperature was maintained at 24 h and room temperature respectively. From Fig. 8, 9, 10, it was observed that for different molar ratio of alcohol to oil, the ester yield increased with the increase in catalyst concentration. Therefore 4% catalyst concentration gave maximum ester yield of 99.25%. The Similar results were obtained by (Edward et al., 2001; Xiaohu and Feng, 2010; Arumugam and Ponnusami, 2017; Jayaprabakar et al., 2019; Febrian et al., 2020).
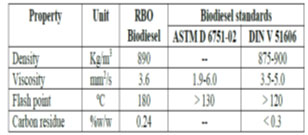
Characterization of RBO biodiesel: The characterization of RBO biodiesel was done as per standard test procedures. All the properties of biodiesel were determined at department of biotechnology and mechanical engineering, KLE Technological University, Hubballi. The flash point and fire point were observed to be 180 oC and 210 oC respectively. Density was found to be 890 Kg/m3. Viscosity of RBO biodiesel was found to be 3.6 mm2/s and carbon residue was found to be 0.24 %w/w. Similar results were obtained by (Joshua, 2013; Nguyen et al., 2019; Veeranna et al., 2020). These results fulfilled ASTM D6751-02 and DIN V51606 biodiesel standards.
Table 1. Characterization of RBO Biodiesel
CONCLUSION
The present study involved rice bran oil extraction by solvent extraction process and enzymatic biodiesel production by transesterification. The optimum conditions obtained were 10:1 solvent to solid ratio, 8 h reaction time for maximum oil yield of 12%. Hexane gave good oil yield when compared to petroleum ether. Different factors like alcohol to oil molar ratio and concentration of catalyst affecting biodiesel production was studied. The optimum values obtained were 6:1 alcohol to oil molar ratio and 4% concentration of catalyst for maximum biodiesel yield of 98.5%. The produced RBO biodiesel was characterized for carbon residue, viscosity, density and flash point. All the values obtained were as per ASTM and DIN international standards. The results showed that the rice bran oil is a potential raw material for biodiesel production. Results indicated that the Rice bran oil can be used for biodiesel production.
ACKNOWLEDGEMENTS
We are thankful to the staff of Biotechnology and Mechanical department, K.L.E. Technological University, Hubballi, India, for their help and support in conducting this research work.
Conflict of Interest: Authors have no conflict of interest
REFERENCES
Anh, T. H., Meisam, T., Mortaza, A., Antonio, P. C., Aykut, I. O., Anh, T. L. and Abbas, G. (2020). Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: A review. Renewable and sustainable energy reviews, 135. http://doi.org/10.1016/j.rser.2020.110204
Anil, R. S., Basavaraj, B. U., Laxmikant, R. P., Veeresh, S. H. and Deepak, A. Y. (2012). Optimization of Biodiesel production and its characterization from soya beans. International Journal of Advances in Engineering, Science and Technology, 2 (3): 322-327.
Anil, R. S., Sashank, S. K., Deepa, D.M., Rajath, S. P. and Jagadish, V. R. (2012). Production and characterization of Biodiesel from cottonseed oil. International Journal of Advances in Engineering, Science and Technology, 2 (4): 328-334.
Arumugam, A. and Ponnusami, V. (2017). Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon, 3(12):1-18.
https://doi.org/10.1016/j.heliyon.2017.e00486
Balaji, M. P., Shrikant, D., Sanjay, D. andMunish, S. (2012). Optimization of extraction of oil and biodiesel from Thespesia populnea seed oil by alkali catalyst in India. International Journal of Green Energy, 13(15): 1634-1639.
Bani, O., Parinduri S. and Ningsih P.R.W. (2018). Biodiesel production from rice bran oil by transesterification using heterogeneous catalyst natural zeolite modified with K2CO3. IOP conf. Ser. Material Science and Engineering, 309: 12107.
Dayang, N. F., Abang, Z., Ida, I. M., Nurul, S., Mohd, D., Nor Azyati, A. M., Nozieana, K., Nurul, A. M. and Lazim. (2019). Production of biodiesel from rice bran oil. Biomass Biopolymer-Based Materials and Bioenergy,409-447, https://doi.org/10.1016/B978-0-08-102426-3.00018-7.
Dharmaraja, J., Nguyen, D. D, Shobana, S., Saratale, G. D., Arvind N. S. and Atabani A.E. (2019). Engine performance, emission and bio characteristics of rice bran oil derived biodiesel blends. Fuel, 239:153-161.
Divine, B. N. and Anuanwen, C.F. (2020). Optimization methods for the extraction of vegetable oils: A review. Processes, 8: 209. https://doi.org/10.3390/pr8020209.
Edward, C., Cirilo, N. H., Genta, K., Kenji, S. and Ayaaki, I. (2001). Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties, 37(1): 65-71.
Fajriyati, M., Fajar, Herman, B., Sri, I., Abigael, T., Suhardi and Muhammad, S. (2019). Model development to enhance the solvent extraction of rice bran oil. OCL, 26:16.
https://doi.org/10.1051/ocl/2019009.
Febrian, R., Vinod, K. J., Ranjna, J., Romanee, T., Masaki, T. and Kazuyuki, O. (2020). Biodiesel Production from Refined Rice Bran Oil Using Eggshell Waste as Catalyst Impregnated with Silver Nanoparticles, 4th International Conference on Green Energy and Applications (ICGEA), Singapore, 134-138. https://doi.org/10.1109/ICGEA49367.2020.239706.
Goga, G., Chauhan, B.S., Mahla, S.K. and Cho, H. M. (2019). Performance and emission characteristics of diesel engine fueled with rice bran biodiesel and n-butanol. Energy Rep, 5:78-83.
Jayaprabakar, J., Karthikeyan, A., Harish, V., Prabhu, A., Nivin, J., Parthipan, J. and Anish, M. (2019). Enzymatic production of rice bran biodiesel and testing of its diesel blends in a four-stroke CI engine. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. https://doi.org/ 10.1080/15567036.2019.1671554
Joshua, F. (2013). Production of Biodiesel (B100) from Jatropha oil using sodium hydroxide as catalyst. Journal of petroleum engineering, 1-6.
https://doi.org/10.1155/2013/956479.
Majid, A., Phull, A. R. and Khaskheli A. H. (2019). Applications and opportunities of supercritical fluid extraction in food processing technologies: a review. Int J Adv Appl Sci, 6: 99-103.
https://doi.org/10.21833/ijaas.2019.07.013
Nguyen, D. D., Dharmaraja, J., Shobana, S., Sundaram, A., Chang, S. W., Kumar, G., Shin, H. S., Saratale, R. G. and Saratale, G. D. (2019). Transesterification and fuel characterization of rice bran oil: A biorefinery path. Fuel, 253: 975-987. https://doi.org/10.1016/j.fuel.2019.05.063
Oliveira, R., Oliveira, V., Aracava, K. K. and Costa Rodrigues, C. E. (2012). Effects of the extraction conditions on the yield and composition of rice bran oil extracted with ethanol—a response surface approach. Food Bioprod Process, 90 (1):22-31.
https://doi.org/10.1016/j.fbp.2011.01.004
Pandey, R. and Shrivastava, S. L. (2018). Comparative evaluation of rice bran oil obtained with two-step microwave assisted extraction and conventional solvent extraction. J Food Eng, 218:106-114. https://doi.org/10.1016/j.jfoodeng.2017.09.009
Rengasamy, M., Pugalenthi, V., Sivasubramanian, V. and Jaya, N. (2018). Synthesis of biodiesel from palm oil & rice bran oil using NA2SIO3 as heterogeneous base catalyst. Eng Reports, 1:11-21.
Sanjay, G., Sam, C. and Sentil kumaran, D. (2011). Process optimization for biodiesel synthesis from jatropha curcas oil. Distributed Generation and Alternative Energy Journal, 26(4):6-16. https://doi.org/10.1080/21563306.2011.10462201.
Shukla, H. S. and Pratap, A. (2017). Comparative studies between conventional and microwave assisted extraction for rice bran oil. J Oleo Sci, 66(9):973-979. https://doi.org/10.5650/jos.ess17067
Syed, W. A., Farhan, J., Sajjad, A., Muhammad, A. and Abdur, R. (2016). Parametric optimization of rice bran oil extraction using response surface methodology. Polish Journal of chemical Technology, 18(3): 103-109.
Tamilarasan, S. and Sahadevan, R. (2012). Optimization and kinetic studies on algal oil extraction from marine macroalgae Ulva lactuca. Bioresource Technology, 107:319-326.
Venkanna, B. K. and Venkataramana, R. (2009). Biodiesel production and optimization from calophyllum inophyllum linn oil – A three stage method. Bioresource Technology, 100(21): 5122-5125.
Veeranna, S. H., Shivlingasarj, V. D. and Sharanappa, A. (2020). Characterization of lipase immobilized on chitosan magnetic micro-particles for economic biodiesel production. International Journal of Scientific and Technological Research, 9(3): 5111-5116.
Xiaohu, F., Xi, W. and Feng, C. (2010). Ultrasonically assisted production of Biodiesel from crude cottonseed oil. International Journal of Green energy, 7(2): 117-127.
Xiulian, Y., Haile, M., Qinghong, Y., Zhenbin, W. and Jinke, C. (2012). Comparison of four different enchancing methods for preparing biodiesel through transesterification of sunflower oil. Applied energy, 91(1): 320-325.


