Shri M. M. Patel Institute of Sciences and Research, Kadi Sarva Vishwavidyalaya, Gandhinagar, India.
Corresponding author email: pjvaghela999@gmail.com
Article Publishing History
Received: 15/10/2020
Accepted After Revision: 13/12/2020
Rheumatoid arthritis (RA) is a joint autoimmune disorder with unknown aetiology, affecting 1% of the world’s population causing permanent deformation of the affected joint. The main objective of this study was to investigate the prevalence of comorbidities in a population-based cohort of individuals suffering from rheumatoid arthritis (RA) as compared to normal population. Data of 877 individuals with 402 RA and 475 Age- and sex- matched controls without RA were taken with their consent from the population of Gujarat. As compared to the controls, most of the investigated comorbidities were more frequent in the individuals with RA. On further dividing them into five different regions within Gujarat based on their origin-it was found that both in the RA and the control population belonging to North Gujarat were maximum and least sample population was belonging to Kutch region.
Severe Anaemia, fatigue and depression were found more in RA than the control population. It was found that in addition to RA, some other disease conditions like Hypertension, Diabetes, Kidney problems, thyroid dysfunction, cholesterol and breathing problems were found in 40.55% of the RA population and 11.58% of the control population. BMI results also showed maximum obesity in RA population as compared to the control population in both genders (73.63% vs 34.74). Females were found to be more prone than the males in most of the comorbidity. Compared to the control population, persons with RA present with increased prevalence of numerous comorbidities. Patients with RA and multimorbidity are at risk of insufficient rheumatologically care and poorer patient-reported outcomes. Thus, a proper understanding of different comorbid condition along with RA can improve the quality of life as well as decrease the mortality rate of the RA patients.
Comorbid Conditions, Gujarat, Rheumatoid Arthritis.
Vaghela P, Shah I, Jarullah B. Rheumatoid Arthritis and Comorbid Conditions; is there A Missing Link?. Biosc.Biotech.Res.Comm. 2020;13(4).
Vaghela P, Shah I, Jarullah B. Rheumatoid Arthritis and Comorbid Conditions; is there A Missing Link?. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/2I5DA08
Copyright © Vaghela et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic disease that affects the joints, connective tissues, muscles, tendons and fibrous tissue. The prevalence of RA is estimated to be ~1% of the population worldwide and is more common in women. The prevalence of RA in India is 0.75% (Malaviya et al., 1993; Handa et al., 2016). Although the aetiology and pathogenic mechanism underlying the development of RA remain unclear, the combination of a susceptible genetic background interplaying with environmental factors has been considered to be associated with the development of this complex disorder. It is well established that there are several risk factors like age, gender, hormonal levels, alcohol, cigarette smoking, socioeconomic status, and dietary habits that is contributing to the initiation and promotion of this complex disorder.
When RA is left uncontrolled, the RA patient may experience joint deterioration, severe disability, decreased quality of life, the onset of comorbidities and premature mortality ( Choy and Panayi, 2001; Handa et al., 2016). It has been observed that RA comes with one or more comorbid conditions. Comorbid conditions can be broadly arisen by – (i) arising due to disease pathology and (ii) due to treatment drugs used. The most affected organs includes eyes, heart, lungs, bones and also psychological disorders are commonly found in addition to RA, (Wolfe et al., 1994 ; Gonzalez et al., 2007; Salliot and van der Heijde, 2008; Emamifar and Hansen, 2016; Singh et al., 2016; Levytskyi et al., 2019).
More comorbid conditions have been seen to be leading to a higher death rate in RA (S E Gabriel, 2008). Moreover other risk factors like smoking might be playing a role in causing comorbidity (Liao and Solomon, 2013). Comorbidity in addition to RA is an economic burden on patients, their families, and society which also a challenge to the rheumatologists (Cross et al., 2014; Onna and Boonen, 2016). Although prevalence of comorbidities is more common in RA than controls, comorbidity is many a times under recognized and undertreated (MacLean et al., 2000; Dougados et al., 2015; Mohan et al., 2017). There are many studies showing comorbidities in RA but some studies lack a comparison with the non-RA population. Moreover, the data regarding medical comorbidities associated with RA in previous studies were mainly from western populations. So, the aim of this study was to determine the prevalence of comorbidities in adult population with RA along with non-RA population of Gujarat, India.
MATERIAL AND METHODS
Total of 402 RA patients classified according to American college of Rheumatology 2010 criteria (Aletaha et al., 2010). Retrospective analysis of the patients attending the rheumatology outpatient clinic between the years December 2014 to December 2015 were analyzed. A detailed questionnaire regarding their pervious health history and other details as well was taken. Prior to participation, the purpose of the study was explained to all the subjects and their informed consent was taken. A total of 475 ethnically matched unrelated healthy volunteers with a negative history of any joint disorders were taken up as control population for the study. Hemoglobin levels were measured using the Sahli’s method (Handa et al., 2016).
RESULTS AND DISCUSSION
It is a well-established fact that the inflammation in RA is also affecting various organs like the bone, lungs, cardiovascular system and many more apart from the joints leading to excess mortality (S E Gabriel, 2008; Gabriel and Michaud, 2009; Dougados et al., 2014).
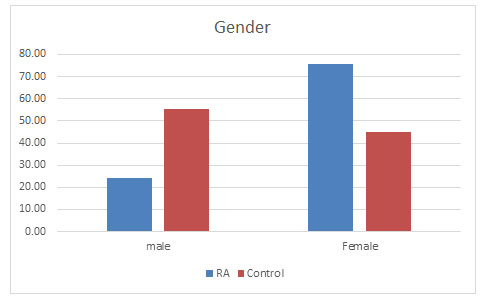
Total population: Total samples collected were 877 out of which 402 were of RA patients and 475 samples belonged to the control population. Out of which 98 (24.38%) were found to be male and 304 (75.62%) were females in the diseased population whereas in the control population 262 (55.16%) were males and 213 (44.84%) were females (Figure 1).
Figure 1: Prevalence of RA in males and females
Supporting previous studies, females were found to be approximately 3 times more prone to the disease than the males (Van Vollenhoven, 2009; Patel, 2011).
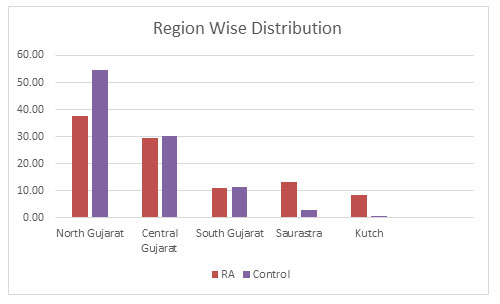
Region wise Distribution: The samples were further divided into five different regions within Gujarat based on their origin which are- North Gujarat, Central Gujarat, South Gujarat, Saurastra and Kutch. It was found that both in the RA and the control population belonging to North Gujarat were maximum with 37.56% and 54.53% respectively. Central Gujarat was consisting of 29.60% RA population and 30.32% of the control population. This was followed by South Gujarat with almost same amount of population in both RA and control population with 11.19% and 11.58% respectively. In Saurastra, RA population was 13.18% and control population was on 2.95%. Least sample population was belonging to Kutch region with 8.40% in RA and 0.63% in the control population as shown in figure 2.
Figure 2: Region Wise Distribution of the population in Gujarat
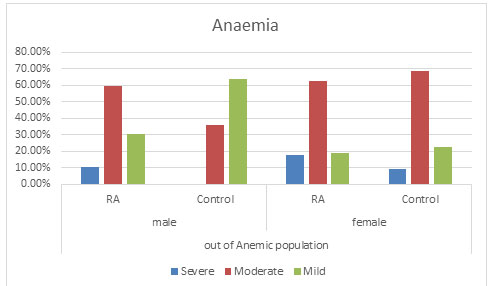
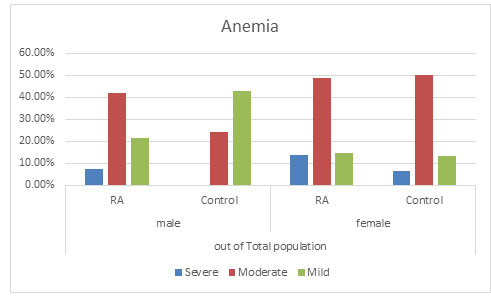
Anaemia: It was observed that severe anaemia was found more in RA population in both the genders with males having 10.14% severe anaemia and 17.65% of severe anaemic female population as compared to the control population with no severe anaemic male population and 8.97% in the female population. Again percentage of moderated anaemia was found to be more which is 59.42% in RA male population as compared to 36.00% in the control male population. No significant change was found in the female population in RA with 62.61% and control population with 68.59%. It was furthermore found that mild anaemia was more in the control population with 64.00% in male population and 22.44% in female population as compare to the RA population with 30.43% in male population and 18.91% in the female population. This suggests that there might be a possibility that due to RA, mild anaemia may lead to moderate or severe anaemia (Figure 3 and 4) (Goyal et al., 2018).
Figure 3: Comparison of the Anaemic population
Figure 4: Distribution of Anaemia in total population
Overall anaemia of chronic disease and iron deficiency anaemia are frequent causes of anaemia in RA patients(Agrawal et al., 2006; Goyal et al., 2018). Anemia was found in 75.87% of the patient population as compared to 69.68% in the control population in our study which correlates with the previous studies of India ( Baer et al., 1990; Agrawal et al., 2006). In this study, severe anaemia was found more in RA population in both the genders having severe anaemia as compared to the control population. Mild Anaemia was seen in the control population more than the diseased population suggesting that there might be a possibility that due to RA, mild anaemia may lead to moderate or severe anaemia supporting various reports implicating anaemia playing an important role in the disease activity (Goyal et al., 2018).
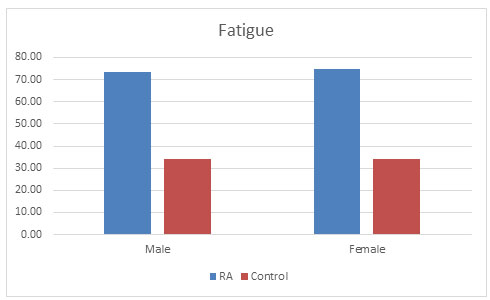
Fatigue: R.P. Riemsma et al. (1998) showed the prevalence of fatigue in RA population to be 80-93%. High fatigue levels are common in RA and are mainly linked to pain and depression (Riemsma, 1998). It has also been observed in one of the study by Pollard, that association with disease activity is secondary, which also correlates with our study that population of RA (74.3%) suffered more from fatigue condition than control population (34.31%) as shown in fig. 5 (Pollard et al., 2006).
Figure 5: Fatigue condition found in both the populations
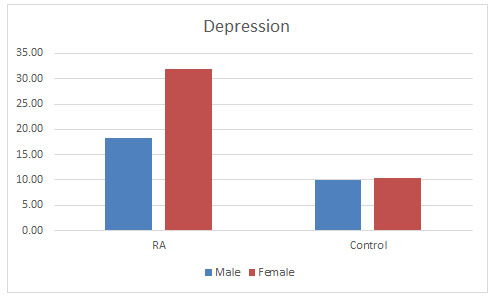
Depression: C. Sheehy et al. (2006) mentioned the prevalence of depressive disorder in patients with RA to be ranging between 13 and 20% (Sheehy et al., 2006). Dickens et al. (2003) found 39.2% had definite symptoms of depression. And further concluded that depression was significantly more common among RA patients than healthy individuals and was influenced by the level of pain but not by demographic factors (Dickens et al., 2003). This study is supporting our study showing that 28.60% of the diseased population were suffering from depression as compared to 10.10% of the control population. Prevalence of depression was found more in the females as compared to males as shown in the figure 6. There is much argument as to whether depression simply reflects a reaction to the pain of RA or whether depression contributes to pain experience. There are studies also stating that depression and psychological stress have been shown to result in immune dysfunction (Herbert TB, 1993; Dickens and Creed, 2001). There are studies suggesting that depression also relates to poorer RA outcomes. So, it is crucial that emphasis on the detection and treatment of depression in RA to improve the lifestyle of the patients (Matcham et al., 2013).
Figure 6: Level of depression found in both the populations
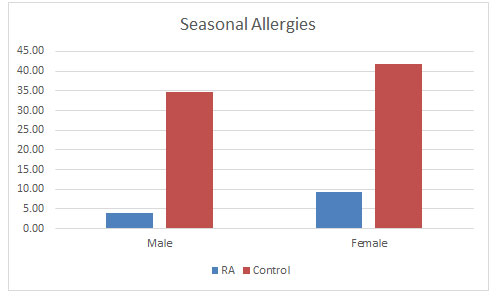
Seasonal Allergies: Surprising to find that seasonal allergies were found to be occurring more in control population than the RA population as seen in figure 7, in oppose to studies that allergy increases with RA (Karsh et al., 2005). Male control population were having 34.73% of seasonal allergies as compared to 41.78% in the male RA population. Also, in the female population in controls were having 41.78% of seasonal allergies as compared to 9.21% of the female RA population. There are no studies supporting this result. So, more studies in this area might broaden the understanding the correlation between the two (Matcham et al., 2013).
Figure 7: Effect of seasonal allergies found in the populations
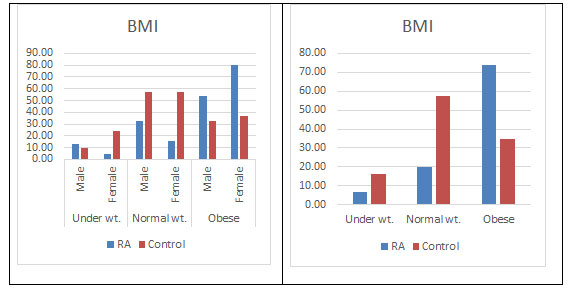
BMI: There have been many studies showing an association between higher body mass and many inflammatory or autoimmune disorders. Overweight or obesity measured by body mass index (BMI) corresponds to an abnormal accumulation of adipose tissue within the body, which secretes proinflammatory and anti-inflammatory metabolically and hormonally active substances, and produces cytokines and chemokines thus contributing in the processes associated with inflammation and immunity (Fantuzzi, 2005; Touyz Rhian M., 2005; Trayhurn and Wood, 2005). There are many reports stating the dominance of obesity in females and its possible role in developing RA (Symmons, 2005). Our study validates this observation with higher BMI found more in female RA population (82.09%) than the male population. The predominance in the female population is still unclear. Moreover higher BMI was also found in the disease population supporting previous studies (Versini et al., 2014; Qin et al., 2015; Albrecht et al., 2016; Hugo et al., 2017) (Figure 10).
Figure 8: Different comorbidities found in the populations
Co-morbidities: There are various reports showing higher death rate in patients with RA might to be the consequence of more serious co-morbid conditions (Gabriel, 2008; Gullick and Scott, 2011). Many studies have showed that comorbid conditions were between 40 and 67% of the RA population ( Innala et al., 2016; Fz, 2019) . In this study, it was found that in addition to RA, some other disease conditions like hypertension, diabetes, kidney problems, thyroid dysfunction, higher cholesterol and breathing problems were prevailing in about 40% of the disease population as compared to the control population (11%) correlating with a study done by Chandrashekara, (2017) in south India. The highest comorbid condition in the RA population was found to be thyroid dysfunction (17.41%), hypertension (14.93%) followed by diabetes (3.98%) (figure 8) (Chandrashekara et al., 2017).
An association between RA and thyroid dysfunction with or without autoimmune origin has been reported in 5% to 34% of patients with RA (Shiroky et al., 1993; Przygodzka and Filipowicz-Sosnowska, 2009; Jeong et al., 2017). This study supports the positive association between RA and thyroid disorder. It was found that RA population was suffering from thyroid dysfunction more as compared to control population. However, this prevalence (17.41%) in the diseased population was found to be quite less as compared to the previous studies (Atzeni et al., 2008) with 10.20% and 19.74% in male and female population respectively. Control population with 0.76% males and 1.41% females were suffering with thyroid dysfunction.
The relationship between RA and thyroid dysfunction is in agreement with the other studies stating the female dominance (Deighton et al., 1992; Bianchi et al., 1993; Andonopoulos et al., 1996). Shiroky (1993) showed the increased number of thyroid disorder in the diseased females than the similar control ones, thus supporting our results (Shiroky et al., 1993; Przygodzka and Filipowicz-Sosnowska, 2009). Hypertension was found to be present in 14.93% of the diseased population as compared to 7.37% in the control population. This percentage is quite less as compared to other studies done but also correlates with various studies showing it to be the most comorbid condition prevailing along with RA (Innala et al., 2016). Physical inactivity is considered as a main cause for hypertension (Aziz and Yadav, 2016; Levytskyi et al., 2019).
Diabetes was found in 3.98% of the disease population which is quite similar to that of a study done by Vij et al. (Vij et al., 2017) , wherein the prevalence was found to be around 4%.However there are studies showing a much higher prevalence of up to 13% in some studies (Tembe et al., 2008; Emamifar and Hansen, 2016; Singh et al., 2016; Levytskyi et al., 2019).
Failure to manage these co-morbidities effectively will have a serious impact on the RA patient. For example, the presence of co-morbidities may add delays to the care pathway, and the co-morbidities themselves may increase the patient’s overall levels of disability, or even their risk of mortality. Moreover, Drugs like DMARDS,TNF i, CS, NSAIDS, are also found to increase the comorbidities thus prompting the judicious use of them by the Rheumatologists (Aziz and Yadav, 2016).
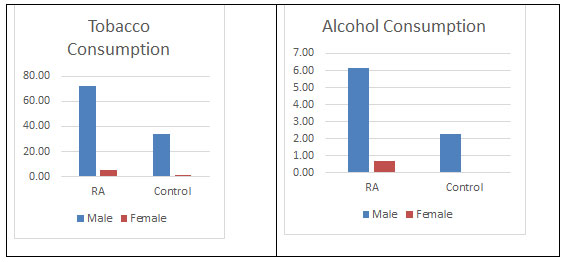
Risk factors: Furthermore, as it can be seen from the figure 9 that tobacco consumption was more prevalent in the males of RA population 72.45% which is supporting the work done by Vij AS. This could be considered as indicative for the causation of RA, at least among males. It has been well established that smoking triggers the genes that may cause an autoimmune disorder leading to RA (Plenge et al., 2007; Vij et al., 2017). There have been mixed reports on alcohol consumptions and causing of RA. In the current study, alcohol consumption was low in the diseased populations which is in support with reports suggesting a protective effect (Scott et al., 2013; Jin et al., 2014). Moreover, it has been suggested that alcohol may been having protective effects in the development of other chronic disorder as well (Fekjaer, 2013). But there is a report as well showing to significant role of it in the development of RA. Here in this study as well no significant association has been found. There has been inverse association with alcohol consumption and cause of RA. But no significant difference has been found in the consumption of alcohol between both the populations (Karlson and Deane, 2012; Di Giuseppe et al., 2014a).
Figure 8: Different comorbidities found in the populations
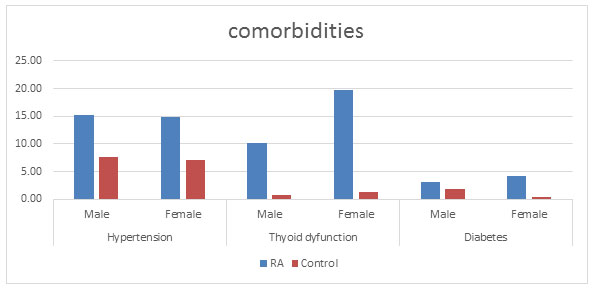
Figure 9: Tobacco and Alcohol consumption found in both the populations
CONCLUSION
To conclude, this study confirms much higher frequency of comorbid conditions present alongside RA as compared to controls. In addition to Anaemia risk, among the most common comorbidities in RA were hypertension, Diabetes, thyroid dysfunction and a higher BMI. There are less studies focusing on the overall lifestyle and health of the RA patients. Future research should address the problem of decreasing coverage by rheumatologic care with an increasing number of comorbidities.
ACKNOWLEDGEMENTS
The authors thank Rheumatologists Dr. Mitul Kotecha and Dr. Reena Sharma for their support.
REFERENCES
Agrawal, S., Misra, R. and Aggarwal, A., (2006) Anemia in rheumatoid arthritis: high prevalence of iron-deficiency anemia in Indian patients. Rheumatol. Int. 26, 1091–1095. https://doi.org/10.1007/s00296-006-0133-4
Albrecht, K., Richter, A., Callhoff, J., Huscher, D., Schett, G., Strangfeld and A., Zink, A. (2016) Body mass index distribution in rheumatoid arthritis: a collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res. Ther. 18. https://doi.org/10.1186/s13075-016-1043-9
Aletaha, D., Neogi, T., Silman, A.J., Funovits, J., Felson, D.T., Bingham, C.O., Birnbaum, N.S., Burmester, G.R., Bykerk, V.P., Cohen, M.D., Combe, B., Costenbader, K.H., Dougados, M., Emery, P., Ferraccioli, G., Hazes, J.M.W., Hobbs, K., Huizinga, T.W.J., Kavanaugh, A., Kay, J., Kvien, T.K., Laing, T., Mease, P., Ménard, H.A., Moreland, L.W., Naden, R.L., Pincus, T., Smolen, J.S., Stanislawska-Biernat, E., Symmons, D., Tak, P.P., Upchurch, K.S., Vencovský, J., Wolfe, F., and Hawker, G. (2010) Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581. https://doi.org/10.1002/art.27584
Andonopoulos, A.P., Siambi, V., Makri, M., Christofidou, M., Markou, C. and Vagenakis, A.G. (1996) Thyroid function and immune profile in rheumatoid arthritis. A controlled study. Clin. Rheumatol. 15, 599–603. https://doi.org/10.1007/BF02238551
Atzeni, F., Atzeni, F., Doria, A., Ghirardello, A., Turiel, M., Batticciotto, A., Carrabba, M. and Sarzi-Puttini, P. (2008) Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: Prevalence and clinical value. Autoimmunity 41, 111–115. https://doi.org/10.1080/08916930701620100
Aziz, M. and Yadav, K., (2016) Review Article – Comorbidities in Rheumatoid Arthritis. J. Nurs. Care 05. https://doi.org/10.4172/2167-1168.1000371
Baer, A.N., Dessypris, E.N. and Krantz, S.B. (1990) The pathogenesis of anemia in rheumatoid arthritis: A clinical and laboratory analysis. Semin. Arthritis Rheum. 19, 209–223. https://doi.org/10.1016/0049-0172(90)90001-V
Bianchi, G., Marchesini, G., Zoli, M., Falasconi, M.C., Iervese, T., Vecchi, F., Magalotti, D. and Ferri, S., (1993) Thyroid involvement in chronic inflammatory rheumatological disorders. Clin. Rheumatol. 12, 479–484. https://doi.org/10.1007/BF02231775
Chandrashekara, S., Shobha, V., Dharmanand, B.G., Jois, R., Kumar, S., Mahendranath, K.M., Haridas, V., Prasad, S., Singh, Y., Daware, M.A., Swamy, A., Subramanian, R., Somashekar, S.A., Shanthappa, A.M. and Anupama, K.R. (2017) Comorbidities and related factors in rheumatoid arthritis patients of south India- Karnataka Rheumatoid Arthritis Comorbidity (KRAC) study. Reumatismo 69, 47. https://doi.org/10.4081/reumatismo.2017.898
Choy, E.H. and Panayi, G.S. ( 2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344, 907–916. https://doi.org/10.1056/NEJM200103223441207
Cross, M., Smith, E., Hoy, D., Carmona, L., Wolfe, F., Vos, T., Williams, B., Gabriel, S., Lassere, M., Johns, N., Buchbinder, R., Woolf, A. and March, L. (2014) The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 1316–1322. https://doi.org/10.1136/annrheumdis-2013-204627
Deighton, C.M., Fay, A., and Walker, D.J. (1992) RHEUMATOID ARTHRITIS IN THYROID DISEASE POSITIVE AND NEGATIVE SAME-SEXED SIBSHIPS. Rheumatology 31, 13–17. https://doi.org/10.1093/rheumatology/31.1.13
Di Giuseppe, D., Discacciati, A., Orsini, N. and Wolk, A. (2014a) Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res. Ther. 16, R61. https://doi.org/10.1186/ar4498
Di Giuseppe, D., Wallin, A., Bottai, M., Askling, J. and Wolk, A. (2014b) Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann. Rheum. Dis. 73, 1949–1953. https://doi.org/10.1136/annrheumdis-2013-203338
Dickens, C. and Creed, F.( 2001) The burden of depression in patients with rheumatoid arthritis. Rheumatology 40, 1327–1330. https://doi.org/10.1093/rheumatology/40.12.1327
Dickens, C., Jackson, J., Tomenson, B. and Creed, F. (2003) Association of Depression and Rheumatoid Arthritis. Psychosomatics 44, 209–215. https://doi.org/10.1176/appi.psy.44.3.209
Dougados, M., Soubrier, M., Antunez, A., Balint, P., Balsa, A., Buch, (2014) Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 73, 62–68. https://doi.org/10.1136/annrheumdis-2013-204223
Dougados, M., Soubrier, M., Perrodeau, E., Gossec, L., and Ravaud, P. (2015) Impact of a nurse-led programme on comorbidity management and impact of a patient self-assessment of disease activity on the management of rheumatoid arthritis: results of a prospective, multicentre, randomised, controlled trial (COMEDRA). Ann. Rheum. Dis. 74, 1725–1733. https://doi.org/10.1136/annrheumdis-2013-204733
Emamifar, A. and Hansen, I.M.J. (2016) FRI0117 The Prevalence of Diabetes Mellitus among Rheumatoid Arthritis Patients Is More than Twice The Prevalence in The Danish Population. Ann. Rheum. Dis. 75, 470–471. https://doi.org/10.1136/annrheumdis-2016-eular.3157
Fantuzzi, G. (2005) Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919; quiz 920. https://doi.org/10.1016/j.jaci.2005.02.023
Fekjaer, H.O. (2013) Alcohol-a universal preventive agent? A critical analysis. Addict. Abingdon Engl. 108, 2051–2057. https://doi.org/10.1111/add.12104
Fz, H. (2019) Comorbidities in rheumatoid arthritis: the RBSMR study 5.
Gabriel, and Sherine E, (2008) Why do persons with rheumatoid arthritis still die prematurely? Ann. Rheum. Dis. 67, iii30–iii34. https://doi.org/10.1136/ard.2008.098038
Gabriel, S.E., Michaud, K. (2009) Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 11, 229. https://doi.org/10.1186/ar2669
Gonzalez, A., Maradit Kremers, H., Crowson, C.S., Nicola, P.J., Davis, J.M., Therneau, T.M., Roger, V.L., and Gabriel, S.E., (2007) The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 56, 3583–3587. https://doi.org/10.1002/art.22979
Goyal, L., Shah, P.J., Yadav, R.N., Saigal, R., Agarwal, A., and Banerjee, S. (2018) Anaemia in Newly Diagnosed Patients of Rheumatoid Arthritis and its Correlation with Disease Activity. J. Assoc. Physicians India 66, 26–29.
Gullick, N.J., and Scott, D.L. (2011) Co-morbidities in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 25, 469–483. https://doi.org/10.1016/j.berh.2011.10.009
Handa, R., Rao, U.R.K., Lewis, J.F.M., Rambhad, G., Shiff, S., and Ghia, C.J. (2016) Literature review of rheumatoid arthritis in India. Int. J. Rheum. Dis. 19, 440–451. https://doi.org/10.1111/1756-185X.12621
Hugo, M., Mehsen-Cetre, N., Pierreisnard, A., Pupier, E., Cherifi, B., Schaeverbeke, T. and Rigalleau, V. (2017) High body mass index in rheumatoid arthritis: why we should promote physical activity. Arthritis Res. Ther. 19. https://doi.org/10.1186/s13075-016-1209-5
Innala, L., Sjöberg, C., Möller, B., Ljung, L., Smedby, T., Södergren, A., Magnusson, S., Rantapää-Dahlqvist, S. and Wållberg-Jonsson, S. (2016) Co-morbidity in patients with early rheumatoid arthritis – inflammation matters. Arthritis Res. Ther. 18, 33. https://doi.org/10.1186/s13075-016-0928-y
Jeong, H., Baek, S.Y., Kim, S.W., Eun, Y.H., Kim, I.Y., Kim, H., Lee, J., Koh, E.-M. and Cha, H.-S. (2017) Comorbidities of rheumatoid arthritis: Results from the Korean National Health and Nutrition Examination Survey. PLOS ONE 12, e0176260. https://doi.org/10.1371/journal.pone.0176260
Jin, Z., Xiang, C., Cai, Q., Wei, X. and He, J. (2014) Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann. Rheum. Dis. 73, 1962–1967. https://doi.org/10.1136/annrheumdis-2013-203323
Karlson, E.W. and Deane, K. (2012) Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 38, 405–426. https://doi.org/10.1016/j.rdc.2012.04.002
Karsh, J., Chen, Y., Lin, M. and Dales, R. (2005) The association between allergy and rheumatoid arthritis in the Canadian population. Eur. J. Epidemiol. 20, 783–787. https://doi.org/10.1007/s10654-005-0704-9
Levytskyi, M., Maier, S. and Schirmer, M. (2019) Management and Comorbidities in Middle-European Patients with Rheumatoid Arthritis: A Retrospective, Cross-sectional Comparison with COMORA Data. Open Rheumatol. J. 13, 94–97. https://doi.org/10.2174/1874312901913010094
Liao, K.P. and Solomon, D.H. (2013) Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatol. Oxf. Engl. 52, 45–52. https://doi.org/10.1093/rheumatology/kes243
MacLean, C.H., Louie, R., Leake, B., McCaffrey, D.F., Paulus, H.E., Brook, R.H. and Shekelle, P.G. (2000) Quality of care for patients with rheumatoid arthritis. JAMA 284, 984–992. https://doi.org/10.1001/jama.284.8.984
Malaviya, A.N., Kapoor, S.K., Singh, R.R., Kumar, A. and Pande, I., (1993) Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatol. Int. 13, 131–134. https://doi.org/10.1007/BF00301258
Matcham, F., Rayner, L., Steer, S. and Hotopf, M. (2013) The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology 52, 2136–2148. https://doi.org/10.1093/rheumatology/ket169
Mohan, V.K., Ganesan, N., Gopalakrishnan, R. and Venkatesan, V. (2017) HLA-DRB1 shared epitope alleles in patients with rheumatoid arthritis: relation to autoantibodies and disease severity in a south Indian population. Int. J. Rheum. Dis. 20, 1492–1498. https://doi.org/10.1111/1756-185X.12948
Patel, M.M., (2011) An Epidemiological survey of arthritis in the population of North Gujarat, India. International Journal of Pharmaceutical Sciences and Research, 2(2), p.325.
Plenge, R.M., Cotsapas, C., Davies, L., Price, A.L. and Altshuler, D. (2007) Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482. https://doi.org/10.1038/ng.2007.27
Pollard, L.C., Choy, E.H., Gonzalez, J., Khoshaba, B. and Scott, D.L. (2006) Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology 45, 885–889. https://doi.org/10.1093/rheumatology/kel021
Porkodi, R., Ramesh, S., Mahesh, A., Kanakarani, P., Rukmangathrajan, S., (2014) Thyroid Dysfunction In Systemic Lupus Erythematosus And Rheumatoid Arthritis 3.
Przygodzka, M. and Filipowicz-Sosnowska, A.(2009) Prevalence of thyroid diseases and antithyroid antibodies in women with rheumatoid arthritis. Pol. Arch. Med. Wewn. 119, 39–43.
Qin, B., Yang, M., Fu, H., Ma, N., Wei, T., Tang, Q., Hu, Z., Liang, Y., Yang, Z. and Zhong, R.(2015) Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res. Ther. 17. https://doi.org/10.1186/s13075-015-0601-x
Riemsma, R., (1998) Fatigue in rheumatoid arthritis: the role of self-efficacy and problematic social support. Rheumatology 37, 1042–1046. https://doi.org/10.1093/rheumatology/37.10.1042
Salliot, C. and van der Heijde, D. (2008) Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann. Rheum. Dis. 68, 1100–1104. https://doi.org/10.1136/ard.2008.093690
Scott, I.C., Tan, R., Stahl, D., Steer, S., Lewis, C.M., and Cope, A.P., (2013) The protective effect of alcohol on developing rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol. Oxf. Engl. 52, 856–867. https://doi.org/10.1093/rheumatology/kes376
Sheehy, C., Murphy, E. and Barry, M. (2006) Depression in rheumatoid arthritis—underscoring the problem. Rheumatology 45, 1325–1327. https://doi.org/10.1093/rheumatology/kel231
Shiroky, J.B., Cohen, M., Ballachey, M.L. and Neville, C. (1993) Thyroid dysfunction in rheumatoid arthritis: a controlled prospective survey. Ann. Rheum. Dis. 52, 454–456. https://doi.org/10.1136/ard.52.6.454
Singh, S., Lihite, R.J., Baruah, C., Lahkar, M. and Singh, P.K., (2016) A pilot study ofcomorbidities in patients with rheumatoid arthritis at a tertiary care hospital in Northeast India. Biomed. Res. Ther. 3, 1. https://doi.org/10.7603/s40730-016-0001-0
Symmons, D.P.M. (2005) Looking back: rheumatoid arthritis—aetiology, occurrence and mortality. Rheumatology 44, iv14–iv17. https://doi.org/10.1093/rheumatology/kei055
Tembe, A., Kharbanda, P., Bhojani, K. and Joshi, V. (2008) Profile of rheumatoid arthritis patients attending a private tertiary hospital rheumatology clinic. Indian J. Rheumatol. 3, 144–147. https://doi.org/10.1016/S0973-3698(10)60140-9
Touyz Rhian M. (2005) Endothelial Cell IL-8, a New Target for Adiponectin. Circ. Res. 97, 1216–1219. https://doi.org/10.1161/01.RES.0000196745.09234.36
Trayhurn, P. and Wood, I.S. (2005) Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem. Soc. Trans. 33, 1078–1081. https://doi.org/10.1042/BST0331078
Van Onna, M. and Boonen, A. (2016) The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet. Disord. 17, 184. https://doi.org/10.1186/s12891-016-1038-3
Van Vollenhoven, R.F.(2009) Sex differences in rheumatoid arthritis: more than meets the eye.. BMC Med. 7, 12. https://doi.org/10.1186/1741-7015-7-12
Versini, M., Jeandel, P.-Y., Rosenthal, E. and Shoenfeld, Y. (2014) Obesity in autoimmune diseases: not a passive bystander. Autoimmun. Rev. 13, 981–1000. https://doi.org/10.1016/j.autrev.2014.07.001
Vij, A.S., Malaviya, A.N. and Kumar, S., (2017) Characteristics of rheumatoid arthritis patients at first presentation to a specialized rheumatology department. Int. J. Res. Med. Sci. 3, 2073–2078. https://doi.org/10.18203/2320-6012.ijrms20150329
Wolfe, F., Mitchell, D.M., Sibley, J.T., Fries, J.F., Bloch, D.A., Williams, C.A., Spitz, P.W., Haga, M., Kleinheksel, S.M., and Cathey, M.A. (1994) The mortality of rheumatoid arthritis. Arthritis Rheum. 37, 481–494. https://doi.org/10.1002/art.1780370408