Department of Biochemistry, Sri Sankar Arts and Science College (Autonomous), Kanchipuram, Tamil Nadu, 31561 India
Corresponding author email: kamudhabio@gmail.com
Article Publishing History
Received: 25/12/2020
Accepted After Revision: 19/03/2021
Nanotechnology is the art and science of manipulating matter at the nanoscale to create new unique materials with enormous potential to change society. Nanoparticles can serve as “magic bullets”, which carry the active ingredient along with it, green synthesis or phytosynthesis of nanoparticle is an eco-friendly approach which is in common practice. In this present study, a mono dispersed Spherical shaped copper oxide nanoparticle was prepared successfully using Cassia auriculata flowers extract without using any harmful reducing agents. The synthesized nanoparticles were characterized by UV-VIS spectroscopy, Fourier Transform Infra-Red spectroscopy and Transmission Electron Microscopy. The antibacterial activity of synthesized copper nanoparticles was compared by agar well diffusion method and minimum inhibitory concentration was also calculated.
The zone of inhibition varied in range of 10 to 30 mm. However, bactericidal effect of copper nanoparticles varies with respect to the organism tested. The phytosynthetic approach is a simple alternative to chemical and physical methods due to low cost and less use of toxic chemicals. This study presents a simple, fast, cheap and eco-friendly method for CuONPs synthesis. The method was based on the reduction of copper (II) sulfate pentahydrate salt by Cassia auriculate flowers ethanol extract. The copper oxide Nps synthesized using the green method showed excellent antioxidant, antibacterial antifungal activity. The exact mechanism and the cytotoxic nature of the nanoparticles should be investigated further for its effective application. These findings showed that green method could be used as a good alternative to the current physical and chemical methods associated with environmental toxicity.
Anti-Bacterial; Anti-Fungal; Cassia auriculata Flower; Copper Oxide Nanoparticles; Phytosynthesis
Amudha K, M. V. D, Bathrunisha B. Phytosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles from Cassia auriculata. Biosc.Biotech.Res.Comm. 2021;14(1).
Amudha K, M. V. D, Bathrunisha B. Phytosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles from Cassia auriculata. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3pljq1Y”>https://bit.ly/3pljq1Y</a>
Copyright © Amudha et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Now nanoparticles have many applications in the commercial world. Recently, the green synthesis of NPs using microorganisms and plants extracts has been achieved (Gunalan et al., 2012). Green synthesis procedures are very simple, rapid, nontoxic, inexpensive (Kumar et al., 2009). Conventionally, copper and its complexes have been used as water purifiers, antibacterial and antifungal agents (Stoimenov et al., 2002; Lee et al., 2009). Copper oxide nanoparticles (CuONPs) have been of great interest, due to their exclusive physical and chemical properties (Varshney et al., 2012).
The growing interest of environmental supporting phenomenon for synthesis nanoparticles, phytogenic reduction methods (Phytosynthesis) are more suitable, effective and eco-friendly. Cassia auriculata Linn., an annual or biennial shrub found throughout India, belongs to the family Caesalpiniaceae. The flowers, leaves, stem, root and unripe fruit are used for treatment, especially in Ayurvedic medicine. The plant has been reported to possess antibacterial, antifungal, and anticancer, antipyretic, antihyperglycaemic, antiperoxidative and hepatoprotective activity (Ahamed et al., 2014; Joshi et al., 2019; Zangeneh et al., 2019; Siddiqi et al., 2020).
Figure 1: Flowers of Cassia auriculata Linn.

Phytosynthesis of copper oxide nanoparticles by various plant extracts has been reported so far. The major advantage of using plant extracts for copper oxide nanoparticles (NPs) synthesis is that they are easily available, safe, and nontoxic in most cases. Several reports have been proven that Copper oxide NPs has the highest antimicrobial activity compared to other metal oxides (Gebremedhn et al., 2019; Renuga etal., 2020). In this study copper oxide nanoparticles have been synthesized using Cassia auriculata flowers for the first time with the help of greener protocols. Synthesized CuONPs were characterized by UV-visible spectroscopy, FTIR, TEM and followed by antioxidant activity by DPPH method. Further its efficiency against bacteria and fungus were analysed using disc diffusion method.
MATERIAL AND METHODS
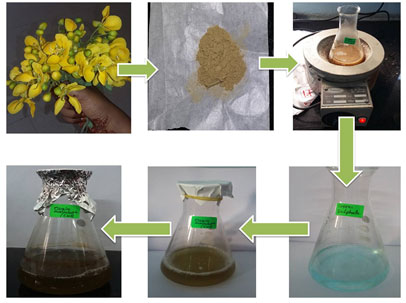
The flowers of the Cassia auriculata were collected from in and around Kanchipuram, Tamil Nadu, India. The taxonomic identities of this plant were determined. They were thoroughly rinsed using normal water, followed by distilled water and then dried in the shade at room temperature. The Cassia auriculata flowers were cut into small pieces and crushed with help of mortar and pestle. 20 grams of powder sample were subjected to Soxhlet extraction at 40-60ºC for 8 cycles in 200 mL of ethanol. The mixture was filtered using Whatman no.1 filter paper and the filtrate solution was concentrated and evaporated on a rotary evaporator (Buchi, R–124 and Switzerland) to obtain a residue which was stored at 4°C to use further.
Figure 2: Ethanolic Extraction and Preparation of CuONPs from Cassia auriculata flower
Antioxidant activity of extract was estimated on the basis of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging effect (Huang et al., 2005). The free radical scavenging activity (FRSA) calculated using the following equation: FRSA (%) = 100 × (AC − AS) / AC where AC is the absorbance of DPPH without sample and AS is the absorbance of DPPH in the presence of sample. The concentration of sample required to scavenge 50% of DPPH radicals was measured as IC50.Various concentrations of copper (II) sulfate pentahydrate solution and the extract in different pH, temperature and incubation time were mixed. The reaction mixture was allowed to stand in a dark room to complete the reaction. The obtained precipitation was purified by repeated centrifugation at 12000 rpm for 20 min, dried in oven at 80o C for 8 h and stored in properly containers (Shiravand and Azarbani, 2017).
The reduction of copper ions in copper (II) sulfate pentahydrate solution to CuONPs was periodically monitored by ultraviolet–visible (UV-Vis) Spectrophotometer. UV-Vis spectral analysis was done by UV-1700 (Shimadzu, Japan) spectrometer at the range of 300–600 nm. The FT-IR spectroscopy were carried out for both the Cassia auriculata flowers ethanol extract and the synthesized CuONPs to identify possible biomolecules in the Cassia auriculata flowers extract that can participate in reduction process of copper ions and capping of the resulting CuONPs. The samples grinded with potassium bromide (KBr) and analyzed by Bruker fourier transform infrared (FTIR) Tensor- 27 spectrophotometer at range of 4000–400 cm−1 with a resolution of 4 cm−1.
The sample morphology and size were examined with Tecnai G2 20 (FEI) S Twin model operating at 200 kV transmission electron microscope (TEM). The images were recorded to confirm the shape of newly synthesized CuONPs (Shiravand et al., 2017). Antibacterial activity of synthesized CuONPs was evaluated against Escherichia coli, Staphylococcus aureus, serratia species, Vibrio harveyi by disc diffusion method. Then antifungal activity was evaluated against Aspergillus niger and Aspergillus fumigatus. Nutrient agar plates were seeded with overnight bacterial and fungal culture. 50 μL of different concentrations (250 −1000 μg/mL) of biosynthesized CuONPs were placed on the surface of the inoculated plates. After incubation at 37°C for 24 h, zone diameters were measured (mm) (Li et al., 2006).
RESULTS AND DISCUSSION
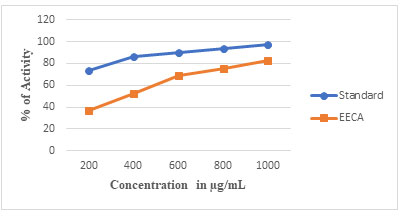
In the present study, the different concentrations of Ethanolic extract of C. auriculata flowers were subjected to DPPH free radical scavenging assay. The antioxidant capacity of the extract was compared with standard ascorbic acid. Results obtained showed that standard antioxidant had higher scavenging activity at all tested concentrations than the extracts. The result showed the Percentage of activity of standard ascorbic acid and extract for 1000 µg/ml as 97.3% and 82.32%, respectively when compared with DPPH assay activity. Therefore, the results indicated that C. auriculata flowers are a rich source of antioxidant compounds. Therefore, their ethanolic extract was used to reduce copper ions present in copper (II) sulfate pentahydrate solution and synthesis of CuONPs.
Figure 1a: DPPH radical scavenging activity of ethanolic extract of Cassia auriculata flower in various concentrations
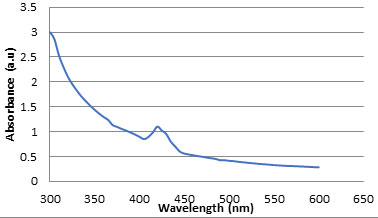
Synthesis of CuONPs using ethanol extract of Cassia auriculata flowers were observed by UV-visible spectroscopy. Reduction of copper sulphate solution to CuONPs was confirmed by measuring the UV-Vis spectrum at the range of 300–600 nm. The UV-Vis absorption spectrum of sample is recorded and shown in Figure 2. As expected, CuO nanoparticles show an absorption peak between 400 and 500 nm i.e. 420 nm that can be contributed to the characteristic absorption of CuONPs. The peak was observed even after one week indicating the stability of CuONPs (Subashini et al., 2019).
Figure 2a: UV-Vis spectrum of CuO nanoparticles phyto-synthesized by Cassia auriculate flower.
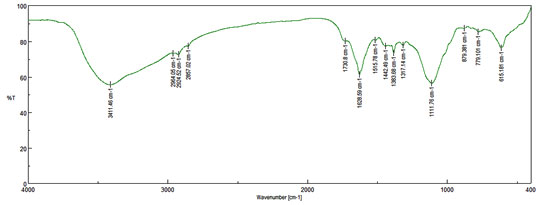
CuONPs solution was centrifuged at 10,000 rpm for 30 minute and obtained solid residue was washed several times with distilled water followed by drying. The solid powder was used for FTIR analysis, which were performed on Bruker fourier transform infrared (FTIR) Tensor- 27 spectrophotometer. The FTIR peaks were identified and expressed in wave numbers (cm-1). The refined CuONPs possessed absorption peaks at 3411, 2964, 2924, 2857, 1730, 1628, 1515, 1442, 1383, 1317, 1111, 879 and 779 cm −1 corresponding to hydroxyl group (OH) stretching, hydroxyl (-OH) bending, and C-O stretching, respectively. (Figure 3). It may be confirmed that the bioactive ingredients of Cassia auriculate flower was the probable reducing agent which was concerned in the Phyto-synthesis of CuONPs and might have organized a layer on the CuONPs (i.e., Phyto-capping) that may have delayed the agglomeration of the Nanoparticles would have stabilized them (Hassanien et al., 2018).
Figure 3: FT-IR spectrum of CuO nanoparticles phyto-synthesized by Cassia auriculate flower
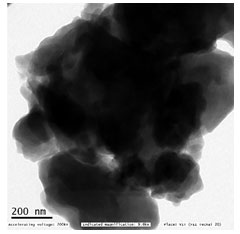
The shape and size of the synthesized CuO-NPs were analysed by TEM analysis. Image III shows the TEM image of biosynthesized CuONPs. The experimental results showed that the shape of prepared CuONPs was spherical with diameters that ranged from 200 nm and found in form of nanocluster. The larger copper particles may be due to the aggregation of the smaller ones, during the TEM analysis.
Figure 3a: Bright field TEM image of phyto-reduced copper nanoparticles. (Vellore Institute of Technology, Vellore, Tamil Nadu, India)
The antibacterial activity of synthesized CuONPs was evaluated against E. coli, S. aureus, Serratia Sp and V. harveyi bacteria. The CuONPs showed activity against all tested organisms (Table 1). It was found that the zone of inhibition increased with increasing the concentration of CuONPs (Figure 5). The exact mechanism behind the biocidal activity of CuONPs is not yet fully known. It was suggested that copper ions originating from the CuONPs may interact with phosphorus and sulfur-containing biomolecules such as DNA and protein to distort their structures and thus disrupt biochemical processes (Ruparelia et al., 2008; Wu et al., 2009).
Effectiveness of CuONPs against both Gram-negative and Gram-positive bacteria proposing as broad-spectrum potential of nanoparticle. Bacterial colony stamp down by cell filaments formation influenced by CuONPs subjected to bacterial cell membrane destruction (Montes-Burgos et al., 2010; Saranya et al., 2020).
Table 1. Antibacterial activity of Phyto-synthesized CuONPs.
| Zone of Inhibition (mm) | ||||
| Concentration (µg/mL) | ||||
| Bacterial Strain | Control | 250 | 500 | 1000 |
| E. coli | 13 | 14 | 15 | 18 |
| S. aureus | 30 | 15 | 23 | 30 |
| Serratia sp | 33 | 10 | 12 | 17 |
| V. harveyi | 24 | 13 | 14 | 15 |
Figure 4: Antibacterial activity of Copper oxide nanoparticles
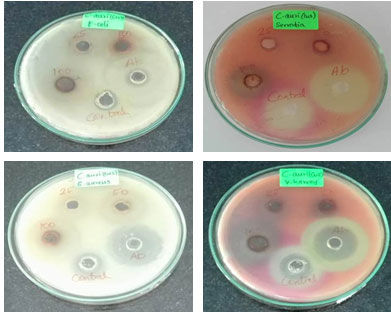
Figure 5: Antifungal activity of copper oxide nanoparticles
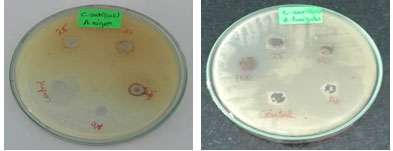
Table 2. Antifungal activity of Phyto-synthesized CuONPs.
| Zone of Inhibition (mm) | ||||
| Concentration (µg/mL) | ||||
| Fungi | Control | 250 | 500 | 1000 |
| A. niger | 16 | 9 | 11 | 13 |
| A. fumigatus | 35 | 16 | 16 | 18 |
The antifungal test of CuO nanoparticles were performed by allowing Aspergillus niger and Aspergillus fumigatus to grow on agar CD medium containing different concentration of CuO nanoparticles respectively (Figure 6). It was found that the growth inhibition of A. niger and Aspergillus fumigatus were observed in a concentration dependent manner (Table 2). Recent advances in the field of nanotechnology, particularly the ability to prepare metal oxide NPs of any size and shape, could lead to the development of new antifungal agents. The use of NPs suggests a new promising approach for fungal infection therapy.
CONCLUSION
The copper oxide Nps synthesized using the green method showed excellent antioxidant, antibacterial antifungal activity. The exact mechanism and the cytotoxic nature of the nanoparticles should be investigated further for its effective application. These findings showed that green method could be used as a good alternative to the current physical and chemical methods associated with environmental toxicity.
REFERENCES
Ahamed, M., Hisham, AA., Majeed Khan, MA., Karuppiah, P., Naif, A., Dhabi, A. (2014) Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles Journal of nanomaterials. 17: 1-4.
Baek, YW and An, YJ. (2011). Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Science of the Total Environment 409: 1603–1608.
Gebremedhn, K,, Kahsay, MH., Aklilu, M. (2019). Green synthesis of CuO nanoparticles using leaf extract of catha edulis and its antibacterial activity Journal of Pharmacy Pharmacology. 7: 327–42
Gunalan, S., Sivaraj, R., and Venckatesh, R. (2012). Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: optical properties. Spectrochimica acta. Part A, Molecular and Biomolecular Spectroscopy 97: 1140-1144.
Hassanien, R., Dalal, Z., Husein, Mostafa, F., Hakkani, A. (2018). Biosynthesis of coppernanoparticles using aqueous Tilia extract: antimicrobialand anticancer activities. Heliyon. 4: 1-21.
Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H., Kahru, A. (2012). Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibro fischeri and crustaceans Daphyna magna and Thamnocephalus platyurus. Chemosphere 71: 1308–1316.
Huang, D., Ou, B., Prior, R. L. (2005). The Chemistry behind Antioxidant Capacity Assays. Journal of Agricultural Food Chemistry, 53: 1841-1856.
Joshi, A., Sharma, A., Bachheti, R.K., Husen, A., Mishra, VK. (2019). Plant-mediated synthesis of copper oxide nanoparticles and their biological applications. Nanomaterials and Plant Potential. 221–37.
Kumar V and Yadav SK. (2009). Plant-Mediated Synthesis of Silver and Gold Nanoparticles and Their Applications. Journal of Chemical Technology and Biotechnology 84 (2):151-157.
Lee., S. Choi, SUS., Li. S., Eastman, JA. (1999). Measuring thermal conductivity of fluids containing oxide nanoparticles. Journal of Heat Transfer 121: 280–289.
Li, Z., Lee, D., Sheng, X., Cohen, RE., Rubner, MF. (2006). Two-Level Antibacterial Coating with Both Release-Killing and Contact-Killing Capabilities. Langmuir. 22: 9820–9823.
Montes-Burgos, D., Hole, WP., Smith, J., Lynch, I., Dawson, KJ. (2010). Characterisation of nanoparticle size and state prior to nanotoxicological studies. Nanoparticle Research. 12: 47–53.
Renuga, D., Jeyasundari, J., Shakthi Athithan, SA., Brightson Arul Jacob, Y. (2020). Synthesis and characterization of copper oxide nanoparticles using Brassica oleracea var. extract for its antifungal application. 7: 1-6.
Ruparelia, JP., Chatterjee, AK., Duttagupta, SP., Mukherji, S. (2008). Strain Specificity in Antimicrobial Activity of Silver and Copper Nanoparticles. Acta. Biomaterialia 4 (3): 707–716.
Saranya, S., Agneeswaran, R., Deepa, P. (2020). Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 5: 1040-1051.
Shiravand, S., Azarbani, F. (2017). Phytosynthesis, characterization, antibacterial and cytotoxic effects of copper nanoparticles, Green Chemistry Letters and Reviews. 10 (4): 241-249,
Siddiqi, K., Husen, A. (2020). Current status of plant metabolite-based
fabrication of copper/copper oxide nanoparticles and their applications: a review. Biomaterials Research. 24: 1-15.
Stoimenov, PK., Klinger, RL., Marchin, RL., Klabunde, KJ. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir 18: 6679–6686.
Subashini, K., Prakash, S., Sujatha, V. (2019). Anticancer Activity of Copper Oxide Nanoparticles
Synthesized from Brassia actinophylla Flower Extract. Asian Journal of Chemistry. 31(9): 1899-1904.
Varshney, R., Bhadauria, S., Gaur, M. S. (2012). A Review: Biological Synthesis of Silver and Copper Nanoparticles. Nano Biomedicine and Engineering 4: 99–106.
Wu, XH., Ye, L., Liu, K., Wang, W., Wei, J., Chen FP., Liu, CS. (2009). Antibacterial properties of mesoporous copper-doped silica xerogels Biomedical Materials 4: 45-48.
Zangeneh, MM., Ghaneialvar, H., Akbaribazm, H., Ghanimatdan, M., Abbasi, N., Goorani, S., Pirabbasi, E., Zangeneh, A. (2019). Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. Journal of Photochemistry and Photobiology. 197:111556.


