Department of Zoology, Asansol Girls’ College, West Bengal, India
Corresponding author email: ray.supriya@gmail.com
Article Publishing History
Received: 20/10/2020
Accepted After Revision: 10/12/2020
Mastacembelus armatus is an indigenous fish species of southern Asia that also resides in the Indian subcontinent. This fish species is facing an alarming decline in their number in the last decade. Due to its moderate cost, it is mainly taken by the lower income group of people within the society. The reproductive care, by artificial breeding, has been taken for those fish species having a high cost in the market or becoming less in number in nature for business purposes or preserving the biodiversity, respectively. The present study was undertaken to characterize different cell types in the pituitary gland because these are ultimately responsible for the maintenance of pituitary-gonadal endocrine cascade.
This work has been done purely on histological techniques. In the present investigation the adenohypophysis is divisible into three component parts viz., antero – dorsal rostral pars distalis (RPD), the middle proximal pars distalis (PPD) and the posterior massive pars intermedia (PI). The acidophilic prolactin cells and ACTH cells are found in the RPD, basophilic GTH cells, TSH cells and acidophilic STH cells are found in PPD whereas MSH and MSH cells are found in PI regions. The neurohypophysis in M. armatus is composed of axonal fibers originating from neuronal cell bodies in the hypothalamus. Understanding the pituitary architecture and cell types for this fish species is of immense importance to save this indigenous variety by artificial breeding, which we are trying to discuss in the detail within this paper of ours.
Acidophilic, Adenohypophysis, Mastacembelus Armatus, Neurohypophysis
Ray S. Characterization of Different Cell Types in The Pituitary Gland of Indian Fresh Water Spiny Eel Mastacembelus armatus (Lacepede). Biosc.Biotech.Res.Comm. 2020;13(4).
Ray S. Characterization of Different Cell Types in The Pituitary Gland of Indian Fresh Water Spiny Eel Mastacembelus armatus (Lacepede). Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3kKxeRj
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The function of pituitary is mostly controlled by the hypothalamus through the synthesis and release of gonadotropin-releasing hormone (GnRH), therefore, acting as a major initiator of the hormonal cascade controlling the reproductive axis. Gonadal activities in teleost fishes primarily depend on the function of pituitary gonadotrophs and that the pituitary and the gonads exist in a mutual state of excitation and inhibition (Farbridge et al.1985; Kaneko et al., 1986). The hypothalamo-hypophyseal complex in vertebrates with their neurosecretory nuclei and long axons, is a coordination point in the vertebrate brain and is known to involve in a complex interaction of a variety of neurotransmitters which modulate the influence of several trophic hormones by controlling their active secretion by releasing or inhibiting hormones within the hypophysis itself (Peter et al., 1991). Pituitary gonadotrophic hormones and GnRH are important in implicating these hormones in gonadal maturation and sex steroid production which plays a very important role in gametogenesis, final maturation of oocytes and spermiation (Parhar et al., 2003; Lethimonier et al., 2004; Chakrabarti and Chowdhury, 2015, Trudeau and Somoza 2020).
The teleost hypophysis is generally composed of a neurohypophysis and the adenohypophysis. A significant feature of the teleost pituitary is the interdigitation between the neurohypophysis and adenohypophysis, and at least in some species a prominent innervation of the adenohypophysis (Da Lage,1955). Among all groups of vertebrates perhaps the teleosts show greater structural diversity in the organization of their pituitary gland. In elucidating pituitary function a logical first step is to identify the specific type of hormone-secreting cells. The identification and distribution of the cell types in the pituitary gland of different teleosts have attracted some investigators from the histochemical, ultrastructural and immunocytochemical techniques (Ball and Baker, 1969; Holmes and Ball, 1974; Joy and Sathyanesan, 1980; Chakrabarti and Chowdhury, 2015 Hassan El-Sayyad et al. 2020).
Most of the authors have pointed out that the secretory cells of the pituitary gland show different patterns of distribution in the rostral pars distalis, proximal pars distalis and pars intermedia zones of adenohypophysis. They pointed that the identification and distribution of the different cell types indicate that the adenohypophysis consisted mainly of two groups: basophilic or PAS positive and acidophilic or PAS negative (Chakrabarti and Chowdhury, 2015). The present studies were undertaken with a view to determine the cytology of the pituitary and to identify and localize the different cell types in pituitary of freshwater spiny eel Mastacembelus armatus (Lacepede) by using various modern staining techniques.
MATERIAL AND METHODS
Adult male (average length 15.2 to 15.8 cm) and mean body weight (50g to 75g) and female (average length 17.5 to 17.7 cm) and mean body weight (55g to 70g) of M. armatus were procured fortnightly throughout the consecutive years from particular pond of Asansol in order to avoid ecological variations than can affect development of hypothalamus, pituitary and gonads. The fishes were collected during the second week of every month from January 2019 to December 2019. As the pituitary gland of M. armatus lodged inside sella turcica, it was difficult to dissect out the pituitary intact along with the brain. The entire brain was exposed by dissection from the dorsal aspect and subsequently immersed in 10% neutral formalin for hardening at the fish collection site.
After 45 minutes, the brain including the hypothalamus and the pituitary gland were carefully dissected out from the cranium and subsequently fixed in Bouin’s fixative, Zenker’s fluid and Eltman fixatives. After proper fixation, pituitary gland throughout the year were placed in 70% ethanol for overnight and subsequently dehydrated through ascending ethanol series followed by acetone and then cleared in benzene. Tissues were then embedded in paraffin wax (560C-580C melting point). Mid sagittal section and frontal section of pituitary gland along with hypothalamus were cut at 4 μm thickness using a Leica RM 2125 RT microtome. Deparaffinized sections of pituitary and hypothalamus were stained by techniques which are as follows:a) Chrome alum haematoxylin phloxin (CAHP) (Gomori 1941).b) Mallory’s triple stain (MT) (Mallory,1936)c) Aldehyde fuchsin (AF) (Gabe, 1953).d) Alcian blue-orange G-acid fuchsin (AB-OFG) (Slidders,1961).Slides were examined under microscope, followed by microphotography.
RESULTS AND DISCUSSION
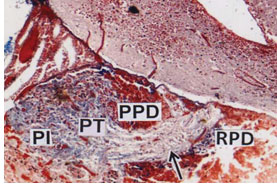
Nerve fibers from hypothalamus pass to the pituitary and thus connecting neurohypophysis (arrow) with the brain (Fig.1). Based on histological features and of its cell types, the adenohypophysis is divisible into three component parts viz., antero – dorsal rostral pars distalis (RPD), the middle proximal pars distalis (PPD) and the posterior massive pars intermedia (PI). Although there is no sharp demarcation between these zones but narrow line of penetration of axonal fibres of neurohypophysis ramified between RPD, PPD and PI (Fig.1). The hypothalamus consists of two nuclei rich areas viz., elongated nucleus preopticus (NPO) and oval shaped nucleus lateralis tuberis (NLT) (Fig. 1). Based on different staining techniques various cell types have been identified in the RPD, PPD and PI.
Figure 1: Pituitary gland (PT) attached to the brain. The PT is divided into rostral pars distalis (RPD), proximal pars distalis (PPD) and pars intermedia (PI), (CAHP) x 50.
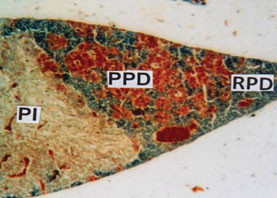
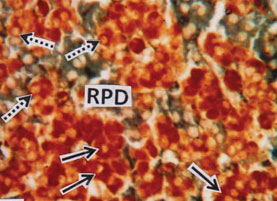
The Rostral pars distalis (RPD) zone is packed closely with mostly acidophilic cells interspersed with a few basophils (Fig.2). For distinguishing the various cell types according to their morphology, size and shape of the cells and specially the stainability of their secretory granules is very important. In the RPD the acidophilic prolactin cells (PRO) occupy the major part of the RPD and stained red with acid fuchsin having densely stained rim of cytoplasm (Fig. 3). Other types of acidophilic cells which are numerically fewer and comparatively less chromophilic are ACTH cells which are dispersed among the PRO cells (Fig. 3).
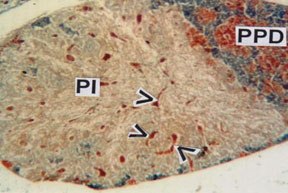
Figure 2: PT showing small RPD, moderate PPD provided with acidophils and basophils and massive PI. (MT) X 100.
Figure 3: RPD showing tubular arrangement of acid fuchsin stained prolactin cells (PRO) (solid arrows) and dispersed ACTH cells (broken arrows). (MT) x 40.
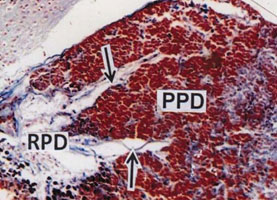
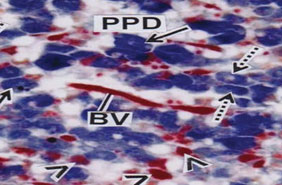
The Proximal pars distalis (PPD) area of pituitary gland is densely occupied by basophilic chromophils and showing clear innervation of axonal fibres (Fig. 4). The anterior and middle part of PPD is provided with alcian blue positive basophilic rounded or oval gonadotroph (GTH) cells. The GTH cells are intermingled with basophilic thyrotrophs (TSH) which are elongated in shape (Fig. 5) and densely stained with alcian blue. The acidophils present in proximal pars distalis are generally identified as somatotrophs (STH) which are stained with orange G. The PPD region is densely distributed by orange G positive blood vessels (Fig. 5).
Figure 4: PPD showing densely packed aldehyde fuchsin positive basophilic cells. Innervation of neurohypophyseal axons (arrows) in the PPD. (AF) x 150.
Figure 5: PPD showing alcian blue positive round or oval GTH cells (solid arrows), elongated TSH cells (broken arrows) and scattered STH cells (arrow heads). BV indicates blood vessels. (AB-OFG) x 400.
Figure 6: PI showing ramification of neurohypophyseal tract interspersed with neurosecretory materials (arrow heads). (MT) x 100.
In Pars intermedia (PI) area the branches of the neurohypophysis interdigitate with the PI than it does in the RPD and PPD. A considerable number of acids fuchsin positive neurosecretory materials and blood vessels of various sizes have been observed in PI region (Fig. 6). The PI contains two types of cells, the larger cells stained with aniline blue and are identified as melanotrophs (MSH) cells. The comparatively smaller cells are provided with scanty cytoplasm and stained with acid fuchsin and are identified as melanocyte concentrating cells (MCH). The pituitary in teleosts is the intricacy and extent of the contact between the nervous system and glandular components, and the division of the latter into three distinct regions, all of which may have contact with the former. The hormones produced by the pituitary gland of teleosts regulate directly/indirectly some fundamental physiological processes like growth, development and reproduction (Agulleiro, 2006).
The teleostean pituitary gland is known for its remarkable structural diversity in topography, size, shape, mode of attachment in nervous component and basic histology (Green and Maxwell, 1959). The pituitary gland in M. armatus is small and lodged in sella turcica and situated between saccus vasculosus and brain. Based on the classical staining methods and distribution of cells the adenohypophysis consists of antero – dorsal rostral pars distalis, middle proximal pars distalis and massive pars intermedia.
In M. armatus the neurohypophysis is rich in blood vessels and neurosecretory materials that lie in close proximity to the rostral pars distalis (RPD) and proximal pars distalis (PPD). Joy and Sathyanesan (1980), Jafri and Ensor (1980) identified precisely the different cell types located in the pituitary of a few teleosts. They categorized various cell types in the teleostean pituitaries on the basis of the staining reaction in the cytoplasmic content adopting different staining technologies. In M. armatus the RPD occupies the antero – dorsal in position of the gland and contains two types of chromophilic acidophils which show a strong affinity to acid fuchsin. The acidophilic prolactin (PRO) cells occupy the major part of RPD and the granules are stained red with acid fuchsin and are frequently attached with the blood vessels advocates their higher secretory activity. In M. armatus prolactin is responsible for the control of a carrier for sodium ion transport in the chloride cells of the gills, stimulation of mucus secretion both the gills and skin and is essential for osmoregulation (Aseem, 2004).
The prolactin cells in the pituitary of Dicentrarchus labrax showed strong affinity to Azan stain to give red colour (Aseem, 2004). Jose and Sathyanesan (1977) and Mandal and Sinha (1985) also advocated about the random distribution of the prolactin cells in the RPD of Labeo rohita and Catla catla. The ACTH cells in the RPD tinctorially may be chromophobic or acidophilic. In M. armatus the carminophilic corticotrophic cells are rounded or oval in shape and generally dispersed among the prolactin cells. Mandal and Sinha (1985) reported that ACTH cells were lead haematoxylin positive and were located in the RPD bordering the neurohypophysis and occurred in groups in Catla catla. Zaki et al. (1996) also reported that the corticotrophic cells are generally found at the interphase between prolactin cells and neurohypophysis.The proximal pars distalis (PPD) is perhaps the most vital part of the pituitary as it shows remarkable variations in its size as well as cellular components at different reproductive phases.
In M. armatus three types of chromophilic cells can distinguished in the PPD on the basis of shape and tinctorial properties. However, the basophilic gonadotrophs formed the main bulk of PPD during maturation and spawning phases. Tinctorially the TSH cells in M. armatus closely resemble the GTH cells. TSH cells are comparatively less in number than that of GTH cells. They are also PAS positive and also stained with aldehyde fuchsin, aniline blue and alcian blue. Aseem (2004) emphasized that the TSH cells were intermingled between GTH cells in Dicentrarchus labrax. In M.armatus the main bulk of TSH cells present in the proximal pars distalis on the ventral aspect of GTH cells. Pickford and Atz (1957) noticed that thyroid follicles of hypophysectomized fish showed histological signs of inactivity. In the present observation the only acidophils stained with orange G in the PPD region considered as somatotroph (STH cells) stained acid fuchsin in some areas. These cells are dispersed among the GTH and TSH cells. This disperse nature of STH cells amongst GTH and TSH cells in Ctenopharyngodon idella is also reported by Hassan El-Sayyad et al. (2020).
Srivastava et al. (1977) emphasized that the acidophils of PPD are deeply stained with azocarmine and correspond to somatotrophs. The present study advocates that the pars intermedia (PI) contains two types of cells, the larger cells provided with rim of cytoplasm around nucleus which stained with aniline blue or orange G i.e. amphiphilic in staining nature and are identified as melanotrophs (MSH) cells. The comparatively smaller cells are provided with scanty cytoplasm and stained with orange G or acid fuchsin and identified as melanocyte concentrating hormone secretory cells or MCH. These cells are acidophilic in nature. The cells of pars intermedia are difficult to demonstrate uniformly in all teleosts by usual standard staining techniques. The PAS – positive MSH cells also been described in the pars intermedia of Clarias batrachus (Joy and Sathyanesan, 1979) and Siganus rivulatus (Zaki et al., 1996). Black melanophores have also been reported in zebra fish by Patterson and Parichy (2019).
The neurohypophysis in M. armatus is composed of axonal fibres originating from neuronal cell bodies in the hypothalamus. These nerve fibres extend as narrow strips into the pituitary gland and found to be closely associated with the blood vessels. The anterior part of the neurohypophysis penetrates the pars distalis with finger like processes, although in a less intricate manner than in the pars intermedia. The neurohypophysis and the neurosecretory cells of the hypothalamus form a functional unit concerned in the synthesis, transport and release of neurosecretory materials. Present findings overlap with the work of Trudeau and Somoza (2020), related to the innervations of hypothalamo-tract into the adenohypophysis.
Secretion of hormones by the different cells qualitatively and quantitatively depends on the nature of formation and discharge of specific secretory granules. The acidophil granules have a strong affinity for acid dyes of all kinds. The granules of basophil cells on the other hand contain glycoprotein as indicated by their staining properties. Parallelism between intensity of staining and hormone content has been employed to support the claim that it is the hormone product itself of these cells which is stained. Staining reactions demonstrate the number of granules present. It is therefore, quite likely that the staining reactions are primarily due to the hormonally active product itself.
CONCLUSION
Fish production in captivity is an essential prerequisite for the increasing population globally. Indigenous fish species of the pond and ditches are the main source of protein in the rural areas of India. With demanding globalization these fish species are facing critical problem of survivalism. Induced breeding has been taken for those fish species which are economically important or number has been reduced drastically. From this research finding it has been established that in Mastacembelus armatus, different cell types are located in particular places of pituitary. The most important one is the GTH secreting cells which is present in the proximal pars distalis region. Seasonal secretion study of this particular hormone can focus light on the Induced breeding program for this fish species.
ACKNOWLEDGEMENTS
I would like to acknowledge Prof. (Retd.) P. Chakrabarti, Department of Zoology, The University of Burdwan and Mainak Banerjee, research scholar Department of Zoology, The University of Burdwan, for guiding me during the time of preparation of the manuscript.
Conflict of Interest: Author does not have any conflict of interest.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Asansol Girls’ College, West Bengal, India.
REFERENCES
Agulleiro, B., M.P. Gorcia-Hernandez, and Ayala A.G., (2006) Teleost adenohypophysis: morph functional and development aspects. In; Reinecke M.P., Zaccone, G., Kapoor, B.G. (eds). Fish Endocrinology, Vol 1 Pages 289-440.
Assem, S.S. (2004). Histochemical studies, cell type distribution and seasonal variation of gonadotropin cells in pituitary gland of female Dicentrarchus labrax in relation to maturation of gonads. Egypt. J. Agua. Res., Vol 30 Pages 374-389.
Chakrabarti, P. and S.H. Chowdhury, (2015). Annual cyclical changes in the histological features and surface ultrastructure in ovaries of the freshwater feather back Notopterus notopterus (Pallas). Proc. Zool. Soc., Springer, Vol 67 No 2 Pages 158-166.
Ball J.N. and B.I. Baker, (1969). The pituitary gland: Anatomy and histophysiology. In: Fish physiology (Eds. W.S. Hoar, D.J.Randall and E.M. Donaldson). Academic Press. New York and London, Vol 2 Pages1 – 110.
Da Lage, C. (1955). Innervations neurosecretoire de I’adenohypophyse chez I’hippocampe. C.R. Ass. Anat., Vol 85 Pages 361-366.
Farbridge, K.M.G. Burke and J.K. Leatherland, (1985). Seasonal changes in the structure of the adenohypophysis of the brown bull head, Ictalurus nebulosus (Leusuer). Cytobios., Vol 44 Pages 49-66.
Gabe M. 1953 ‘‘Sur quelques applications de la coloration par la fuchsine paraldehyde’’. Bull. Micr. Appl, Paris, Vol 3 Pages 153-162.
Gomori G. (1941). ‘‘Observations with differential stains on human islets of Langerhans’’. Amer. J. Path, Vol 17 No 3 Page 395.
Green, J.D. and D.S. Maxwell, (1959). Comparative anatomy of the hypophysis and observations on the mechanisms of neurosecretion. Comp. Endo. Ed. A. Gorbman, Willey, New York. Pages 368-392.
Hassan El-Sayyad, Abd-Elhakim E. El- Gamal, Samah T. Darwih and Mohamed A. Sheha. (2020). The correlation between the hypophysial gonadal axis with special reference to the role of fatty acids and isoenzyme during the ovarian maturation in female grass carp, Ctenopharyngodon Idella. Egyptian Journal of Aquatic Biology & Fisheries. Vol 24 No 1 Pages 311 – 328.
Holmes, R.L. and J. N. Ball, (1974). The pituitary gland: A comparative account. CUP Archive, Cambridge University Press., Vol 4 Pages 170-220.
Jafri S.I.H. and D.M. Ensor, (1980). Cell types in the pituitary of the roach, Rutilus rutilus (L.). J. Anat., Vol 130 No 4 Pages 667-672.
Jose, T.M. and A.G. Sathyanesan, (1977). Pituitary cytology of the Indian carp Labeo rohita (Ham.). Anat. Anz., Vol 142 No 4 Pages 410-423.
Joy. K.P. and A.G. Sathyanesan, (1979). Functional cytology of the pituitary gland of the teleost Clarias batrachus (L).Endokrinologie, Vol 73 Pages 82-90.
Joy, K.P. and A.G. Sathyanesan, (1980). Pituitary cytology of the teleost fish Tilapia mossambica. Zeit.Mikrosk. Anat. Forsch., Vol 94 Pages 337-344.
Kaneko, T., K. Aida and J. Haryu, (1986). Ultrastructural changes in the pituitary gonadotrophs during the annual reproductive cycle of the female chichibu goby, Triclenfiger obscurus. Cell Tissue Res., Vol 246 Pages 137-144.
Lethimonier, C., T. Madigou, J.A. plunoz-cueto, J.J. Lareyre and O. Kah, (2004). Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. Gen. comp. endocrinol., Vol 135 No 1 Pages 1-16.
Mallory, Y.B. (1936). The aniline blue collages stain. Stain Technology, Vol 11 Page 101.
Mandal, S. and G.M. Sinha, (1985). Adenohypophysial cells types in an Indian freshwater major carp, Catla catla (Ham.). Gegenbaurs Morphor Jahard. Vol 131 Pages 81-92.
Parhar, I.S., T. Soga, S. Ogawa and Y. Sakuma, (2003). FSH and LH-β subunits in the preoptic nucleus: Ontogenic expression in teleost. Gen. Comp. Endocrinol., Vol 132 No 3 Pages 369-378.
Patterson, L. B., and Parichy, D. M. (2019). Zebrafish pigment pattern formation: Insights into the development and evolution of adult form. Annual Review of Genetics, Vol 53 No 1 Pages 505–530.
Peter, R.E., V.L. Trudeau and B.D. Sloley, (1991). Brain regulation of reproduction in teleosts. Bull. Inst. Zool., Acad. Sinica (Monograph), Vol 16 Pages 89-118.
Pikford, G. E. and J.W. Atz, (1957). The physiology of the pituitary gland of the fishes. New York Zoological Society, New York. Pages 1-613.
Slidders, W. (1961). The OFG and Br AB-OFG methods for staining the adenohypophysis. J. Pathol. Vol 82 No 2 Pages 532-539.
Srivastava, S., B.P. Rai and K. Swarup, (1977). Histomorphology of the tinctorial cells in the pituitary gland of Channa striatus (Bloch). Archives de biologic, Vol 88 No 1 Pages 101-116.
Trudeau, V. L., and Somoza, G. M. (2020). Multimodal hypothalamo-hypophysial communication in the vertebrates. General and Comparative Endocrinology, Vol 293 Page 113475.
Zaki,M.L.Z., A.A.M. Massoud, G.M. Madkour, N.E.F.El-Fiky and G.E.E. Elmesiry. (1996). The cyclic changes in the pituitary gland and gonads of Siganus rivulatus from the Red sea. JAKU marine science special issue. Symposium on Red sea marine environment, Jeddah, Pages 271-287.