Department of Botany, Union Christian College Aluva, Ernakulam, Kerala, India
Corresponding author email: drmakumar@gmail.com
Article Publishing History
Received: 29/10/2020
Accepted After Revision: 21/12/2020
Drugs derived from plants have been used routinely in modern medicines, especially in the treatment of chronic disorders. Identification, isolation and characterization of active principles from plant derived drugs are of prime importance now a days. It demands an extensive pharmaceutical study of the different extracts of medicinal plants to identify their biological properties. The present study was designed to assess the anti-inflammatory potential of Litsea quinqueflora (Dennst.) Suresh. Leaf paste of L. quinqueflora have been used by many traditional healers as a remedy for inflammatory disorders. But scientific validation of this practice still remains undone and hence an attempt has been made to evaluate the anti-inflammatory potential of different leaf extracts of L. quinqueflora. Dried powder of leaves was sequentially extracted with hexane, chloroform, ethyl acetate, methyl ethyl ketone, methanol and water. Preliminary phytochemical screening revealed the presence of flavonoids, steroids, terpenoids, phenols, alkaloids, resins, glycosides etc. in the various extracts.
Ethyl acetate, methyl ethyl ketone and methanol extracts were selected for the further studies due to the presence of most of the phytochemicals. Anti-inflammatory activity of the extracts was tested by inhibition of protein denaturation, proteinase inhibition and Human Red Blood Cell membrane stabilization assays. Percentage of inhibition and IC50 values were calculated. The assay concluded that ethyl acetate, methyl ethyl ketone and methanol extracts showed significant inhibition (P≤0.05) in a concentration dependent manner and there by the anti-inflammatory property. Among them methanolic extract (LM) showed highest activity. The results of this study supported the efficacy of L. quinqueflora as herbal anti-inflammatory agent.
Litsea quinqueflora, Phytochemicals, Anti-Inflammatory, Protein Denaturation, Proteinase Inhibition, Membrane Stabilization
Jose S. M, Anilkumar M. In vitro Anti-inflammatory analysis of leaf extracts of the Litsea quinqueflora Suresh. Biosc.Biotech.Res.Comm. 2020;13(4).
Jose S. M, Anilkumar M. In vitro Anti-inflammatory analysis of leaf extracts of the Litsea quinqueflora Suresh. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/2KcB5db”>https://bit.ly/2KcB5db</a>
Copyright © Jose and Anilkumar This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The term inflammation springs out from a Latin word “inflammare” which means to burn. Inflammation is a defending process of body against noxious stimuli, infection or traumatic conditions. Inflammations can be acute or chronic with characteristic symptoms such as pain, heat, redness, swelling and loss of function (Raghavendra et al., 2015).
It is an indication of injury or disease that occurs in living system and an alert to start healing process. Though it is a security measure of body, loss of proper control and its persistence in the tissues for a long time leads to various inflammatory disorders such as asthma, allergy, multiple sclerosis, systemic lupus erythematosus, arthritis, psoriasis, arthrosclerosis, diabetes, Crohn’s disease, ulcerative colitis, etc. Inflammatory responses are associated with changes in homeostatic balances of living body (Anilkumar, 2010; Raghavendra et al., 2015). Two types of anti-inflammatory drugs viz. steroidal and non-steroidal are available to treat inflammatory disorders. But their long term use lead to adverse side effects. Steroidal drugs such as betamethasone, prednisolone and dexamethasone cause adrenal atrophy, osteoporosis, euphoria, cataracts, glaucoma and suppression of response to infection and injury. Non-steroidal drugs inhibit both physiological and inflammatory prostaglandins and cause peptic ulcers and bronchospasm (Chaible et al., 2017). The side effects of commercial anti-inflammatory drugs prompted the need for a safer medicine with better efficacy, economic feasibility and easy availability. Thus, plants with a wide range of biologically active compounds became novel sources of crude as well as pure compounds in different disease conditions (Abdulkhaleq et al., 2018; Ogunmefun, 2018).
The investigation and identification of phytochemicals led to the development of new plant-based drugs (Abdulkhaleq et al., 2018; Ogunmefun, 2018). In the present investigation, the solvent extraction of leaves of Litsea quinqueflora was done successively with different solvents of non-polar to polar nature to identify the phytochemicals. Anti-inflammatory potential of leaves has been studied through different anti-inflammatory assays such as inhibition of protein denaturation, proteinase inhibition and membrane stabilization. The genus Litsea that belongs to the family Lauraceae is predominant in tropical and subtropical regions. Species of Litsea are important traditional folk medicines of those areas and sources of important secondary metabolites and essential oils. They possess various ethno pharmacological properties such as antioxidant, antimicrobial, anti-inflammatory, antitumor and cytotoxicity. There are few reports on the anti-inflammatory and antioxidant properties of L. quinqueflora (Anilkumar and Johny, 2015; Jose and Anilkumar, 2018; Kamle et al., 2019).
Traditional utilization of Litsea species in inflammatory disorders such as oedema, rheumatic arthritis and gastroenterologia have been reported (Wang et al., 2016). The recent study on antioxidant activity and DNA protective effect of fruits of L. cubeba is another evidence to confirm the ethnomedicinal importance of the genus Litsea (Seal et al., 2020). There are only very less scientific reports available on the folklore use of L. quiqueflora and its validation. The present study attempts to scientifically validate the anti-inflammatory properties of this plant via in vitro methods.
MATERIAL AND METHODS
Leaves of Litsea quinqueflora (Dennst.) Suresh were obtained from Kurianad of Kottayam district, Kerala, India. Plant specimen was identified and authenticated at Kerala Forest Research Institute, Kerala, India and voucher specimen was deposited with serial number KFRI 13057. Leaves were washed thoroughly in running water and shade dried. The dried leaves were powdered using a mechanical blender and used for extraction using different solvents. The leaf powder was extracted sequentially in a soxhlet apparatus with different solvents such as hexane, chloroform, ethyl acetate, methyl ethyl ketone, methanol and water. Each extract was collected, filtered and dried using a vacuum evaporator followed by redissolving in 5% Dimethyl sulfoxide (DMSO) and was used for further studies. Preliminary phytochemical screening to identify different classes of phytochemicals was done as per the method of Harborne, (1998).
Based on the results obtained from preliminary phytochemical screening, further anti-inflammatory assays were carried out using ethyl acetate (LE), methyl ethyl ketone (LMEK) and methanol (LM) since all other extracts contained negligibly lesser number of phytochemicals. Three screening assays such as inhibition of protein denaturation, proteinase inhibition and HRBC membrane stabilization activity were done to assess the anti-inflammatory activity of different solvent extracts of L. quinqueflora. Inhibition of protein denaturation was done as per the method of Mizushima and Kobayashi (1968). The reaction mixture contained 0.45 ml of 1 % aqueous solution of bovine serum albumin (BSA) with 0.05 ml of different leaf extracts having concentrations (62.5, 125, 250, 500 µg/ml) and the pH was maintained at 6.3. It was incubated at 37 ° C for 20 minutes and later temperature was raised up to 57 ° C for 3 minutes. It was then allowed to cool at room temperature. Then added 2.5 ml of phosphate buffered saline (PBS) of pH 6.3 and measured the optical density (OD) at 660 nm using UV- Vis spectrometer (Shimadzu – UV 1800) with PBS as blank. Diclofenac sodium was used as the standard anti-inflammatory drug. Test control was mixed with distilled water instead of extract and distilled water was added instead of BSA in the product control. The percentage of inhibition of protein denaturation (Kiranmayi, 2018) was calculated by the formula,
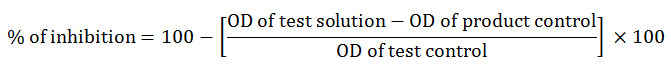
Proteinase inhibitory activity was executed according to the method of Oyedapo et al., (1995) with slight modifications. The reaction mixture consisted of 2 ml of 20 mM Tris HCl buffer (pH 7.4), proteinase (0.06mg), and 62.5, 125, 250 or 500 µg/ml of test solution. After incubating for 5 minutes at 37ºC, 1 ml of 0.8 % (w/v) casein was mixed with the reaction mixture. It was kept under incubation for 20 minutes and the reaction was terminated by the addition of 2 ml of 70 % perchloric acid. The cloudy suspension thus obtained was centrifuged at 3000 rpm for 10 minutes. The optical density of the clear supernatant was measured at 210 nm in a UV- visible spectrophotometer against Tris – HCl buffer as blank. The following formula was used to calculate the percentage of inhibition,
![]()
Where, product control was devoid of casein and the test solution and test control without the drug. Anti-inflammatory activity was also tested using human red blood cell (HRBC) membrane lysis and hypotonicity and heat induced membrane lysis methods as per Oyedapo et al., (1995) with minor modifications. Blood sample was taken from a healthy volunteer who had not been administered any anti-inflammatory drug for the past two weeks of experiment. It was centrifuged at 3000 rpm for 10 minutes at room temperature. The red blood cells thus obtained were repeatedly washed isosaline (0.85% w/v Sodium chloride) till a colourless supernatant appeared. From the final wash, a 10% RBC suspension was prepared using isosaline. The centrifugation and washing were continued repeatedly until the supernatant became colorless. HRBC suspension of 10% was prepared using isosaline. Hypotonicity induced hemolysis was performed by preparing a reaction mixture with 2 ml hyposaline (0.25% w/v NaCl), 1 ml 0.15M sodium phosphate buffer (pH 7.4), 0.5 ml 10 % blood cell suspension and 1 ml of extract in different concentrations (62.5,125, 250, 500 µg/ml) and the final volume was made up to 4.5 ml using isosaline. Diclofenac sodium was used as the standard drug and the reaction mixture was incubated for 45 min at 40°c. The tubes were then centrifuged at 3000 rpm for 10 min and the absorbance was measured was spetrophotometrically at 560nm. The percentage of inhibition was calculate using the formula
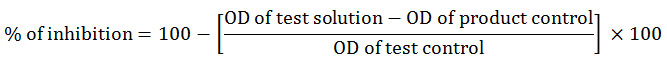
where, product control means the absorbance of drug without HRBC and test control contained the HRBC but not the test sample. Heat induced hemolysis was done by preparing the reaction with 1 ml of HRBC suspension (10%) and 1 ml test samples of different concentrations (62.5, 125, 250, 500 µg/ml). Test control contained 1 ml saline instead of test sample and product control omitted HRBC. Diclofenac sodium served as standard drug. The reaction mixture was incubated at 56 º C for 30 minutes followed by cooling under tap water. Then centrifugation was carried out at 2500 rpm for 5 min. The optical density of supernatant was measured in UV- visible spectrophotometer. Percentage of inhibition was calculated using the formula,
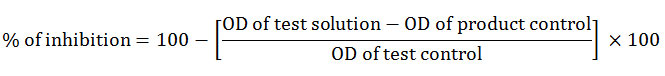
Statistical analysis was performed in triplicates and the results were expressed in mean with standard division. Regression analysis was done in Microsoft excel 2007 version. The inhibitory percentages obtained with each concentrations of each assays were statistically analyzed through one way analysis of variance (ANOVA) followed by Tukey’s post-hoc test using IBM spss statistics 25.
RESULTS AND DISCUSSION
Results of the preliminary screening of different extracts revealed the presence or absence of different phytochemical compounds is shown in Table (1). Methanol extract contained more number of compounds such as flavonoids, coumarins, tannins, saponins, steroids, terpenoids, phenols, alkaloids, resin, carbohydrates and reducing sugar or glycosides when compared to others. This can be correlated with the earlier study of Anilkumar and Johny in L. quinqueflora (Anilkumar and Johny, 2015). LM, LMEK and LE contained most of the phytochemicals tested when compared with hexane, chloroform and water. Preliminary phytochemical screening of leaves of L. monopetala showed the presence of same compounds that exhibited pharmacological properties such as anti-microbial and anti-inflammatory (Ali-Ahmmad et al., 2012). LM, LMEK and LE are selected for the further anti-inflammatory studies because of the presence of most of the phytochemical constituents especially the flavonoids, phenols and alkaloids than other extracts. The medicinal properties of Litsea species can be attributed to the secondary metabolites present in it (Kamle et al., 2019).
Table 1. Preliminary Phytochemical Screening
| Components | Hexane | Chloroform | Ethyl acetate | Ethyl methyl ketone | Methanol | Water |
| Flavonoids | – | – | + | + | + | – |
| Coumarins | – | – | + | – | + | – |
| Tannins | – | – | + | + | + | + |
| Saponins | – | + | + | – | + | – |
| Steroids/terpenoids | – | – | – | + | + | + |
| Phenols | – | – | + | + | + | – |
| Alkaloids | – | – | + | + | + | – |
| Quinines | – | – | – | – | – | – |
| Anthraquinones | – | – | – | – | – | – |
| Protein | – | – | – | – | – | – |
| Carbohydrates | + | + | + | + | + | + |
| Resin | – | – | – | + | + | – |
| Reducing sugar/ Glycosides | – | – | + | + | + | – |
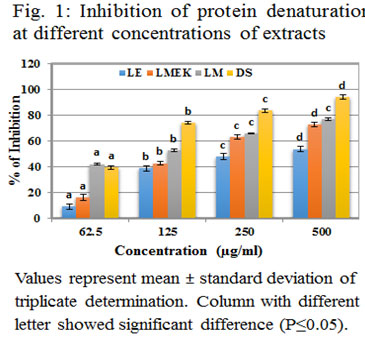
Inflammation can be accompanied with gradual denaturation of protein. Here, a protein candidate has been subjected to heat induced denaturation by applying 57ºC. The capacity of samples to inhibit the protein denaturation was evaluated and shown in figure (1). LE showed inhibition against protein denaturation at IC 50 value of 390.73 μg/ml as obtained from the linear curve with regression equation y = 0.082x + 17.96; R2 = 0.637. In the case of LMEK the IC 50 value was observed at 248.6μg/ml with regression equation y = 0.114x + 21.66; R2 = 0.783. LM exhibited an IC 50 value of 114.8 μg/ml from the linear curve with regression equation y = 0.075x + 41.39; R2 = 0.919. From this it was clear that LM has more activity than that of LE and LMEK. Percentage of inhibition of all the three extracts was significant (P≤0.05) on a dose dependent manner. Highest inhibitory percentage of LM is 76.7 % at 500μg/ml, LMEK at 500μg/ml is 72.51% and that of LE is 53.55%. The IC50 values of leaf extracts of Aegle marmelos and Ocimum sanctum in protein denaturation assay were 95.64μg/ml and 42.17μg/ml respectively (Reshma and Brindha, 2014) and is an indication of its higher activity than that of LM, LMEK and LE. The results obtained in Polyalthia longifolia and Pergularia daemia (Iffath Hina and Rose, 2018; Ogbomade et al., 2019) can be compared with that of L. quinqueflora. In a study using ethanolic leaf extract of Nauclea latifolia Sm (Iheagwam et al., 2020), the highest activity of 70.54 % was observed at 5 mg/ml.
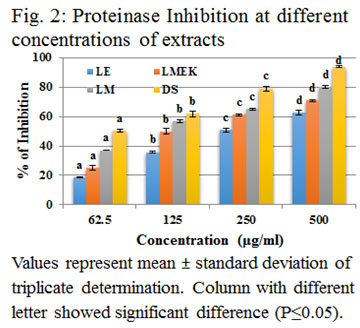
From this it is clear that L. quinqueflora has greater inhibition of protein denaturation than most of the previously reported similar cases. All the three extracts used in the present study showed significant inhibitory activity against proteinase action when compared with standard diclofenac sodium as shown in figure (2). The IC 50 value of LE was 324.18 μg/ml (y = 0.0914x + 20.50; R2 = 0.87), LMEK was 215.39 μg/ml (y = 0.089x + 30.83; R2 = 0.762) and that of LM was 122.21μg/ml (y = 0.086x + 39.49; R2 = 0.868). Statistically, all the three extracts showed increase in percentage of inhibition with increase in concentration and each concentration levels were significantly different (P≤0.05). The proteinase inhibitors have significant role in inflammation. Inflammation caused the infiltration of neutrophils which can be a rich source of serine proteases. Intracellular release of these proteases causes damage of microbes. But extracellular release leads to tissue damage during inflammatory conditions (Pham, 2008). The alcoholic leaf extract of Justicia gendarussa inhibit proteinase activity at 209μg/ml which was less than that of LM and thus noticed higher activity of LM. But the IC 50 value of root extract of J. gendarussa showed inhibition at 54μg/ml and higher activity than that of LM, LMEK and LE (Patel and Zaveri 2014; Naz et al., 2017).
The IC50 value in proteinase inhibition of methanolic extract of Aristolochia indica, Cuscuta pedicellata, Melilotus indicus and Tribulus terrestris were higher than that of present extracts of L. quinqueflora (Naz et al., 2017). The IC50 value of leaf extracts of Moringa oleifera (Saleem et al., 2020) was higher than that of LMEK and LM. Hence the study revealed that all the three extracts were able to inhibit trypsin or achieve 50% of inhibition with less amount of extract when compared with lesser concentration of already mentioned plants. In membrane stabilization assay, HRBC membrane acts as an imitation of lysosomal membrane. Lysosomal membrane is significant in inflammation as its breakage causes infiltration of various inflammatory mediators into the inflammatory site. Accumulation and persistence of these intracellular lysosomal constituents gradually lead to chronic inflammation. A drug which is able to prevent breakage of HRBC membrane can contribute to the stability of lysosmal membrane and thus can be considered as an anti-inflammatory agent. Here, HRBC was exposed to heat and hypotonicity as two stimulants in order to create an inflammatory condition (Amrutkar et al., 2017).
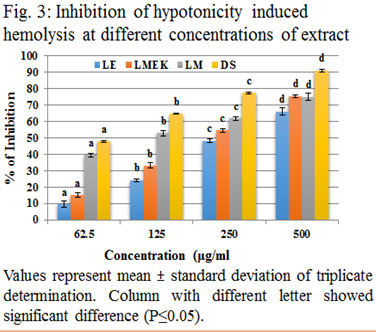
In both conditions, membrane started to lyse and as a result of lysis, haemoglobin leaks out. The amount of haemoglobin came out the cell due to cell lysis is proportionate to the grade of lysis. The samples exhibited effective inhibition against hypotonicity induced hemolysis as showed in figure (3) and was significant with an IC50 value of 340.28 μg/ml (y = 0.124x + 7; R2 = 0.926) for LE. LMEK showed 276.1 μg/ml as IC 50 value (y = 0.131x + 13.83; R2 = 0.935) and LM as 137.4 μg/ml (y = 0.073x + 39.97; R2 = 0.762). Membrane stabilization property of direct methanolic extract of leaves of L. quinqueflora has been studied and reported where 400μg/ml exhibited a 60% inhibition (Anilkumar and Johny, 2015). Here, the sequentially extracted LM shows 61.89% at 250μg/ml. This results point to the fact that LM obtained via sequential extraction is more purified and potentially active than that of the crude one. Blood samples or HRBC membrane is intact in isosaline conditions. When placed in hypotonic solution, osmotic stress results thus causing gradual lysis of the outer membrane to ooze out haemoglobin. The inhibitory potential of sample can be compared with that of ethanolic leaf extract of Dalbergia sissoo which exhibited a similar level of inhibition (Amrutkar et al., 2017). The study of methanolic leaf extract of Mucuna pruriens expressed less inhibition (Anosike et al., 2019) when compared with that of LM.
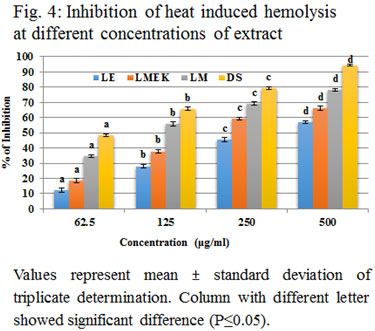
LE, LMEK and LM could effectively inhibit hemolysis at each concentration level. The results obtained after heat induced HRBC lysis is shown in figure (4). LE showed an IC 50 value of 389.15 μg/ml (y = 0.094x + 13.42; R2 = 0.875), LMEK at 282.73 μg/ml (y = 0.099x + 22.01; R2 = 0.792) and that of LM at 127.93 μg/ml (y = 0.087x + 38.87; R2 = 0.792).When HRBC membrane is exposed to an elevated temperature, thermal osmolysis can occur. It leads to swelling of cells due to the increase in turbidity of suspending solution. The rapid increase in temperature and swelling causes slow rupture of cell membrane and driving out of cell constituents. This prolonged hemolysis diverts the normal effect of inflammation into inflammatory disorders. This assay enables the measurement of inhibition of hemolysis by the leaf extract of L. quinqueflora and thus inflammation (Tsong and Kingsley, 1975). Methanolic extract of whole plant of Enicostemma axillare (Leelaprakash and Dass, 2011) also effectively inhibit heat induced hemolysis and less potent than LM (Kumar et al., 2011).
The inhibitory percentage of leaf extracts of Baselia alba against membrane lysis is almost similar to that of LE but less than that of LMEK and LM (Kumar et al., 2011). The membrane stabilization activity of Vitex leucoxylon showed highest percentage of inhibition with lowest concentration and the inhibitory activity of its alcoholic extracts are higher than that of L. quinqueflora extracts (Faimum et al., 2013). Gunathilake et al reported heat induced membrane stabilization assay in methanolic extracts of Cassia auriculata, Passiflora edulis, Sesbania grandiflora, Olax zeylanica, Gymnema lactiferum and Centella asiatica (Gunathilake et al., 2018). Though they inhibit hemolysis, its percentage activity is less when compared to LM, LMEK and LE. In most of the earlier studies, anti-inflammatory screening assays were reported using methanol extract of plant parts. Here, ethyl acetate, methyl ethyl ketone and methanolic extracts were used for each assay. Methyl ethyl ketone was preferred to avoid hindrance due to mucilage. All of them showed significant inhibition (P≤0.05) on a dose dependent manner. Their inhibitory percentage is directly proportional to increase in concentration. Methanolic extract showed highest activity when compared with LMEK and LE (Faimum et al., 2013).
CONCLUSION
This present study supported the use of L. quinqueflora as a traditionally used medicinal plant against inflammatory disorders. Though there are scanty reports available on the bioactivity of this plant, it has gained importance as among the local remedy against inflammation. This study thus revealed its potency as an anti-inflammatory agent by inhibiting protein denaturation, proteinase activity and membrane lysis. The presence of various classes of phytochemicals contributes to its pharmacological properties. Further studies to evaluate the pharmacological activities of the isolated components of L. quinqueflora are under progress in our lab.
Conflict of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
ACKNOWLEDGEMENTS
The first author gratefully acknowledges the financial support received from Mahatma Gandhi University, Kottayam, Kerala, in the form of Junior Research Fellowship for carrying out this research work.
REFERENCES
Abdulkhaleq, L.A., Assi, M.A., Abdullah, R., Zamri-Saad, M., Taufiq-Yap, Y.H. and Hezmee, M.N.M., (2018). The crucial roles of inflammatory mediators in inflammation: A review. Veterinary World, 11(5), p.627.
Ali-Ahmmad, M., Islam, T., Sultana, I., Mahmood, A., Akther, J., Hossain, Z.H., Ibrahim, M. and Chowdhury, M.M.U., (2012). Pharmacological and phytochemical screening of ethanol extract of Litsea monopetala (Roxb.) Pers. IOSR Journal of Pharmacy, 2(3), pp.398-402.
Althaf Faimum, D., Sudaroli, M., and Mohammed Salman, I. (2013). In Vitro anti-inflammatory activity of Vitex leucoxylon Linn. leaves by HRBC membrane stabilization. International Journal of Pharmacy & Life Sciences, 4(1).
Amrutkar, Y., Hajare, S., Ingawale, M.V., Bhojane, N.M., Madhuri, H. and Ingole, R.S., (2017). Anti-inflammatory activity of ethanolic leaf extract of Dalbergia sissoo in vitro and in vivo. Advances in Tissue Engineering and Regenerative Medicine, 2(3), pp.171-174.
Anilkumar, M. (2010). Ethnomedicinal plants as anti-inflammatory and analgesic agents. in: Chattopadhyay, D. pp.267-293 (Ed.) Ethnomedicine: A source of complementary therapeutics. Research Signpost, Trivandrum.
Anilkumar, M. and Johny, J., (2015). Evaluation of in vitro anti-Inflammatory activity of the methanolic extract of Litsea quinqueflora (Dennst.) Suresh. Journal of Pharmacy and Biological Sciences, 10(2), pp.32-36.
Anosike, C.A., Igboegwu, O.N. and Nwodo, O.F.C., (2019). Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. Journal of Traditional and Complementary Medicine, 9(4), pp.278-284.
Chaible, L.M., Kinoshita, D., Corat, M.A.F., and Dagli, M.L.Z., (2017). Genetically Modified Animal Models. in: Animal Models for the Study of Human Disease, pp. 703-726.Academic Press.
Gunathilake, K.D.P.P., Ranaweera, K.K.D.S. and Rupasinghe, H.P., (2018). In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines, 6(4), p.107.
Harborne, J. B., (1998). Photochemical methods: a guide to modern techniques of plant analysis, pp. 4-84 (third ed). Chapman A. & Hall, London, UK.
Iffath Hina, M. and Rose, C., (2018). In Vitro anti-Inflammatory and antiarthritic activity of Pergularia daemia Leaves and Roots. International Journal of Drug Development and Research10, p. 1.
Iheagwam, F.N., Israel, E.N., Kayode, K.O., DeCampos, O.C., Ogunlana, O.O. and Chinedu, S.N., (2020). Nauclea latifolia Sm. Leaf Extracts Extenuates Free Radicals, Inflammation, and Diabetes-Linked Enzymes. Oxidative Medicine and Cellular Longevity, 2020.
Jose, S.M. and Anilkumar, M., (2018). In vitro antioxidant activity of Litsea quinqueflora (Dennst.) Suresh. Journal of Pharmacognosy and Phytochemistry, 7(4), pp.3217-3221.
Kamle, M., Mahato, D.K., Lee, K.E., Bajpai, V.K., Gajurel, P.R., Gu, K.S. and Kumar, P., (2019). Ethnopharmacological properties and medicinal uses of Litsea cubeba. Plants, 8(6), p.150.
Kiranmayi, G.V.N., (2018). Preliminary phytochemical screening and in vitro evaluation of anti-inflammatory, antiarthritic, and thrombolytic activities of ethanolic leaf extract of Bauhinia purpurea. International Journal of Green Pharmacy, 12(01).
Kumar, V., Bhat, Z. A., Kumar, D., Bohra, P., and Sheela, S. (2011). In-vitro anti-inflammatory activity of leaf extracts of Basella alba Linn. var. alba. International Journal of Drug Development and Research, 3(2), 176-179.
Leelaprakash, G. and Dass, S.M., (2011). In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. International Journal of Drug Development and Research, 3(3), pp.189-196.
Mizushima, Y. and Kobayashi, M., (1968). Interaction of anti‐inflammatory drugs with serum proteins, especially with some biologically active proteins. Journal of Pharmacy and Pharmacology, 20(3), pp.169-173.
Naz, R., Ayub, H., Nawaz, S., Islam, Z.U., Yasmin, T., Bano, A., Wakeel, A., Zia, S. and Roberts, T.H., (2017). Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complementary and Alternative Medicine, 17(1), p.302.
Ogbomade, R. S., Asara, A. A. and Eboh, A. S., (2019). In vitro anti-inflammatory properties of leaf extract of Polyalthia longifolia extract. The Pharmaceutical and Chemical Journal6, pp. 112-115.
Ogunmefun, O.T., (2018). Phytochemicals—God’s Endowment of Curative Power in Plants, in: Asao, T., Md Asaduzzaman. pp.7-22 (Eds.) Phytochemicals: Source of Antioxidants and Role in Disease Prevention. Intech open, London.
Oyedapo, O.O. and Famurewa, A.J., (1995). Antiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. International journal of Pharmacognosy, 33(1), pp.65-69.
Patel, S. S., and Zaveri, M. N. (2014). Trypsin and Protein Denaturation Inhibitory Activity of Different Fractionation and Isolated Compound of Leaf and Root of Justicia Gendarussa. Asian Journal of pharmaceutical science and technology, 5(2014), 5564-71.
Pham, C.T., (2008). Neutrophil serine proteases fine-tune the inflammatory response. The International Journal of Biochemistry & Cell Biology, 40(6-7), pp.1317-1333.
Raghavendra, G.M., Varaprasad, K. and Jayaramudu, T., (2015). Biomaterials: design, development and biomedical applications. In Nanotechnology applications for tissue engineering, William Andrew Publishing (pp. 21-44).
Reshma, A. K., and Brindha, P. (2014). In vitro anti-inflammatory, antioxidant and nephroprotective studies on leaves of Aegle marmelos and Ocimum sanctum. Asian Journal of Pharmaceutical and Clinical Research, 7(4).
Saleem, A., Saleem, M. and Akhtar, M.F., (2020). Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: An ethnomedicinal plant of Moringaceae family. South African Journal of Botany, 128, pp. 246-256.
Seal, T., Chaudhuri, K., Pillai, B., Chakrabarti, S., Mondal, T., and Auddy, B. (2020). Evaluation of antioxidant activities, toxicity studies and the DNA damage protective effect of various solvent extracts of Litsea cubeba fruits. Heliyon, 6(3), e03637.
Tsong, T.Y. and Kingsley, E., (1975). Hemolysis of human erythrocyte induced by a rapid temperature jump. Journal of Biological Chemistry, 250(2), pp.786-789.