1Department of Urology, Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine(TCM), Xianyang ,712000,China
2Biological of Shaanxi University of Traditional Chinese Medicine, Xianyang, 712000,China
Corresponding author email: wangwenjuan1225@126.com
Article Publishing History
Received: 05/12/2020
Accepted After Revision: 29/03/2021
The objective of the present study was to determine the effects of norcantharidin on the invasion of PC-3 cells. In this study, norcantharidin was applied to PC-3 cells, after which the real-time fluorescence quantitative PCR and Western-blot were used to detect the expression level of cortactin and MMP-9, scratch test and invasion tests were also used to detect the invasion of PC-3 cells. Transmission electron microscopy was used to observe the morphological changes of pseudopods of PC-3 cells. We found the mRNA expression of cortactin and MMP-9 in PC-3 cells of norcantharidin group was significantly lower than that in PC-3 cells of blank control group, The protein expression of cortactin and MMP-9 in PC-3 cells of the norcantharidin group was also significantly lower than that of the blank control group (P < 0.05). the scratch test results showed that the scratch healing degree of PC-3 cells in the norcantharidin group was significantly lower than that of the blank control group (P < 0.05). Similarly, the invasion of PC-3 cells in the norcantharidin group was also significantly lower than that of the blank control group (P < 0.05), and observation of transmission electron microscope showed that the invasion of pseudopodia of PC-3 cells in the norcantharidin group was significantly less than that in the blank control group. The results of this study indicate that when norcantharidin acts on PC-3 cells, the invasion of PC-3 cells decreases, which may be related to the decrease of the expression level of cortactin and MMP-9. The results also demonstrate that norcantharidinmay may be a new treatment for prostate cancer.
Norcantharidin; Prostate Carcinoma; Cortactin; MMP-9; Migration; Invasion
Luo X, Wang W, Li K, You J, Du H. Effect of Cortactin and MMP-9 Expression Inhibited by Norcantharidin on Invasion of PC-3 Cells. Biosc.Biotech.Res.Comm. 2021;14(1).
Luo X, Wang W, Li K, You J, Du H. Effect of Cortactin and MMP-9 Expression Inhibited by Norcantharidin on Invasion of PC-3 Cells. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3r4QPQn”>https://bit.ly/3r4QPQn</a>
Copyright © Luo et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
A recent study has indicated that norcantharidin (NCTD) can inhibit PC-3 cell proliferation and induce PC-3 cells apoptosis (Luo, 2020). As well, few studies have also shown that NCTD can inhibit the invasiveness of some tumor cells (Fan, 2020; Yang, 2020 and Gao,2020). Cortactin and MMP-9 have been shown to be associated with prostate carcinoma cell invasion (Ma, 2016 and Liu, 2016). This study aims to confirm if NCTD can inhibit the expression of cortactin and MMP-9 and then reduce the invasion of PC-3 cells. Prostate carcinoma is the most common malignant tumor of the male reproductive system, which is more common in the elderly men over 60 years . In recent 10 years, the incidence rate of prostate carcinoma in China has been increasing year by year. Compared with developed countries, the incidence of prostate carcinoma in China is quite higher than before, (Li,2021).
The incidence rate of advanced carcinoma is also higher in China than in developed countries, (Xu,2020), and many patients lose the chance of radical operation. The vast majority of patients with advanced prostate carcinoma are initially sensitive to androgen inhibition. However, after an average of 2.5 years of androgen suppression endocrine therapy, all of these patients will be transformed into hormone resistant prostate carcinoma (HRPC), that is, it has no response to the endocrine therapy of inhibiting androgen. The latter has been developed rapidly, with a median survival of no more than 18 months. Cortactin, a protein that interacts directly with cytoplasmic microfilaments, served as the tyrosine protein kinase SRC 80 in the early 1990s.The direct substrate of 85-kDa (Wu,1991), encoded by CTTN gene located in 11q13 region of chromosome, contains four structural regions: N-terminal acid region, tandem repeat region, carboxyl proline rich region and SH3 region, which are located in invasive pseudopodia and play an important role in the function of invasive pseudopodia (Ren,2018).
At the same time, matrix metalloproteinases (MMPs) have been confirmed to be involved in the procedure of invasion and distant metastasis of various tumors. At present, 26 different kinds of MMPs have been found, in which MMP-9 can degrade and destroy tumor extracellular matrix, and promote the formation of invasive pseudopodia, (Murphy and Courtneidge 2011). Norcantharidin (NCTD) is a demethylated analogue of cantharidin extracted from cantharidin(Tang,2010). NCTD has strong anti-cancer activity and has been used in the clinical treatment of a variety of malignant tumors. This experiment focuses on whether NCTD can down regulate the expression of cortactin and MMP-9 protein in PC-3 cells, and then inhibit the invasion of PC-3 cells.
MATERIAL AND METHODS
Cell line PC-3 was purchased from the National Cell Bank of Shanghai Institute of Cell Technology. Main reagents used were: NCTD (Aladdin company, batch number d131603-1g), cisplatin (MCE company, batch number hy-17394) cytoplasmic nucleoprotein Extraction Kit (Nanjing Kaiji biological company, batch number kgp150),BCA protein concentration determination kit(biyuntian biological technology company, batch number p0010), mouse monoclonal antibody β-actin(Wuhan PhD Bioengineering Co.,Ltd, batch number bm0627), rabbit monoclonal antibody C ortactin (Abcam, batch number: ab81208), rat monoclonal antibody MMP-9 (Abcam, batch number: ab58803), Transwell culture dish (Corning, batch number: 658042)Instruments used were: Real-time fluorescent quantitative PCR instrument (ABI company of America), CO2 constant temperature incubator (Sanyo company of Japan), inverted microscope (Olympus company of Japan), 3001 enzyme labeling instrument (Thermo Fisher Scientific Company of America).
Cell culture PC-3 cells were resuspended in a complete medium containing 10% fetal bovine serum and placed at 37 ℃ and 5% CO2 Culture in cell incubator. Take the PC-3 cells in logarithmic growth period and add the culture medium containing NCTD with different concentrations prepared in advance,The final concentration of NCTD was 12.5 μg/ml, 25 μg/ml and 50 μg/ml respectively, the blank control group and positive control(cisplatin concentration 2.5 μmol/L).
The total RNA of PC-3 cells in each group was extracted by Trizol reagent in real-time PCR experiment, and then the operation instructions of real-time PCR kit were followed. PCR primers were synthesized by Xi’an Kehao Bioengineering Co., Ltd. the upstream primer sequence of cortactin was 5 ‘-CGATGAGTACGAGAACGAT-3’, the downstream primer sequence was 5 ‘-GCAACACGAACACAAGAGA-3’, the upstream primer sequence of MMP-9 was 5 ‘- GCTACCACCTCGAACTTTGAC -3’, and the downstream primer sequence was 5 ‘- TCAGTGAAGCGGTACATAGGG -3′, the upstream primer sequence of β-actin was 5′ – agcgagcatcccaaagtt-3′, and the downstream primer sequence was 5’ – gggcacaggcatcatcat3 ‘. PCR reaction conditions: 50 °C 2min, 95 °C 10min; 95 °C 30 sec, 60 °C 30sec, 40cycles. In the experiment, each sample was tested three times, and the dissolution curve was drawn. The final data was analyzed with 2-△Ct.
Western-blot was used to collect PC-3 cells from five groups, add the lysate and place it on ice for 30min, 4℃, 12000rpm for 5min, and use BCA protein concentration test kit to determine the protein content in the supernatant. Take 40μg of total protein for SDS-PAGE electrophoresis, transfer membrane, 5% skimmed milk powder, add anti-cortactin rabbit monoclonal antibody and mouse monoclonal antibody MMP-9 4℃ overnight, take β-actin mouse monoclonal antibody as reference, and then add corresponding anti IgG (HRP labeled Sheep anti rabbit and HRP labeled Sheep anti mouse) to incubate at room temperature for 1h. ECL exposure imaging, chemiluminescence system analysis results.
In the cell scratch test, PC-3 cells of blank control group, NCTD 12.5 μg/ml group, NCTD 25 μg/ml group, NCTD 50 μg/ml group and cisplatin 2.5 μmol/l group were taken respectively, and the cell density was adjusted to 2.5×105 cells/ml with 1640 medium, and 6-well plates were connected with 2ml cell suspension of each hole, which was cultured overnight at 37 ℃; cells were washed with PBS for three times to remove the scratched cells, and serum-free medium was added. After 24 hours of treatment, samples were taken for photos.
Transwell experiment, 2.5×105 cells were taken from the blank control group, 12.5 μg/ml, 25 μg/ml, 50 μg/ml NCTD group and positive control group( 2.5 μmol /L cisplatin) PC-3 cells respectively. 100μl Matrigel (final concentration was 1 mg/ml) was added vertically to the center of the upper chamber bottom of the Transwell, chamber of Matrigel was incubated at 37 ℃ for 4-5h to make it dry and gelatinous. After Matrigel dry and gelatinous, 200 μL cell suspensions of the above groups were respectively connected into the upper chamber of Transwell, cultured in 5% CO2 incubator at 37 ℃ for 24h and fixed with 70% ice ethanol solution h. Staining with 0.5% crystal violet dye solution, cleaning with PBS once, wiping the non-migrated cells on one side of the upper chamber with clean cotton ball, observing and taking photos under the microscope.
Transmission electron microscope was used to observe 10 μl of PC-3 cell suspension in blank control group, 12.5 μg/ml, 25 μg/ml, 50 μg/ml NCTD group and 2.5 μmol/L cisplatin positive control group. After 50 times dilution with 1×PBS, 10 μl of suspension was added to copper mesh, dried at 25℃ for 12 hours, and then re-stained with uranyl acetate. The morphology of pseudopodia was observed and photographed under transmission electron microscope,under accelerating voltage 200kV, ×5000.
Statistical analysis: The measurement data was expressed as mean±standard error . The difference between the two groups of independent samples was compared by t-test. The difference between the multi-factor groups was compared by single factor analysis of variance. P<0.05 was statistically significant. All the statistical analyses were performed using Statistical Package forthe Social Sciences 16.0 (IBM, New York, USA).
RESULTS AND DISCUSSION
Real time fluorescent quantitative PCR was used to detect the content of cortacin and MMP-9 mRNA in PC-3 cells of each group. The results showed that the content of cortactin mRNA in12.5 μ g/ml,25 μg/ml and 50 μg/ml NCTD decreased by 20%, 38% and 59% respectively, P<0.05, and the content of cortacin mRNA in 50 μg/ml NCTD group decreased by 37% compared with that in the positive control group ,P<0.05, The content of MMP-9 mRNA decreased by 13%, 39% and 58% respectively compared with the blank control group,P<0.05.and the content of MMP-9 mRNA decreased by 45% in the 50 μg/ml NCTD group compared with the positive control group ,P<0.05. Real-time quantitative PCR detect the amplification curve and dissolution curve of cortactin, MMP-9, and β-actin internal parameter, the amplification curves of cortactin, MMP-9 and β-actin internal parameter were smooth s-shaped with clear inflection points;dissolution of cortactin, MMP-9 and β-actin internal parameter showed a single peak.Indicating that the specificity of amplification was good and the results were reliable.See Figure 1.
Figure 1: the curves of amplification and dissolution
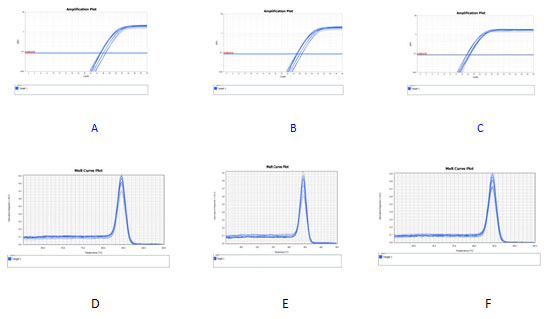
A-C:amplification curves of cortactin ,MMP-9 and β-actin
D-F: dissolution curves of cortactin,MMP-9 and β-actin
Western-blot showed that the expression levels of cortactin and MMP-9 in 12.5 μ g /ml, 25 μg/ml and 50 μg/ml NCTD group were lower than those in the blank control group, and the expression levels of cortactin and MMP-9 in the 50 μg/ml NCTD group were lower than those in the blank control group with the increasing of NCTD concentration ,P<0.05. see Figure.2
Figure 2: Electrophoresis of NCTD effect on expressions of cortactin and MMP-9 in PC-3
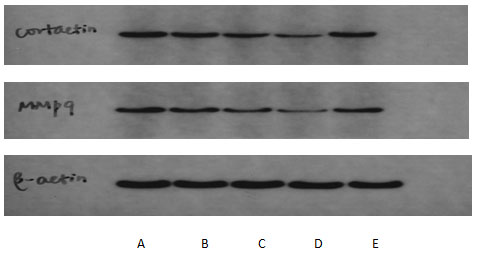
A:blank control group ;B-D:NCTD group( 12.5、25、50 μg/mL);E:positive control group(cisplatin 2.5 μmol/L)The effect of NCTD on the migration and invasion of PC-3 cells showed that the scratch healing area of 12.5 μg /ml, 25 μg/ml and 50 μg/ml NCTD group was smaller than that of the blank control group, as shown in Figure 3. At the same time, Transwell chamber method also found that the number of invasive cells in 12.5 μg / ml, 25 μg/ml and 50μg/ml NCTD groups were (66.4 ± 12.4), (53.4 ± 8.1), (31.6 ± 4.6) respectively, which were significantly lower than that in the blank control group (84.2 ± 7.3),P<0.05, as shown in Figure.4
Figure 3: Effect of NCTD on migration of PC-3 cells( inverted microscope,× 100)
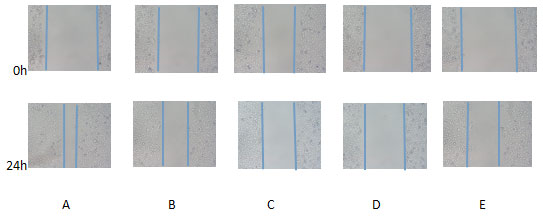
A:blank control group ;B-D:NCTD group( 12.5、25、50 μg/mL);E:positive control group(cisplatin 2.5 μmol/L)
Figure 4: Effect of NCTD on invasion of PC-3 cells( crystal violet,×200 )
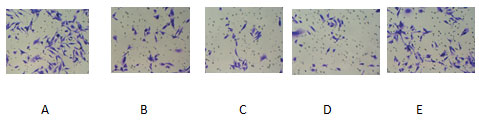
A:blank control group ;B-D:NCTD group( 12.5、25、50 μg/mL);E:positive control group(cisplatin 2.5 μmol/L)
Transmission electron microscopy showed that the diameter of PC-3 cells was about 4 μ m, the nucleus was large, the nucleolus was obvious, and there were many pseudopodoid processes on the cell surface. After 24 hours of incubation with 12.5 μ g / ml, 25 μ g / ml and 50 μ g / ml NCTD, the number of pseudopodia on the surface of PC-3 cells decreased. Compared with negative control, with the increase of NCTD concentration,the number of pseudopodia of PC-3 cells decreased gradually.What’s more,the number of pseudopodia decreased obviously in 50 μ g / ml NCTD group compared with positive control group. See Figure.5
Figure 5: Effect of NCTD on pseudopodia morphology of PC-3 cells (transmission electron microscopy, accelerating voltage 200kV, ×5000)
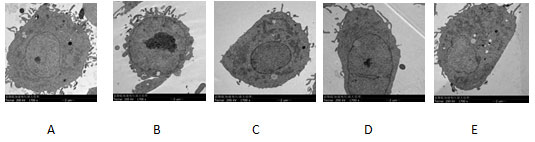
A:blank control group ;B-D:NCTD group( 12.5、25、50 μg/mL);E:positive control group(cisplatin 2.5 μmol/L)
The existing evidence (Marioni,2018;Zhao,2016;Horn,2018;Wen,2019) shows that the overexpression of cortactin may increase the formation of pseudopodia of various cancer cells, accelerate the degradation of peripheral matrix components of cancer cells, and facilitate the invasion and diffusion of cancer cells. Recent studies (Ma,2016;Qi,2020) have also shown that the expression of cortactin in prostate cancer is higher than that in benign prostatic hyperplasia, and the expression of cortactin gradually increases with the development of prostate cancer, and is related to the distant metastasis of prostate cancer. After knockout of the expression of cortactin in PC-3 cells, the invasion ability and extracellular matrix degradation ability of PC-3 cells were significantly reduced, (Horn,2018).
Recent studies of Ren (2019) have shown that the expression of MMP-9 mRNA in prostate cancer tissue is significantly higher than that in benign prostate tissue, and the expression of mm-9 mRNA in invasion and metastasis prostate cancer tissue is significantly higher than that in non invasion and metastasis prostate cancer tissue, indicating that MMP-9 may play an important role in invasion and metastasis of prostate cancer. MMP-9 can degrade and destroy tumor extracellular matrix and promote the formation of invasive pseudopodia of cancer cells, which suggests that MMP-9 may have synergistic effect with cortactin protein in the development of prostate cancer. There are few studies of NCTD in the treatment of prostate cancer. Recent clinical studies of scholars have found (Song,2018)that NCTD combined with paclitaxel is better than paclitaxel alone in the treatment of HRPC patients, and may reduce the adverse reactions caused by paclitaxel, but the specific mechanism is not clear. A recent study indicated that norcantharidin (NCTD) can inhibit PC-3 cells proliferation and induce PC-3 cell apoptosis (LUO,2020).
In this study, after the PC-3 cells were treated with different concentrations of NCTD, real-time fluorescence quantitative PCR and Western blot were used to detect the content of cortacin and MMP-9 mRNA and protein expression. The results showed that the content of cortacin and MMP-9 mRNA and protein expression of PC-3 cells in each concentration group of NCTD were lower than those in the blank control group. At the same time, with the increase of NCTD concentration, the mRNA content and protein expression of cortin and MMP-9 in PC-3 cells decreased gradually, and there was a dose-response relationship. The results of scratch test and Transwell test indicate that NCTD can inhibit the peripheral migration and invasiveness of PC-3 cells, and there is a dose-response relationship between the concentration of NCTD and the invasiveness of PC-3 cells. At last, the ultrastructural observation of PC-3 cells was carried out by means of transmission electron microscopy. Compared with the blank control group, the number of pseudopodoid protrusions on the surface of PC-3 cells decreased in the NCTD group. With the increase of NCTD concentration, the number of pseudopods decreased significantly. It indicates that NCTD would inhibit the formation of pseudopodia on the surface of PC-3 cells.
CONCLUSION
NCTD can inhibit the peripheral migration and invasiveness of PC-3 cells, and its mechanism may be related to the down-regulation of mRNA content and protein expression of cortactin and MMP-9 in PC-3 cells and the inhibition of the formation of pseudopods on the surface of PC-3 cells. At the same time, there may be some synergistic effect between cortactin and MMP-9 in promoting the invasion and metastasis of prostate cancer.
Author contributions: W.J. conceived the study. K. and J.J. and H.H. performed the experiments and analyzed the data. X.N. wrote the manuscript.
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
We thank Prof. Xiaojun Ma for advice about cell culture. We are grateful to Ms. Ning Li for help in the procedure of transmission electron microscopy.
REFERENCES
Fan, L and J.W. Xu,X.X. Wang, et al(2020)Effects of norcantharidin on proliferation and apoptosis and its mechanism in human undifferentiated thyroid carcinoma FRO cells.Guangxi Medical Journal.42(10)1257-1260.DOI:10.11675/j.issn.0253-4304.2020.10.17
Gao,Y. and L.N. Cong, Y. Cheng, et al (2020)Norcantharidin induced apoptosis of melanoma M14 cells and its action mechanism, Hebei Medical Journal.42(2):179-183. DOI:10.3969 /j.issn.1002-7386.2020.02.004
Horn, Dominik and Madeleine Gross,Gerhard Dyckhoff,et al.(2018) Cortactin expression: Association with disease progression and survival in oral squamous cell carcinoma,Head Neck.40 (12) 2685-2694. DOI:10.1002/hed.25515.
Li,X and X.Y. Zeng (2021) Advances in epidemiology of prostate cancer in China.Cancer.Res Prev Treat.48(1):98-102.DOI:10.3971/j.issn.1000-8578.2021.20.0370
Liu, B.D and X. Gu,G.C. Zhou, X.F. Ding (2016) Effects of TMPRSS2-ERG and MMP-9 gene on the invasiveness of prostate cancer.Basic and Clinical Medicine.36(4):508-512. DOI:10.16352/j.issn.1001-6325.2016.04.016
Luo, X.N and W.J. Wang,K. Li, et al (2020) Effect of norcantharidin on proliferation and apoptosis of anfrogen independent prostate cancer PC-3 cells.Shaanxi Medical Journal,49(8):928-939. DOI:10.3969/j.issn.1000-7377.2020.08.004
Ma,P.D and B. Sheng, X.M. Wang, et al (2016) Expression changes of cortactin in benign and malignant prostatic tissues and its effect on cell invasive ability and extracellular matrix degradation ability of prostate cancer cells PC-3.Shandong Medical Journal.56(23):9-12.DOI: 10.3969 /j.issn.1002-266X.2016.23.003
Marioni,Gino and Marco Lionello,Rosario Marchese-Ragona (2018) Cortactin and phosphorylated cortactin tyr421 and tyr466 expression in supraglottic laryngeal carcinomas and lymph node metastases,The International Journal of biological markers.33 (1)79-86.DOI: 10.5301/ijbm.5000297
Murphy DA and Courtneidge SA. (2011) The’ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function,Nat Rev Mol Cell Biol.12 (7) 413-426.DOI:10.1038/nrm3141
Qi, T.Y and H.Y. Cao H.G. Sun, et al (2020) piR-19166 inhibits migration and metastasis through CTTN/MMPs pathway in prostate carcinoma.Aging.12(18):18209-18220.DOI: 10.18632/aging.103677
Ren, L and D. Yang, P.C. Zhao, et al (2019) Expression and significance of matrix metalloproteinase-9 and Raf-kinase inhibitor protein mRNA in prostate cancer,Chinese J Clinical Urology.34 (7) 533-537. DOI:10.13201/j.issn.1001-1420.2019.07.008
Ren,X.L and Y.D. Qiao, J.Y. Li,et al (2018) Cortactin recruits FMNL2 to promote tactin polymerization and endosome motility in invadopodia formatio,Cancer Letters.419 (2) 245-256. DOI:https://doi.org/10.1016/j.canlet.2018.01.023
Song, Z.G and P.C. Zhao, W.G. Wang, et al (2018) Clinical efficacy of norcantharidin combined with paclitaxel in patients with hormone refractory prostate cancer,Oncology Progress.16 (6)719-721.DOI:10.11877/j.issn.1672-1535.2018.16.06.15
Tang,H.Q and R Bi,X.H. Liu,et al (2010) Clinical application of cantharides and its preparations,Chinese journal of ethnomedicine and ethnopharmacy.12 (21) 54-55.DOI:10.3971/j.issn.1000-8578.2010.12.2107
Wen,D and W.L. Liu, Y.M. Cao, et al (2019) The expressions of cortactin and N-WASP in thyroid papillary carcinoma and their significance.China Oncology.29(9):688-692.
DOI:10.19401/j.cnki.1007-3639.2019.09.002
Wu,H and Reynolds AB,Kanner SB, et al (1991) Identification and characterization of a novel cytoskeleton -associated pp60src substrate,Mol Cell Biol.11 (10):5113.DOI: 10.1128/MCB.11.10.5113
Xu,L and J.M. Guo (2020) The standard and the latest development of the treatment of advanced prostate cancer,Journal of Practical Oncology.35 (2) 100-106.DOI:10.13267/j.cnki.syzlzz.2020.02.002
Yang,R.Y and C.Y. Zhang,Y. Cui, et al (2020) Norcantharidin inhibits ovarian cancer cell proliferation and invasion by regulating miR-182-5p. Chinese Immunological Journal.36(15):1848-1852.DOI:10.3969 /j.issn.1000-484X.2020.15.012
Zhao,G and H.Y. Zhang, Z.M. Huang,et al (2016) Cortactin and Exo70 mediated invasion of hepatoma carcinoma cells by MMP-9 secretion, Molecular Biology Reports.43 (5) 407-414.DOI:https://doi.org/10.1007/s11033-016-3972-4


