1Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam, India
2Department of Pharmaceutical Sciences, North East Frontier Technical University, Along, Arunachal Pradesh, India
3Department of Pharmacology, Himalayan Pharmacy Institute, Majhitar, Sikkim, India
4Department of Pharmacy, Govt. Girls Polytechnic, Raipur, Chhattisgarh, India
Corresponding author email: nhussain116@gmail.com
Article Publishing History
Received: 21/10/2020
Accepted After Revision: 16/12/2020
Cordia dichotoma Forst. has been used in the management of diabetes in traditional medicine. However, the antidiabetic activity of the methanolic extract of C. dichotoma (MECD) bark has not been reported so far. In this study, the antidiabetic activity of C. dichotoma was assessed in alloxan-induced diabetic Wistar rats. The acute toxicity study indicated that the MECD was safe up to the dose level of 2000 mg/kg body weight. In oral glucose tolerance (OGT) test, the pre-treatment of MECD showed partial protection from hyperglycemia induced by a glucose load (2 g/kg, body weight) in rats at the dose levels of 250 and 500 mg/kg, body weight. The MECD significantly reduced the blood glucose levels in the alloxan-induced diabetic rats at the dose levels of 250 & 500 mg/kg, body weight as compared to normal control animals. Analysis of biochemical parameters and histopathological investigations also demonstrated the antidiabetic potential of the MECD with significant improvement of biochemical parameters including body weight, serum lipid profile and antioxidant enzymes/ biomarkers in comparison to the normal control. The activities of the MECD (500 mg/kg body weight) were comparable to some extent with that of the standard drug, glibenclamide (5 mg/kg). Our study scientifically validates the folkloric claim as well as traditional uses of C. dichotoma as antidiabetic medicine. It is suggested that the antidiabetic activity of C. dichotoma may be due to the presence of phenolic phytoconstituents or plant flavonoids in the methanolic bark extract.
Cordia dichotoma, Methanolic Extract, Antidiabetic Activity, Flavonoids, Antioxidant Property
Hussain N, Kakoti B. B, Rudrapal M, Rahman Z, Rahman M, Chutia D, Sarwa K. K. Antidiabetic Activity of the Bark of Indian Cherry, Cordia dichotoma. Biosc.Biotech.Res.Comm. 2020;13(4).
Hussain N, Kakoti B. B, Rudrapal M, Rahman Z, Rahman M, Chutia D, Sarwa K. K. Antidiabetic Activity of the Bark of Indian Cherry, Cordia dichotoma. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/3mXCdzU”>https://bit.ly/3mXCdzU</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Diabetes mellitus (or diabetes) is a chronic metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemic condition of diabetes may lead to several health complications including cardiovascular (cardiomyopathy), neurological (neuropathy), renal (nephropathy) and ocular (retinopathy) (Junejo et al., 2020a; Junejo et al., 2020b). In diabetic patients, the hyperglycemic condition is mainly because of the decreased insulin secretion due to the abnormal functioning of insulin producing beta cells (present on the islets of Langerhans in pancreas) which thereby fail to produce enough insulin resulting in unstable blood glucose level followed by the occurrence of insulin resistance by metabolizing tissues (Tanwar et al., 2020; Yeh et al., 2003).
The prevalence of diabetes is increasing with the global rise of obesity and lifestyle disorders. According to World Health Organization (WHO) reports, it has been estimated that there were 422 million adults living with diabetes mellitus with 1.6 million deaths each year globally. The type 2 diabetes has been accounted for the majority (> 90-95%) of diabetes with approximately 1.5 million death annually (WHO, 2020). Various synthetic hypoglycemic agents have been used to control the elevated blood sugar level in patients with diabetes mellitus. Some common hypoglycemic agents approved by FDA include sulfonylureas, biguanides, thiazolidinediones and so on. Despite the availability of many effective oral hypoglycemic agents, diabetes is still life-threatening because of the limited clinical efficacy of existing drugs. Some common side effects that are associated with synthetic antidiabetic drugs include weight gain, weakness, fatigue, headache, palpitation, increased LDL chlosterol level etc. (Tanwar et al., 2020).
Plant-based traditional remedies have been used for the treatment of human diseases for thousands of years. About 80% of world population rely on traditional herbal medicines for primary health care. Traditional medicines derived from plants play a significant role in the management of a variety of human disorders including cancer, neurological disorders, diabetes and pain and inflammatory disorders, just to name a few. WHO has recommended the antidiabetic evaluation of traditional plant-based remedies or herbal preparations because they are effective with less or no toxicities as compared to synthetic antidiabetic drugs. Moreover, herbal medicines have the property of synergistic action due to the presence of a variety of active constituents in a single drug/ medicinal preparation (Jaiswal, 2016). Many indigenous Indian medicinal plants have been found to be useful in the treatment of diabetes mellitus. Ayurveda, Unani and Siddha, are the notable systems of medication documented in ancient practice basically utilizing plants/ plant-based preparations as medicines for curing human ailments/ diseases like diabetes. In view of their traditional and ethnopharmacological importance, herbal medicine may have potential role in the management of diabetes as well as its complications.
Cordia dichotoma Forst. (also known as Indian cherry) belonging to the family Boraginaceae is an average-sized tree of tropical and subtropical origin. It is widely found in Sri Lanka, India, and other tropical regions of the world. The use of this plant has been on ancient practice for the management of a variety of human disorders. It is also an important plant species found in the traditional Indian system of medicine including Ayurveda, Unani and Siddha. Seeds of C. dichotoma are used for the treatment of various inflammatory disorders. Fruits are used as expectorant, astringent, laxative and anthelmintic. Some common ethnomedinal uses of C. dichotoma includes antidiabetic, immunomodulator, diuretic, anthelmintic, wound healing, antiulcer, gastro-protective, anti-inflammatory, antileprotic, antidiabetic, and hepatoprotective and antioxidant activities. The bark of C. dichotoma has been reported to possess betulin, α-amyrins, octacosanol, β-sitosterol, lupeol-3-rhamnoside, β-sitosterol-3-glucoside, hentricontanol, taxifolin-3,5-dirhmnoside, α-amyrin, hentricontane and hesperitin-7-rhamnoside (Hussain et al., 2020).
There has been no scientific study on the the antidiabetic activity of the C. dichotoma bark previously reported in literature. The present study was, therefore, aimed at investigating the antidiabetic activity of the methanolic extract of C. dichotoma bark with a view to justify the traditional use of the plant in the treatment of diabetes.
MATERIAL AND METHODS
Drugs and chemicals: Alloxan monohydrate was purchased from Sigma-Aldrich, Mumbai, India. Glibenclamide was procured as a gift sample from Sun Pharmaceutical Industries Ltd., India. All other chemicals, reagents and solvents used were of analytical grade.
Plant material, extraction and phytochemical screening: The barks of C. dichotoma Forst. were collected during the month of April-May, 2012 from the Duhai forest of Ghaziabad, Uttar Pradesh, India. The plant material was identified from CSIR-National Institute of Science Communication and Information Resources (CSIR-NISCAIR), New Delhi. A voucher specimen (NISCAIR/RHMD/ Consult/2012-13/2025/33) of the bark of C. dichotoma was submitted at the herbarium for future reference. The shade-dried barks of C. dichotoma were pulverized to coarse powder, defatted using petroleum ether, and extracted with methanol. The methanolic extract was subsequently evaporated to dryness and the concentrated extract so obtained was preserved in a refrigerator at 4 oC for further use. The percentage yield of the methanolic bark extract of C. dichotoma (MECD) was found to be 7.11% w/w on dry weight basis. The MECD was subjected to preliminary phytochemical screening to identify the presence of phytoconstituents as per the standard procedure previously described in literature (Ajiboye et al., 2020; Junejo et al., 2018).
Experimental animals: Adult Wistar albino rats of either sex weighing around 300-330 g were obtained from the Institutional Animal House for the experimental study. Animals were acclimatized to the standard laboratory conditions (temperature: 25 ± 2 oC, relative humidity: 50 ± 5 %) with a 12 h light/12 h dark cycle for a week before the beginning of experiments, and were provided with free access to the standard pellet diet and drinking water ad libitum. The experimental protocol was approved by the IAEC vide approval no. IAEC/DU/58 dated. 24/09/2013.
Acute oral toxicity:The acute toxicity test of MECD was performed on Swiss albino mice (40-45 g) as per the OECD guidelines (Junejo et al., 2014b). After overnight fasting, the animals were given a fixed maximum dose 2000 mg/kg body weight via intraperitoneal route of administration. Animals were under observation for 48 hours for signs of mortality or morbidity or death. One group was maintained as normal control and was given only vehicle.
Oral glucose tolerance (OGT) test: Overnight fasted rats were randomly divided into five groups with six animals each. Group 1 rats were given normal saline by oral route. Group 2 animals were administered glucose (2 gm/kg body weight) load orally. Group 3 and group 4 rats received MECD (250 and 500 mg/kg body weight, respectively) by oral route followed by the administration of glucose (2 gm/kg body weight) load after 30 minutes. Similarly, group 5 rats received glibenclamide (5 mg/kg body weight) followed by the glucose (2 gm/kg body weight) load. Blood was collected from the tail vein of animals at 30, 60, 90 minutes interval after the glucose administration. The blood glucose level was measured with the help of a glucometer (Lifescan, Milpitas, CA) (Amira et al., 206; Junejo et al., 2020b).
Evaluation of hypoglycemic activity: Diabetes was induced in overnight fasted rats withalloxan monohydrate (120 mg/kg body weight, in normal saline) administered by a single intraperitoneal injection. The animals confirmed as diabetic (after 72 h of alloxan injection) by the elevated plasma glucose levels (> 150 mg/dl) was used for the experiment. The animals were randomly divided into five groups of six rats each. Group 1 animals received normal saline orally. Group 2 received alloxan monohydrate (120 mg/kg body weight) by intraperitoneal route. Group 3 and group 4 received alloxan monohydrate (120 mg/kg body weight) intraperitoneally followed by the oral administration of MECD (250 and 500 mg/kg body weight, respectively). Similarly, group 5 received alloxan monohydrate (120 mg/kg body weight) followed by the standard drug, glibenclamide (5 mg/kg body weight) (Mishra and Garg, 2011; Raut and Naresh, 2006).
The blood samples were collected from the tail-tip of animals for measuring the blood glucose levels on 1st, 7th, 14th and 21st day. The blood glucose level was determined using the glucometer and the results were expressed as mg/dl (Banerjee et al., 2017; Junejo et al., 2020a).
Initial and final body weights were measured on 0 and 21st day (Junejo et al., 2017). The serum was separated by centrifugation (2000 rpm, 10 minutes) for the estimation of various biochemical parameters. Serum lipid profile was estimated using commercially available kits. Triglycerides (TG), total cholesterol (TC), high density lipoprotein (HDL) and low density lipoprotein (LDL) were determined (Junejo et al., 2014a). The levels of different antioxidant enzymes and/or markers were also determined. The activities/ levels of reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and malonaldehyde (MDA) were measured. Malonaldehyde is formed in vivo as a result of lipid peroxidation in the tissue under oxidative stress (Junejo et al., 2017; Chaulya et al., 2010).
Histopathological investigation: At the end of 21 days of treatment, the animals were fasted for 12 hours, anaesthetized and sacrificed. Pancreas of rats from control, diabetic control, MECD treated, and standard drug treated (glibenclamide) groups were quickly removed and processed for histopathological studies. Pancreatic tissues removed from control and treated rats were washed in saline, the sections stained in haematoxylin-eosin were observed under a light microscope for histopathological investigations. Tissues were further fixed in Hollande‐Bouin fixative for 48 hours. Fixed tissues were processed for paraffin embedding (Junejo et al., 2020c).
Statistical analysis : Results are presented as mean ± standard error of mean (SEM). The one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test was used to analyse and compare data. Statistical analysis was performed using the IBM SPSS 19.0 statistical software package, for Windows. Statistical differences at 1% (p < 0.01) level of probability between the groups were considered statistically significant.
RESULTS AND DISCUSSION
Preliminary qualitative phytochemical analysis showed the presence of alkaloids, steroids, phenolic compounds, flavonoids, tannins, saponins and carbohydrate in the MECD.The animals showed no signs of adverse effects during the period of observation. No gross behavioural changes like drowsiness, restlessness, writhing, convulsions and symptoms of toxicity and mortality were observed in animals up to 48 hours of experimentation. The body weight and food consumption were normal as compared to control animals. The MECD was found to be safe up to the test dose of 2000 mg/kg body weight.
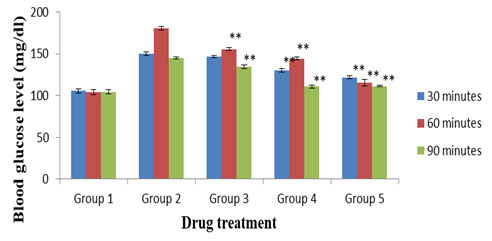
Figure 1 displays the results of OGT test. The MECD treated groups showed significant (p < 0.01) reduction of blood glucose levels at both the doses (250 and 500 mg/kg) as compared to the normal control group. Similarly, the standard, glibenclamide (5 mg/kg) treated group also exhibited significant (p <0.05) activity in comparison to normal control.
Figure 1: OGT test. Values were expressed as mean ± SEM (n = 6). Activities of MECD and standard drug, glibenclamide are statistically significant at **p < 0.01 as compared to normal control. Group 1: Normal control; Group 2: Diabetic (alloxan) control; Group 3: MECD, 250 mg/kg; Group 4: MECD, 500 mg/kg; Group 5: Glibenclamide, 5 mg/kg.
Results of the effect of MECD on blood glucose level of alloxan-induced diabetic rats are depicted in Figure 2. After treatment with MECD, the blood glucose levels were significantly (p < 0.01) reduced at both the doses (250 and 500 mg/kg) as compared to the diabetic control group. The glibenclamide (5 mg/kg) treated group also showed significant (p < 0.01) reduction in the blood glucose level when compared to normal control group.
Figure 2: Effect of MECD on blood glucose level in alloxan-induced diabetic rats. Values indicate mean ± SEM (n = 6). Activities of MECD and standard drug, glibenclamide are statistically significant at **p < 0.01 as compared to normal control. Group 1: Normal control; Group 2: Diabetic (alloxan) control; Group 3: MECD, 250 mg/kg; Group 4: MECD, 500 mg/kg; Group 5: Glibenclamide, 5 mg/kg.
After 21 days of treatment with MECD (250 and 500 mg/kg), the body weight of animals were significantly (p <0.05) increased as compared to diabetic rats. The effects of MECD on body weight and lipid profile of diabetic rats are presented in Table 1. In diabetic rats, the levels of TG, TC, and LDL were significantly increased, and the HDL level was significantly decreased. In MECD (250 and 500 mg/kg body weight) treated groups, the TG, TC and LDL levels were significantly (p < 0.01) reduced and the HDL level was significantly (p < 0.01) increased as compared to diabetic control rats. The activities of MECD at the dose of 500 mg/kg body weight were somewhat comparable with that of glibenclamide (5 mg/kg).
Table 1. Effect of MECD on body weight and lipid profile of diabetic rats
| Group | Body weight (g) | Lipid profile (mg/dl) | ||||
| 0 day | 21st day | TG | TC | HDL | LDL | |
| Group 1
(Normal control) |
300.23 ± 3.56 | 302.38 ± 2.49 | 85.32 ± 2.45 | 156.71 ± 4.10 | 36.14 ± 3.45 | 98.14 ± 4.41 |
| Group 2
(Diabetic control) |
300.12 ± 3.86 | 270.90 ± 3.46 | 215.14 ± 4.61 | 250.34 ± 5.20 | 36.10 ± 4.20 | 189.51 ± 4.20 |
| Group 3
(MECD, 250 mg/kg) |
298.34 ± 2.43** | 290.30 ± 4.21** | 147.21 ± 2.35** | 160.44 ± 4.73** | 43.71 ± 5.62** | 118.21 ± 7.21** |
| Group 4
(MECD, 500 mg/kg) |
300.23 ± 5.42** | 292.96 ± 4.72** | 38.34 ± 3.91** | 148.31 ± 4.00** | 49.32 ± 4.19** | 102.42± 6.34** |
| Group 5
(Glibenclamide, 5 mg/kg) |
299.24 ± 3.79** | 298.59 ± 3.72** | 116.56 ± 7.16** | 147.32 ± 2.16** | 56.11 ± 4.10** | 76.34 ± 3.62** |
Results are expressed as mean ± SEM (n = 6); **p < 0.01, compared to normal control. TG: Triglyceride, TC: Total cholesterol, HDL: High density lipoprotein, LDL: Low density lipoprotein.
The MECD restored significantly (p < 0.01) the levels/ activities of GSH, SOD and CAT at both the doses (250 & 500 mg/kg body weight) as compared to the normal control. Additionally, the MECD significantly (p < 0.01) improved the levels of LPO marker component i.e., MDA in comparison to the normal control group. Results of the effects of MECD on antioxidant enzymes/ markers of diabetic rats are displayed in Table 2. Results also reveal that the antioxidant activity of MECD (500 mg/kg body weight) was comparable with that of the standard drug, glibenclamide (5 mg/kg body weight).
Table 2. Effect of MECD on antioxidant enzymes/ markers of diabetic rats
| Groups | GSH (µmoles of GSH/mg protein) | SOD (U/mg protein) | CAT (U/mg protein) | MDA (nmoles of MDA/mg of protein) |
| Group 1 (Normal control) | 4.99 ± 0.17 | 3.79 ± 0.13 | 5.09 ± 0.18 | 3.53 ± 0.17 |
| Group 2 (Diabetic control) | 3.11 ± 0.14 | 2.20 ± 0.26 | 2.01 ± 0.25 | 5.29 ± 0.26 |
| Group 3 (MECD, 250 mg/kg) | 4.82 ± 0.18** | 4.08 ± 0.25** | 4.85 ± 0.23** | 4.83 ± 0.15$ |
| Group 4 (MECD, 500 mg/kg) | 5.58 ± 0.12** | 4.75 ± 0.20** | 5.07 ± 0.13** | 4.16 ± 0.19** |
| Group 5 (Glibenclamide, 5 mg/kg) | 4.25 ± 0.18** | 3.93 ± 0.25** | 3.62 ± 0.24** | 4.13 ± 0.18** |
Results are expressed as mean ± SEM (n = 6); **p < 0.01, compared to normal control. GSH: Reduced glutathione, SOD: Superoxide dismutase, CAT: catalase, MDA: Malonaldehyde.
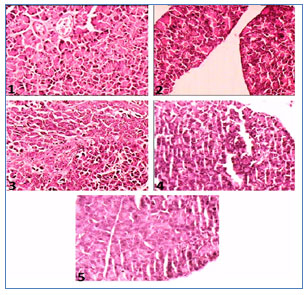
Histopathological investigations of pancreas of alloxan-induced diabetic rats (Figure 3) exhibited pathophysiological features with the reduction in the number of islets, damaged β-cell population and extensive necrotic changes followed by fibrosis and atrophy (Slide 2). In the normal control group, normal cellular population in the islets of Langerhans were observed (Slide 1). MECD (250 and 500 mg/kg) (Slide 3 and Slide 4, respectively) and glibenclamide (5 mg/kg) (Slide 5) treated rats restored the necrotic and fibrotic changes and also increased the number and the size of the islets.
Figure 3: Photographs showing histopathology of pancreas of experimental rats. Slide 1: Treated with normal saline, Slide 2: Treated with alloxan (120 mg/kg), Slide 3: Treated with MECD (250 mg/kg), Slide 4: Treated with MECD (500 mg/kg), Slide 4: Treated with standard drug, glibenclamide (5 mg/kg).
Cellular oxidative stress (OS) induced by the reactive oxygen species (ROS) produced from the action of free radicals in the biological matrix may be increased abnormally during diabetes, causing an imbalance between the cellular metabolism and the antioxidant system of the body. The oxidative stress produces inflammatory cascades that damage the cellular components such as beta cells of islets of Langerhans (Tanwar et al., 2020).
Further oxidative stress is undoubtedly claimed to have significant involvement in the pathogenesis of chronic diabetic mellitus along with various disease complications. The oxidative stress can be reduced to a considerable extent by the action of various antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GSH) and glutathione peroxidase (GPx) (Junejo et al., 2017, Debasis et al., 2010). Besides hypoglycemic activity, the antioxidant activity of herbal drugs can help reduce the long term complications of diabetes. Restoring the levels of antioxidant enzymes, herbal medications could act as free-radical scavengers and eventually prevent generation of ROS and OS induced cellular damages. Many studies have investigated the antioxidant potential of plant polyphenols and flavonoids. Phenolic phytoconstituents and flavonoids have been attributed to exhibit pharmacological effects against heart diseases, cancer, neurological disorders, diabetes, inflammatory disorders and so on owing to their radical scavenging actions (Sekhin-Loodu and, Rupasinghe, 2019; Shaheen et al., 2017).
In this study, the antioxidant potential of MECD may be the underlying reason behind its hypoglycaemic action. Phenolic compounds and flavonoids of MECD could prevent cellular oxidative stress and eventual tissue damages associated with diabetes. However, the probable mechanism of hypoglycemic action may be due to the potentiating effect of insulin either by stimulating the pancreatic insulin secretion from the β-cells of the islets of Langerhans or by its action on the body tissues.
CONCLUSION
It is concluded that the metabolic extract of C. dichotoma (MECD) bark possesses antidiabetic activity in alloxan-induced diabetic rats. Our study scientifically validates the folkloric claim as well as traditional uses of C. dichotoma as antidiabetic medicine. It could be attributed that the antidiabetic activity of C. dichotoma may be due to the presence of phenolic phytoconstituents or plant flavonoids in the methanolic bark extract. Further studies can be carried out in order to explore the specific phytochemical(s) responsible for the antidiabetic potential C. dichotoma.
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of the experimental protocol was approved by the IAEC vide approval no. IAEC/DU/58 dated. 24/09/2013.
REFERENCES
Ajiboye BO, Shonibare MT, Oyinloye BE.(2020) Antidiabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats. J Diabetes Metab Disord 19: 343-352
Amira R, El B. Samy AH, Abeer AA, Yehia AH, Tarek MM. Anti-diabetic activity of Holothuria thomasi saponin. Biomed Pharmacother 84: 1472-1487
Banerjee A, Maji B, Mukherjee S, Chaudhuri K, Seal T. (2017) In Vitro Antidiabetic and Anti-oxidant Activities of Methanol Extract of Tinospora sinensis. J Appl Biol Biotechnol 5: 061-067
Chaulya NC, Haldar PK, Mukherjee A.(2010) In vitro free radical scavenging activity of methanol extract of rhizome of Cyperus tegetum Roxb. (Cyperaceae). Int J Curr Pharm Res 2(3): 39-43
Debasis DE, Chatterjee K, Ali MK, Mandal S, Barik B, Ghosh D. (2010). Antidiabetic and antioxidative effects of hydro-methanolic extract of sepals of Salmalia malabarica in streptozotocin-induced diabetic rats. J Appl Biomed 2010; 8: 23–33
Diabetes. Available from URL: https://www.who.int/health-topics/diabetes (accessed on 22/07/2020)
Hussain N, Kakoti BB, Rudrapal M, Laskar MA.(2020) A Concise Review on Cordia dichotoma Forst. J Global Trends Pharm Sci 11(4): 8744-8747
Jaiswal Y, Tatke PSY, Gabhe A, Vaidya DB.(2016) Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med 7(4): 421-427
Junejo JA, Gogoi G, Islam J, Rudrapal M, Mondal P, Hazarika H, et al.(2018) Exploration of antioxidant, antidiabetic and hepatoprotective activity of Diplazium esculentum, a wild edible plant from North Eastern region of India. Future J Pharm Sci 4: 93-101
Junejo JA, Mondal P, Rudrapal M, Zaman K. (2014) Antidiabetic assessment of the hydro-alcoholic leaf extracts of the plant Tetrastigma angustifolia (Roxb.), a traditionally used North-Eastern Indian vegetable. Biomed Pharmacol J 7(2): 635-644
Junejo JA, Rudrapal M, Zaman K. (2020) Antidiabetic activity of Carallia brachiata Lour. leaves hydro-alcoholic extract (HAE) with antioxidant potential in diabetic rats. Indian J Nat Prod Resour 11(1): 18-29
Junejo JA, Zaman K, Rudrapal M, Hussain N.(2020) Antidiabetic and Antioxidant Activity of Hydro-alcoholic Extract of Oxalis debilis Kunth Leaves in Experimental Rats. Biosci Biotech Res Comm 13(2):860-867
Junejo JA, Zaman K, Rudrapal M, Khan A, Sarwa KK, Suryawanshi VK, et al. (2020) Antidiarrheal and Antipyretic Activity of Ethyl Acetate and Hydro-Alcoholic Extracts of Diplazium Esculentum Leaves. Biosci Biotech Res Comm 13(1): 169-173
Junejo JA, Rudrapal M, Nainwal LM, Zaman K. (2017) Antidiabetic activity of hydro-alcoholic stem bark extract of Callicarpa arborea Roxb. with antioxidant potential in diabetic rats. Biomed Pharmacother 95: 84-94
Junejo JA, Zaman K, Rudrapal M, Mondal M, Singh KD, Verma VK. (2014) Preliminary phytochemical and physicochemical evaluation of Carallia brachiata (Lour.) Merr. Leaves. J App Pharm Sci 4(12):123-127
Mishra A, Garg GP.(2011) Antidiabetic activity of Alangium salvifolium in Alloxan induced diabetic rats. Int Res J Pharmacy 2(6): 101-105
Raut NA, Naresh J. Gaikwad. (2006)Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 77(7): 585-588
Sekhin-Loodu S, Rupasinghe HPV (2019) Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front Nutr 6: 53. DOI: 10.3389/fnut.2019.00053
Shaheen U, Shoeib NA, Temraz A, Abdelhady MIS. (2017) Flavonoidal Constituents, Antioxidant, Antimicrobial, and Cytotoxic Activities of Dipterygium glaucum Grown in Kingdom of Saudi Arabia. Pharmacogn Mag 13(Suppl 3):S484-488
Tanwar A, Zaidi AA, Bhardwaj M, Chakotiya AS, Sharma N, Sharma D, et al.(2020) Herbal informatics approach for the selection of natural compounds targeting diabetes mellitus. Indian J Tradit Know 17(2): 270-275
Yeh GY, David ME, Ted JK, Russell SP. (2003) Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26(4): 1277-1294