Vasantrao Naik College of Agricultural Biotechnology, Yawatmal, MS India
Article Publishing History
Received: 12/03/2016
Accepted After Revision: 01/05/2016
The geographical distribution of Zika virus has expanded tremendously since 2007. The consequences of transmission of dengue and chikungunya , zika virus is the latest culprit in a long list of arbovirus epidemics that emerged as a global public health threat over the last decade. Zika virus (ZIKV) is a mosquito-borne flavivirus, first isolated in Uganda from a sentinel monkey in 1947, which is transmitted by Aedes genus mosquitoes, also transmits diseases such as chikungunya, dengue, and yellow fever. Mosquito and sentinel animal surveillance studies have demonstrated that ZIKV is endemic to Africa and Southeast Asia. It causes dengue-like syndromes but with milder symptoms. Among common clinical manifestations fever, arthralgia, conjunctivitis, myalgia, headache, and maculopapular rash. Newborn microcephaly, the most devastating and insidious complication associated. ZIKV, has been described in the offspring of women who became infected while pregnant. However, Zika virus infection in pregnant women has a suspected link with newborn microcephaly by mother to child transmission. In very few cases Guillain-Barre syndrome (GBS) is a rare condition in which immune system attacks the nerves, leading to muscle weakness and even paralysis. A number of countries have issued travel warnings and the outbreak is expected to have an impact on the tourism industry. This review is focused on zika virus transmitted by Aedes (Stegomyia) mosquitoes their symptoms, diagnosis, prevention ,control strategy and treatment as an important challenge to public health emergency, monitoring environmental and climatic precursors of vector-borne diseases linked to integrated surveillance of human cases and vectors. Given the rapid geographic spread of ZIKV in recent years, a coordinated local, regional, and global effort is needed to generate sufficient resources and political traction to effectively halt and contain further expansion of the current outbreak. Certainly, raising awareness and increasing knowledge among the general public, public health practitioners, and policy makers about disease vectors and their relationship with infectious diseases. In addition, much more work needs to be done to produce an effective vaccine.
Aedes, Flavivirus, Microcephaly, Zika Virus
Bobade S. S, Gade R. M. Zika Virus –A Vector Borne Zoonotic Disease: an International Public Health Emergency. Biosc.Biotech.Res.Comm. 2016;9(2).
Bobade S. S, Gade R. M. Zika Virus –A Vector Borne Zoonotic Disease: an International Public Health Emergency. Biosc.Biotech.Res.Comm. 2016;9(2). Available from: https://bit.ly/2MLfJRM
Introduction
Zika virus is an emerging virus that was first identified in Uganda in 1947 in rhesus monkeys through a monitoring network of sylvatic yellow fever. Zika virus (ZIKV) is an mosquito-borne pathogen belonging to the genus Flavivirus of the Family Flaviviridae (Kuno et al.,1998) .The virus was subsequently isolated from a pool of Aedes africanus mosquitoes collected region from the Zika forest (Dick, 1952). ZIKV isolates were obtained from Aedes spp. in Africa (Aedes africanus) and Malaysia (Ae. aegypti), implicating these species as likely epidemic or enzootic vectors. In 2007, during the Yap island outbreak, it was estimated that approximately 20% of ZIKV cases were symptomatic, while indigenous transmission of ZIKV to humans was reported for the first time in Latin America in 2015 (Zanluca et al., 2015, WHO 2015).
Recent phylogeographic research estimates that the virus was introduced into the region between May and December 2013 (Faria et al., 2016). This recent rapid spread has led to concern that the virus is following a similar pattern of global expansion to that of dengue and chikungunya (Musso et al., 2015a). After substantial spike of cases of microcephaly and Guillian Barres syndrome over the Americas, on 2016 February 1, Zika virus was declared as (public health emergency of International concern) PHEIC, (WHO 2016). As a largely neglected disease, little is known about the basic biology of the virus and about the disease until the past decade when it made its mark outside Africa. (Wong et al., 2016).
The WHO declared that cluster of microcephaly (GBS) Guillain-Barré syndrome cases reported in Brazil are strongly suspected to be associated with the Zika virus outbreak. Non human primates were implicated as the reservoir hosts of ZIKV in Africa and Asia (Wolfe et al., 2001, Hayes, 2009). In Brazil it has been reported that it causes dengue fever-like symptoms and is transmitted by Aedes (Stegomyia) mosquitoes, emphasizing the need for retrieval of the vectorial role of these mosquitoes in diagnosis and disease control of Zika virus (Zanluca et al.,2015).
Zika virus is a flavivirus, originally isolated in 1947 from a rhesus monkey utilized while study of YFV in the Zika forest, near Kampala, Uganda. It has been isolated in several African countries (Uganda, Tanzania, Egypt, Central African Republic, Sierra Leone, and Gabon), Asian countries (India, Malaysia, the Philippines, Thailand, Vietnam and Indonesia), and in Micronesia (Lanciotti et al., 2008). In humans, ZIKA viruses causes a mild infection manifested by a rash, fever, joint and muscle pain, headache and peri-orbital pain, which are characteristic signs and symptoms of flavivirus infections (Simpson, 1964; Duffy et al., 2009).
The first human ZIKV infection was reported in Uganda in 1964 (Simpson DI, 1964). Although the isolation of ZIKV has so far been confined to the African continent (Moore et al., 1975, Monlun et al., 1993), serological evidence has shown widespread distribution of the virus even in Asian countries such as Malaysia, India, Philippines, Thailand, Vietnam, Indonesia, and Pakistan (Smithburn, 1954; Hammon et al., 1958; Bhatt, 1960; Pond, 1963; Olson et al., 1981; Darwish et al., 1983). From 2011 to 2014, concomitant infections with DENV, CHIKV, and ZIKV (Asian strain) were observed in the Pacific region (Roth et al., 2014), expected that these viruses use the same vectors (Ae. aegypti and Ae. albopictus) (Marcondes and Ximenes 2016).
We have herewith summarized what is currently known about the infection, highlighting the uncertainties and the approaches for prevention and control of arthropod borne infections which are pertinent to areas with risks of disease introduction and transmission as a global health emergency.
Epidemiology
Arbovirus is a term applied to hundreds of predominantly RNA viruses that are transmitted by arthropods, notably mosquitoes and ticks. Only a few arboviruses have caused human diseases which are clinically significant, including mosquito-borne alphaviruses such as chikungunya and flaviviruses such as dengue, West Nile fever and yellow fever. The prototype agent, yellow fever virus, is indeed the first human virus discovered and found to be transmitted by an arthropod vector. Epidemiologically, the arthropod-borne flaviviruses can be divided into mosquito-borne and tick-borne viruses. Flaviviruses are enveloped, single-stranded RNA viruses measuring about 50 nm in size. The viral genome is about 10.5 to 11 kbp in size.( Cook and Holmes, 2006) .
The Zika virus (ZIKV) belongs to Genus Flavivirus (Fig.1) one of the four members of Flaviviridae family, There are currently four genera in Flaviviridae, Flavivirus (53 species), Hepacivirus (one species, the hepatitis C virus), Pegivirus (two species), and Pestivirus (four species). With the exception of the hepatitis C virus, most clinically relevant pathogens belong to the genus Flavivirus. Flaviviruses with no known vectors are also found in animals. The viral genome produces a polyprotein with more than 3000 amino acids; this polyproptein is then cleaved into three structural and seven non-structural proteins. ( Lindenbach et al., 2007; Wong et al., 2016)
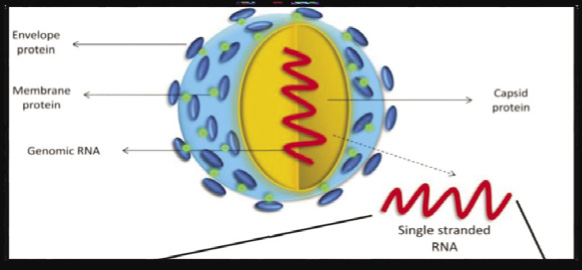 |
Figure 2: Zika virus structure (Kaur et al., 2016) |
 |
Figure 2: Aedes mosquito |
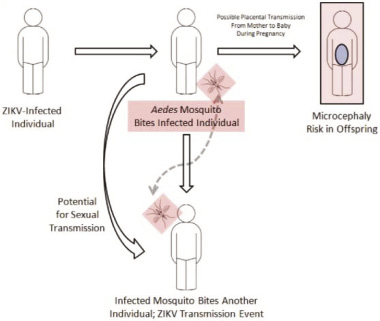 |
Figure 3: Schematic representation of Zika virus spread, including transmission via sexual intercourse and blood transfusion, possibility of placental transmission (Sikka et al., 2016) |
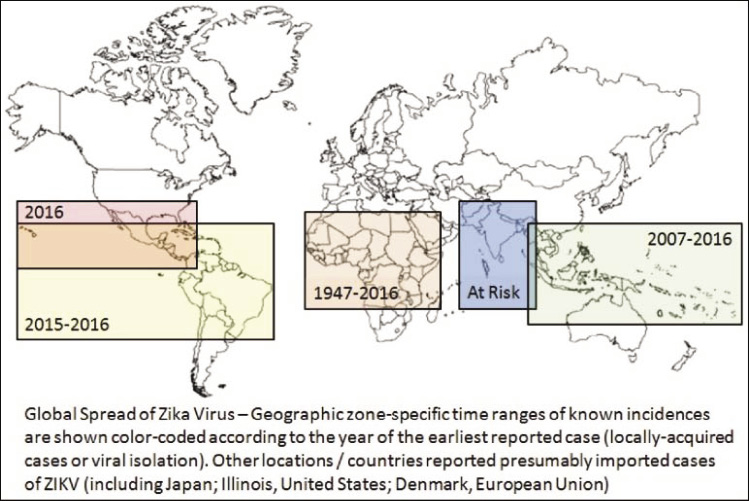 |
Figure 4: A schematic reconstruction of the geographic spread of the Zika virus. (Sikka et al., 2016) |
The pathogenesis of Zika virus infection in humans is poorly understood, a study on the effects of Zika virus suggested that the human skin cells, the fibroblasts, keratinocytes, and dendritic cells are all permissive to infection with replication of the virus. (Hamel et al., 2015). Molecules such as DC-SIGN, AXL, Tyro3, and TIM-1 are involved in cell entry and both type I and type II interferons inhibit viral multiplication. The utilization of multiple cellular receptors for entry is similar to dengue virus. (Tappe et al., 2015)
Symptoms
The symptoms of Zika are similar to those of dengue and chikungunya, which spread through the same mosquitoes that transmit Zika. Signs and symptoms of Zika are mild and last approximately two to seven days. The incubation period for Zika virus disease is not confirmed Zika virus infection presents initially with headache, a descending maculopapular rash involving palms and soles, fever, malaise, myalgia, anorexia, conjunctivitis, arthralgia, muscle and joint pains ,limb oedema and sometimes abdominal symptoms (abdominal pain, diarrhoea, (Ioos
et al., 2014). Zika virus usually remains in the blood of an infected person for about a week but it can be found longer in some people. The most unexpected sequel of Zika virus infection is congenital abnormalities, particularl, microcephaly. In some cases, intrauterine or neonatal death may ensure. (PAHO/WHO, 2015). Affected foetuses and infants also have cerebral calcification seen in imaging, (Oliveira Melo et al., 2016; Ventura et al., 2016a) In microcephalic infants, retinal abnormalities manifesting as macular neuroretinal atrophy, macular pigment mottling, foveal reflex loss, and chorioretinal atrophy, as well as optic nerve hypoplasia were also observed,( Ventura et al., 2016a; Ventura
et al., 2016b)
Clinically, Zika virus infection reliably cannot differentiate from other arbovirus infections such as dengue and chikungunya as the symptoms and signs are not pathognomonic. The clinical and epidemiological features are also confounded by cocirculation of different arboviruses in the same geographical area, (Grard et al., 2014).
Transmission
In February 2016, there were over 2,000 confirmed and over 118,000 suspected cases in the Americas (PAHO/WHO, 2015). The exact time and route of spread of the virus to Brazil is unknown, but importation during the 2014 World Cup has been postulated. (Zanluca et al., 2015), probably, when many tourists visited the Natal and other Brazilian capitals, possibly contributing to the infection of Aedes (Stegomyia) mosquitoes. Because dengue fever occurred in several cities where the games were played, tourists could also have acquired the viruses, carrying them when going back to their respective homes. Rapid travel and commerce link urban centres allowing for the rapid movement of both vector mosquitoes and infected humans. Established vectors can rapidly transmit allowing a hop scotch type of viral transmission, resulting in rapid and randomly spread outbreaks. Zika virus is transmitted to people through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti in tropical regions. This is the same mosquito that transmits dengue, chikungunya and yellow fever. The virus is epizootic and enzootic in non-human primates in Africa (sylvatic cycle), and these mammals are the most important natural reservoir hosts (Wong et al., 2016) .
In addition to mosquitoes, other routes of transmission of Zika virus are possible, although these are unlikely to be of major epidemiological significance under natural circumstances.
Direct transmission from primates to human via animal bites has been suggested though not proven. (Leung et al., 2015). Coincidentally, Zika virus has also been detected in the saliva of 19.2% of infected individuals but the epidemiological significance should remains to be determined. (Musso et al., 2015b). Sexual transmission has been documented, and the virus has been detected in the semen up to 62 days after onset of febrile illness. (Foy et al., 2011; Musso et al., 2015b; Atkinson
et al., 2016). Perinatal and congenital infections can occur.(Besnard et al., 2014). Transmission through transfusion and transplantation is another major clinical and public health concern. In Ae. aegypti, high levels of viruses could be found within the mosquitoes from days 20-60 after infection, though the average lifespan of female Ae. Aegypti adults is shorter than this in the tropical field conditions. (Goindin et al., 2015).
Zika virus disease outbreaks were reported for the first time from the Pacific in 2007 and 2013 (Yap and French Polynesia, respectively), and in 2015 from the Americas (Brazil and Colombia) and Africa (Cape Verde). In addition, more than 13 countries in the Americas have reported sporadic Zika virus infections indicating rapid geographic expansion of Zika virus. It has been suggested that Zika virus originated in east Africa near Uganda, which subsequently spread to west Africa and central Africa (the African lineage). (Faye et al., 2014).
The Uganda strain also spread to Malaysia and the Micronesia, thereby establishing the Asian lineage. The Yap outbreak in 2007, French Polynesian outbreak in 2013-2014, and the Latin American epidemic since 2015 were due to viruses belonging to the Asian lineage, probably originating from a strain from Southeast Asia.(Haddow et al., 2012; Enfissi et al., 2016). Species from which the virus has been isolated or found to be capable of transmitting the virus include Ae. unilineatus (found inAfrica and parts of Asia, including India, Pakistan, and Saudi Arabia), Ae. aegypti, and Ae. albopictus. Ae. africanus (chiefly a forest-dwelling mosquito feeding on non-human primates), Ae. opok (an African mosquito species described in Uganda), Ae. hensilli (endemic species in Micronesia, implicated in the outbreaks of dengue, chikungunya, and Zika virus infections in Yap Island of Micronesia), Ae. apicoargenteus (an African mosquito species), Ae. luteocephalus (an African mosquito species), Ae. furcifer (an African mosquito species), Ae. vittatus (worldwide distribution), (Haddow et al., 1964; Wong et al., 2013; Grard et al., 2014; Berthet et al., 2014; Ledermann et al., 2014; Savage et al., 2015).
Diagnosis
It is difficult to diagnose Zika virus infection based on clinical signs and symptoms alone due to overlaps with other arboviruses that are endemic to similar areas (Fauci et al., 2016). The methods currently available to test for Zika antibodies cross react with dengue antibodies. IgM positive result in a dengue or Zika IgM ELISA test should be considered indicative of a recent flavivirus infection. Plaque reduction neutralization tests can be performed and may be specific (CDC, 2016). The RT-PCR technique is used for identification of Zika virus infection in acute illness. The diagnosis is also done through PCR (polymerase chain reaction) and virus isolation from blood samples. It is difficult to diagnose serologically as the virus can crossreact with other flaviviruses such as dengue, West Nile and yellow fever. Zika virus is phylogenetically closest to the Spondweni virus, which is also a mosquito-borne flavivirus that has been found to cause a febrile illness in Africa (Wolfe et al., 1982). Zika virus can be cultured in a number cell lines such as Vero and LLC-MK2 or by intracerebral inoculation of suckling mice. (Way et al., 1976), Zika virus is classified as a biosafety level 2 organism according to the US CDC and human pathogen hazard group 3 according to the UK Advisory Committee on Dangerous Pathogens. (CDC, 2009). These diagnosis technique are useful for virological studies and research, they are impractical for most clinical laboratories. Laboratory-acquired infections of Zika virus have also been reported. (Hayes, 2009).
Antibody detection and nucleic acid amplification using RT-PCR are the usual diagnostic tests of choice. A commercial system (EUROIMMUN AG, Lubeck, Germany) detecting anti-Zika IgG and IgM using ELISA (with recombinant NS1 antigen) and indirect immunofluorescence assay (which also allows differentiation between Zika, chikungunya, and dengue viruses) was marketed in January 2016. (Tappe et al., 2014).
An in-house ELISA antibody test was developed during the Yap outbreak by the Centers for Disease Control and Prevention, USA. As expected, IgG and IgM antibody testing using ELISA shows cross-reaction with other flaviviruses, especially in patients with prior flaviviral infections. IgM antibodies are detectable from 3-8 days after onset of illness. Antibody testing by the plaque reduction neutralization test is more specific. In-house indirect immunofluorescent assays have also been described for antibody detection. Although antibody testing suffers from cross reactivities with other flaviviruses, it remains an essential diagnostic means, especially in patients who presented late in the course of disease where RT-PCR tests could be negative (about 5-7 days after onset of disease). The usual laboratory test of choice for acute Zika virus infections is RT-PCR of clinical samples, most commonly the peripheral blood. In the Yap outbreak, one third of the sera collected within 10 days after disease onset were still positive by RT-PCR.( Duffy, 2009). PCR protocols have been developed using primers directed towards various targets including E, NS5, and prM/E, and M (Balm et al., 2012; Buathong et al., 2015). RT-PCR allows accurate differentiation of Zika virus from other pathogens which may share similar clinical manifestations and this is especially useful in areas where co-circulaion of different arboviruses is prevalent. Genotyping of the viral strains is also possible
| Table 1: Population living in areas suitable for ZIKV transmission within each major world region and top four countries contributing to these populations at risk. (Messina et al., 2016). | |
| Region/Country | Population living in areas suitable for ZIKV transmission (millions) |
| Africa | 452.58 |
| Nigeria | 111.97 |
| Democratic Republic of the Congo | 68.95 |
| Uganda | 33.43 |
| United Republic of Tanzania | 22.70 |
| Americas | 298.36 |
| Brazil | 120.65 |
| Mexico | 32.22 |
| Colombia | 29.54 |
| Venezuela | 22.22 |
| Asia | 1,422.13 |
| India | 413.19 |
| Indonesia | 226.04 |
| China | 213.84 |
| Bangladesh | 133.29 |
| World | 2,173.27 |
Zika virus RNA has also been detected in the saliva, urine, and semen of some patients; the positive rate of RT-PCR in saliva is higher than that of blood (57.1% vs. 28.1%). (Musso et al., 2015a; Musso et al., 2015b; Shinohara et al., 2016). A generic test (the Generic reverse transcription (RT)-nested polymerase chain reaction (PCR) test for flaviviruses), followed by sequencing for specific viruses was proposed and specific diagnosis of ZIKV in field-collected mosquitoes accomplished by quantitative real time PCR (Faye et al., 2013).
Prevention And Control
Zika virus primarily encompasses mosquito vector control. Mosquitoes and their breeding sites pose a significant risk factor for Zika virus infection. However, its role against the Aedes vectors of Zika virus depends on the behaviours of the vectors in specific geographical areas. In general, Ae. aegypti mosquitoes are endophilic (resting indoors), endophagous (biting indoors), anthropophagic (preferentially biting humans), and diurnal and crepuscular in their activities. Ae. albopictus mosquitoes are generally exophilic (resting outdoors), exophagous (biting indoors), and anthropophilic, and are aggressive daytime biters. (Delatte et al., 2010; Higa, 2011; Bonizzoni et al., 2013).
A thorough knowledge of the local mosquitoes and their behaviours are therefore crucial to the control of vector borne diseases, and this underlines the importance of long-term local vector surveillance. Prevention and control relies on reducing mosquitoes through source reduction (removal and modification of breeding sites) and reducing contact between mosquitoes and people. Which can be done by using insect repellent; wearing clothes (preferably light-coloured) that cover as much of the body as possible, using physical barriers such as screens, closed doors and windows; and sleeping under mosquito nets. It is also important to empty, clean or cover containers that can hold water such as buckets, flower pots or tyres, so that places where mosquitoes can breed are removed. Special attention and help should be given to those who may not be able to protect themselves adequately, such as young children, the sick or elderly. During outbreaks, health authorities may advise that spraying of insecticides can be carried out. Insecticides recommended by the WHO Pesticide Evaluation Scheme may also be used as larvicides to treat relatively large water containers. However, it is known that the endophilic/ exophilic and endophagous/exophagous behaviours are not absolute and these can be variable in different geographical areas.
Genetic-Modification of male Aedes aegypti mosquitoes by Intrexon Corp.’s Oxitec unit has been shown to cause the premature death of offspring preventing a new crop of mosquitoes from reproducing and spreading disease. Oxitec’s gene-modified mosquito was approved by Brazil’s biosecurity commission in April 2014 and is awaiting final clearance from the Ministry of Health to authorize the sale of the mosquitoes to local authorities and private operators (Gale, 2016). Sterilizing male mosquitoes by exposing them to specific RNA molecules during the larvae stage has been shown to reduce insect populations in experiments.. Alternative tools for controlling mosquito-borne diseases, like the utilization of Wolbachia bacteria (Moreira et al, 2009) and of sterile males associated to insecticides (Thome et al, 2010) must be investigated further, but the careful reduction of potential breeding places is probably the most important method for their control.Travellers should take the basic precautions to protect themselves from mosquito bites. Zika virus can be found in the blood and passed from an infected person to a mosquito through mosquito bites and can be spread the virus to other people. The only flaviviral vaccines available for human use are yellow fever (live attenuated), Japanese encephalitis (inactivated, live attenuated, and chimeric), tick-borne encephalitis (inactivated) vaccines, and the newly marketed dengue vaccine (live attenuated, recombinant, tetravalent; marketed since 2015).
Nucleic acid testing (NAT) of samples of all donations was implemented routinely suggested that ZIKAV NAT should be used to prevent blood transfusion-transmitted ZIKAV. As recommended by the European Centre for Disease Prevention and Control, blood safety authorities need to be vigilant and should consider deferral of blood donors returning from areas with an outbreak of ZIKAV infection (ECDC, 2014)
The virus is known to circulate in Africa, the Americas, Asia and the Pacific. Recently in Brazil, local health authorities have observed an increase in Zika virus infections in the general public as well as an increase in babies born with microcephaly cases in northeast Brazil. Agencies investigating the Zika outbreaks are finding an increasing body of evidence about the link between Zika virus and microcephaly. However, more investigation is needed before we understand the relationship between microcephaly in babies and the Zika virus. Other potential causes are also being investigated that, there are also a lot of calcium deposits. Those can cause seizures and causeimpairment in terms of function for the brain.
In the absence of vaccines or chemoprophylaxis, the prevention of Zika virus infection follows the general rules for other vector borne infections. N,N-diethyl-m-toluamide (DEET) remains the gold standard in insect repellents against which other newer compounds are benchmarked. Effective and safe alternatives to DEET are available. The most commonly used ones are ethyl butylacetylaminopropionate (IR3535, more effective against Aedes and Culex than Anopheles mosquitoes), picaridin (also known as icaridin; concentrations of 20% are needed), p-menthane- 3,8-diol (PMD), and possibly 2-undecanone (BioUD). (Lupi et al., 2013) .Vector control is the only long-term solution to the control of vector borne diseases. During outbreak situations, emergency measures such as the use of space spray (fogging) may be deployed to rapidly bring down the number of biting adults and terminate disease transmission. Other means of mosquito control, some of which are still experimental or in the early phases of field trials, include the use of biological measures such as entomopathogenic fungi and genetic measures including sterile insect techniques (Baldacchino et al., 2015; Carvalho et al., 2015).
In the prevention of Zika virus infection, perhaps a more realistic approach is to strengthen education of travellers prior to departure. This may involve travel medicine specialists in travel clinics as part of the pre-travel consultation, as well as reinforcing publicity and education in airports and other departure points. Pre-travel counselling of pregnant women is importance intending to go to endemic areas for various arthropod-borne infections. This group of travellers may pose special difficulties in terms of vaccination, chemoprophylaxis, and choice of personal protection. Exclusion of individuals with a recent history of travel to endemic areas from blood and organ donation would be a prudent precautionary measure. Given the risk of sexual transmission of Zika virus, abstinence or barrier contraceptives should be used by persons with recent visits to endemic areas and patients recovered from the illness. Wolbachia is a naturally occurring bacterium that researchers, funded by the Bill & Melinda Gates Foundation, are using to infect Aedes aegypti mosquitoes. Wolbachia has been shown in experiments to block transmission of dengue and may also stop the spread of Zika and other mosquito-borne viruses (Moreira et al, 2009).
Zika virus disease is usually relatively mild and requires no specific treatment. People sick with Zika virus should get plenty of rest, drink enough Drink fluids to prevent dehydration, and treat pain and fever with common medicines. If symptoms worsen, they should seek medical care and advice. There is currently no vaccine available. Treatment of Zika virus infection is primarily supportive. Nonsteroidal anti-inflammatory drugs should be avoided unless dengue has been excluded (PAHO, 2016; Oduyebo et al., 2016). A few currently available drugs such as the tetracyclines, chloroquine, amodiaquine, and mefenamic acid have shown in vitro inhibitory activities against flavivirus (mostly with dengue virus), but it is still too early to comment on their potential clinical benefits.( Rothan et al., 2014; Boonyasuppayakorn et al., 2014). Acetylsalicylic acid and non-steroidal anti inflammatory drugs are not recommended due to the increased risk of hemorrhagic syndrome described with other arboviruses.
There is no specific treatment and no vaccine against Zika fever. Claims were made by an Indian biotechnology company that two Zika virus vaccine candidates (recombinant and inactivated) can be tested soon; however, no details on the vaccine preparations are currently available in the scientific literature (Science Alert, 2016). Clinical trials reveal inventions like ZIKAVAC claimed by Hyderabad based firm have been developed which showed positive test results on animal tests but not yet tested on humans and for individuals affected with Zika virus disease (Kaur et al., 2016) . In any case, a normal vaccine development cycle usually requires years of preclinical and clinical studies and a Zika virus vaccine for human use is unlikely to be available in the near future.
Conclusion
The unique challenge of Zika virus infection lies not only on disease control, but the potential sequelae of congenital infection and severe neurological complications. Further studies may provide insights to the pathogenic mechanisms, earlier and more sensitive predictors of congenital abnormalities, and the possibilities of vaccination. Cross-reaction with related flaviviruses (e.g., dengue or yellow fever) is common with anti-body testing, and thus it might be difficult to distinguish Zika virus infection from other flavivirus infections. Due to similar clinical presentation with other arbovirosis and to the lack of laboratory capacities in most of the potential endemic areas, the incidence and prevalence of Zika infections are probably underestimated. Laboratory capacities to confirm ZIKV infections should be strengthened in areas where competent mosquito vectors for ZIKV are established. With the increasing recognition of wildlife as a major source of emerging viral infections, more and more attention is being directed to the One Health approach for investigation and prevention. It is imperative that governments, health workers and scientists at every level and in every nation work together to further nurture and maximise emergence of vector borne zoonotic disease so that we can be more effective in our future so as to fight against emerging and re-emerging viral zoonotic infection. Development of a general use prophylactic vaccine for Zika virus induced disease will require considerable time and careful evaluation of safety, effectiveness, and risk/benefit ratio for the population at large. This is particularly true for a vaccine designed to protect against a virus apparently associated with both neurologic teratogenic effects and neurologic autoimmune disease (GBS) and which belongs to a genus notorious for antibody mediated enhancement of infection. In the absence of currently available vaccines, the likely long timeline for vaccine development, and the open questions about the basic pathogenesis of Zika virus infection, parallel development of other prophylactics and therapeutics must be explored. Possible pathophysiologic interactions between Zika virus infection, microcephaly, other birth defects and GBS are not understood. The ultimate goal of the world public health community should be the containment and the subsequent elimination of ZIKV as a global health security threat.
References
Atkinson B., Hearn P., Afrough B., Lumley S., Carter D., Aarons E.J. (2016) Detection of Zika virus in semen. Emerg. Infect. Dis. [In press].
Baldacchino F., Caputo B., Chandre F., Drago A., della Torre A., Montarsi F. (2015) Control methods against invasive Aedes mosquitoes in Europe: A review Pest. Manag. Sci.71: 1471-85.
Balm M.N., Lee C.K., Lee H.K., Chiu L., Koay E.S., Tang J.W.
(2012) A diagnostic polymerase chain reaction assay for Zika virus. J. Med. Virol. 84:1501-5.
Berthet N., Nakoune´ E., Kamgang B., Selekon B., Descorps- Decle`re S., Gessain A. (2014) Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis.; 14:862-5
Besnard M., Lastere S., Teissier A., Cao-Lormeau V., Musso D.(2014) Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro. Surveillance 19(13). pii: 20751.
Bhatt P.N. (1960) Mosquito-borne virus diseases of India known and potential. Proc. Haffkine. Inst. Diamond Symposia: 57–61.
Bonizzoni M., Gasperi G., Chen X., James A.A.( 2013) The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol.; 29:460-8.
Boonyasuppayakorn S., Reichert E.D., Manzano M., Nagarajan K., Padmanabhan R. (2014) Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral. Res.;106:125-34.
Buathong R, Hermann L, Thaisomboonsuk B, Rutvisuttinunt W, Klungthong C, Chinnawirotpisan P, et al.,(2015) Detection of Zika virus infection in Thailand, 2012-2014. Am. J. Trop. Med. Hyg.;93:380-3.
Carvalho D.O., McKemey A.R., Garziera L., Lacroix R., Donnelly C.A., Alphey L., et al., (2015) Suppression of a field populationof Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis.;9:e0003864.
CDC (2016) Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. Memorandum of 13 January 2016. CDC, Division of Vector Borne Disease ,Arboviral Disease and Dengue.
CDC. CDC health advisory: Recognizing, managing, and reporting Zika virus infections in travelers returning from Central America, South America, the Caribbean and Mexico. 2016. [Last accessed on January 24, 2016]. http://emergency.cdc.gov/han/han00385.asp
Centers for Disease Control and Prevention. Biosafety in Microbiological and Biomedical Laboratories. 5th edition 2009 Available at:. http://www.cdc.gov/biosafetypublications/bmbl5/bmbl.pdf [accessed 13.02.16.].
Cook S. and Holmes E.C. (2006) A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch. Virol.,151: 309-25.
Darwish M.A., Hoogstraal H., Roberts T.J., Ghazi R., Amer T. (1983) A seroepidemiological survey for Bunyaviridae and certain other arboviruses in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 77: 446–450.
Delatte H., Desvars A., Bouetard A., Bord S., Gimonneau G., Vourch G., Fontenille D.( 2010) Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis.;10:249-58.
Dick G.W. (1952) Zika virus pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg.;46:521–34. DOI: 10.1016/0035-9203 (52)90043-6.
Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., et al., (2009) Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl. J. Med. 360: 2536–2543.
Enfissi A., Codrington J., Roosblad J., Kazanji M., Rousset D. (2016) Zika virus genome from the Americas. Lancet.;387:227-8.
European Centre for Disease prevention and Control (ECDC). Zika virus infection outbreak, French Polynesia. 14 February 2014. Stockholm: ECDC; 2014. Available from: http://www.ecdc.europa.eu/en/publications/Publications/Zika-virus-French-Polynesia-rapid-risk-assessment.pdf.
European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus epidemic in the Americas: Potential association with microcephaly and Guillain Barré syndrome. 2015. [Last accessed January 24, 2016].
Faria, N. R., R. D. Azevedo, M. U. Kraemer, et al., (2016) Zika virus in the Americas: Early epidemiological and genetic findings. Science.
Fauci, Anthony S.; Morens, David M. (2016). Zika Virus in the Americas – Yet Another Arbovirus Threat. New England Journal of Medicine: 160113142101009. doi:10.1056/NEJMp1600297. PMID 26761185.
Faye O., Faye O., Diallo D., Diallo M., Weidmann M., Sall A.A.(2013) Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J;10:311.
Faye O., Freire C.C., Iamarino A., Faye O., de Oliveira J.V., Diallo M (2014) Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl. Trop. Dis. 8: e2636.
Foy B.D., Kobylinski K.C., Chilson Foy J.L., Blitvich B.J., Travassos da Rosa A., Haddow A.D., Lanciotti R.S., Tesh R.B.(2011) Probable nonvector- borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis.;17:880-2.
Goindin D., Delannay C., Ramdini C., Gustave J., Fouque F.( 2015) Parity and longevity of Aedes aegypti according to temperatures in controlled conditions and consequences on dengue transmission risks. PLoS One.;10:e0135489.
Grard G., Caron M., Mombo I.M., Nkoghe D., Mboui Ondo S., Jiolle D. ( 2014) Zika virus in Gabon (Central Africa)-2007: a new threat from Aedes albopictus. PLoS Negl. Trop. Dis. 2014 – 8: e2681.
Haddow A.D., Schuh A.J., Yasuda C.Y., Kasper M.R., Heang V., Huy R.( 2012) Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012 – 6:e1477.
Haddow A.J., Williams M.C., Woodall J.P., Simpson D.I.H., Goma L.K.H.(1964) Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ.;31:57e69
Hamel R., Dejarnac O., Wichit S., Ekchariyawat P., Neyret A., Luplertlop N. (2015) Biology of Zika virus infection in human skin cells. J. Virol;89:8880-96.
Hammon W.M., Schrack W.D., Jr., Sather G.E .(1958) Serological survey for a arthropod-borne virus infections in the Philippines. Am. J. Trop. Med. Hyg. 7: 323–328.
Hayes E.B.( 2009) Zika virus outside Africa. Emerg Infect Dis; 15:1347- 1350.
Higa Y. (2011) Dengue vectors and their spatial distribution. Trop. Med. Health.;39(4 Suppl):17-27.
Ioos S., Mallet H.P., Leparc G. I., Gauthier V., Cardoso T., Herida M.( 2014) Current Zika virus epidemiology and recent epidemics. Med Mal Infect;44:302-7.
Jason Gale (4 February 2016). The Best Weapon for Fighting Zika? More Mosquitoes. Bloomberg.com. Bloomberg News.
Kaur G., Kumar A., Anand T., Jha D. (2016) ZIKA VIRUS-An Update with Indian Perspective Epidem. Int. 1(1):31-35.
Kuno G., Chang G.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. (1998) Phylogeny of the genus Flavivirus. J. Virol. 72: 73–83.
Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J., (2008) Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis.; 14:1232-1239.
Ledermann J.P., Guillaumot L., Yug L., Saweyog S.C., Tided M., Machieng P., (2014) Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl. Trop. Dis.;8: e3188.
Leung G.H., Baird R.W., Druce J., Anstey N.M. (2015) Zika virus infection in Australia following a monkey bite in Indonesia. Southeast Asian J. Trop. Med. Public. Health;46:460e4.
Lindenbach B.D., Thiel H.J., Rice C.M.. Flaviviridae (2007) the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Philadelphia, P.A.: Lippincott, Williams & Wilkins;. p. 1102-52.
Lupi E., Hatz C. and Schlagenhauf P.( 2013) The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. d a literature review.Travel. Med. Infect. Dis.;11:374-411.
Marcondes C. B. and Ximenes M.F.(2016) Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes Revista da Sociedade Brasileira de Medicina Tropical 49(1):4-10. http://dx.doi.org/10.1590/0037-8682-0220-2015.
Messina J.P., Kraemer M.U., Brady O.J., Pigott D.M., Shearer F.M., Weiss D.J., Golding N ., Ruktanonchai C.W, Gething P.W, Cohn E, Brownstein J.S,Khan K, Tatem A.J, Jaenisch T, Murray C.J, Marinho F, Scott T.W, Hay S.I. (2016) Mapping global environmental suitability for Zika virus. Elife. 2016 Apr 19;5. pii: e15272. doi: 10.7554/eLife.15272.
Monlun E., Zeller H., Le Guenno B., Traore-Lamizana M., Hervy J.P.(1993) [Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal]. Bull. Soc. Pathol. Exot. 86: 21–28.
Moore D.L., Causey O.R., Carey D.E., Reddy S., Cooke A.R.(1975) Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 69: 49–64.
Moreira L.A., Iturbe-Ormaetxe I., Jeffery J.A., Lu G., Pyke A.T., Hedges L.M. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell; 139:1268-1278.
Musso D., Roche C., Nhan T.X., Robin E., Teissier A., Cao- Lormeau V.M. (2015b) Detection of Zika virus in saliva. J. Clin. Virol.;68:53-5.
Musso D., Roche C., Robin E., Nhan T., Teissier A., Cao-Lormeau V.M.(2015a) Potential sexual transmission of Zika virus. Emerg. Infect. Dis;21:359-61. Erratum. in Emerg. Infect. Dis;21:552.
Oduyebo T., Petersen E.E., Rasmussen S.A., Mead P.S., Meaney-Delman D., Renquist C.M., (2016) Update: Interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposuredUnited States. MMWR Morb. Mortal. Wkly. Rep.;65:122-7.
Oliveira Melo A.S., Malinger G., Ximenes R., Szejnfeld P.O., Alves S. S., Bispo de Filippis A.M.( 2016) Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol.;47:6-7.
Olson J.G., Ksiazek T.G., Suhandiman, Triwibowo (1981) Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg.75: 389–393.
PAHO/WHO PAHO Statement on Zika virus Transmission and Prevention. 2016. [Last accessed on January27, 2016]. http://www.paho.org/hq/index.php?optioncom_content&viewarticle&id11605&Itemid0&langen .
Pan American Health Organization/World Health Organization. Epidemiological Alert. Neurological syndrome, congenitalmalformations, and Zika virus infection. Implications for public health in the Americas. 1 December 2015. Available at: http://www.paho.org/hq/index.php?optionZcom_docman& taskZdoc_view&ItemidZ270&gidZ32405&langZen.
Pond W.L. (1963) Arthropod-Borne Virus Antibodies in Sera from Residents of South-East Asia. Trans. R. Soc. Trop. Med. Hyg. 57: 364–371.
Retrievedfrom:http://ecdc.europa.eu/en/publications/Publications/zikavirusamericasassociationwithmicrocephalyrapidriskassessment.pdf .
Roth A., Mercier A., Lepers C., Hoy D., Duituturaga S., Benyon E.(2014) Concurrent outbreaks of dengue, chikungunya and Zika virus infections an unprecedented wave of mosquito-borne in the Pacific 2012-2014. Euro. Surveill.; 19: pii:20929.
Rothan H.A., Mohamed Z., Paydar M., Rahman N.A., Yusof R. (2014) Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch. Virol.;159:711-8.
Savage H.M., Ledermann J.P., Yug L., Burkhalter K.L., Marfel M., Hancock W.T. (2015) Incrimination of Aedes (Stegomyia) hensilli Farner as an epidemic vector of Chikungunya virus on Yap Island, Federated States of Micronesia, 2013. Am. J. Trop. Med. Hyg.;92:429-36.
ScienceAlert (2016). An Indian company says they have two Zika vaccine candidates ready for pre-clinical trials. Available at: http://www.sciencealert.com/an-indian-company-saysthey-have-2-zika-vaccines-ready-for-pre-clinical-trials.[accessed 13.02.16.].
Shinohara K., Kutsuna S., Takasaki T., Moi M.L., Ikeda M., Kotaki A., Yamamoto K., Fujiya Y., Mawatari M., Takeshita N., Hayakawa K., Kanagawa S., Kato Y., Ohmagari N.(2016) Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J. Travel. Med.;23. pii: tav011.
Sikka V. , Chattu V. K., Popli R.K., Galwankar S. C., Kelkar D., Sawicki, Stanislaw S. G., Stawicki P., and Papadimos T. J. (2016) The Emergence of Zika Virus as a Global Health Security Threat: A Review and a Consensus Statement of the INDUSEM Joint working Group (JWG) J. Glob. Infect. Dis.; 8(1): 3–15.doi: 10.4103/0974777X.176140
Simpson D.I. (1964) Zika Virus Infection in Man. Trans. R. Soc. Trop. Med. Hyg. 58: 335–338.
Smithburn K.C. (1954) Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am J Hyg 59: 157 163.
Tappe D., Perez-Giron J.V., Zammarchi L., Rissland J., Ferreira D.F., Jaenisch T., (2015) Cytokine kinetics of Zika virusinfected patients from acute to reconvalescent phase. Med Microbiol Immunol [In press].
Tappe D., Rissland J., Gabriel M., Emmerich P., Gunther S., Held G. (2014) First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro. Surveill. Jan 30;19(4). pii: 20685.
Thome R.C.A., Yang H.M., Esteva L. (2010) Optimal control of Aedes aegypti mosquitoes by the sterile insect technique and insecticide. Mathem. Biosc.; 223:12-23.
Ventura C.V., Maia M., Bravo-Filho V., Go´is A.L., Belfort Jr R. (2016b) Zika virus in Brazil and macular atrophy in a child with microcephaly The Lancet 387:228.
Ventura C.V., Maia M., Ventura B.V., Linden V.V., Arau´jo E.B., Ramos R.C. (2016a)Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol. 79:1-3.
Way J.H., Bowen E.T., Platt G.S. (1976) Comparative studies of some African arboviruses in cell culture and in mice. J. Gen Virol 30:123-30.
WHO Media Centre: WHO Statement on the meeting of the international health regulations Emergency Committee Regarding 2016 Zika Outbreak in Americas. [Internet] 2016 Accessed on 2016, Jan 12; Updated on 2016, Jan 14 Available from http://www.who.int/mediacentre/ news/statements/2016/emergency-committee-zika.
Wolfe M.S., Calisher C.H., McGuire K.(1982) Spondweni virus infection in a foreign resident of Upper Volta. Lancet;2:1306-8.
Wolfe N.D., Kilbourn A.M., Karesh W.B., Rahman H.A., Bosi E.J., et al.,(2001) Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 64: 310–316.
Wong P.S., Li M.Z., Chong C.S., Ng L.C., Tan C.H. (2013) Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013 7:e2348.
Wong S.S., Poon R.W., Wong S. C.(2016) Zika virus infectiondthe next wave after dengue? J. of the Formosan Medical Association 115 :226-242
Zanluca, C., V. C. A. de Melo, A. L. P. Mosimann, G. I. V. dos Santos, C. N. D. dos Santos & K. Luz (2015) First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo. Cruz. 110: 569-572.
Zika Virus: Fact Sheet. 2016 [cited 2016 Jan 31] Available from http://www.who.int/mediacentre/factsheets/zika/en/.
Zika Virus: Fact Sheet. 2016 [cited 2016 Jan 31]; Available from http://www.who.int/mediacentre/factsheets/zika/en/.
Zika virus: WHO declares global emergency “BBC News. Retrieved1 February 2016


