1Department of Zoology, Netaji Mahavidyalaya, Arambagh, Hooghly, West Bengal, India
2Department of Zoology, University of Burdwan, Burdwan, West Bengal, India
3School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
4Department of Zoology, Netaji Mahavidyalaya, Arambagh, Hooghly, West Bengal, India
Corresponding author email: sahanvcbu@gmail.com
Article Publishing History
Received: 05/10/2020
Accepted After Revision: 10/12/2020
The diplomonad fish parasite of the Hexamitadae family frequently infects the fish Anabas testudineus during the warm season, leading to economic loss in the fish farming industry. Parasitic infection causes the generation of a large number of free radicals that promote oxidative stress in the fish body. This oxidative stress may cause direct tissue damage and affects the natural health condition of the fish population. Metronidazole benzoate (MB) is a widely accepted anti-protozoan drug, used to treat the protozoan infection in fish farming. The neurohormone melatonin is a potent free radical scavenger that is well known for its antioxidant, anti-inflammatory, and wound healing properties which can decrease the free-radical damage in liver tissue and reduce oxidative stress in fish body. The use of melatonin alone or in combination with other drugs to treat parasitic infection in fish has not been reported previously. Our current study shows a strong therapeutic potentiality of MB in combination with melatonin to treat the parasitic infection.
The combination therapy caused a significant reduction of the lesion marks and the formation of new skin over the scar area. Complete recovery of liver histopathology was observed in the treated groups. The combination therapy also significantly improved blood cell counts to maintain body homeostasis recovery after infection. MB in combination with melatonin treatment gradually decreased the level of oxidative stress biomarker in parasite-infected fish. The level of antioxidative enzymes likes, CAT, SOD, and GPx was also significantly increased after treatment, which promotes the health recovery of infected fish. Thus, our study demonstrates that combination therapy of MB and melatonin effectively controls parasitic infection in Anabas testudineus which can be used to enhance the productivity in the fish farming industry.
Aquaculture, Hexamitadae, Melatonin, Metronidazole benzoate (MB), Oxidative Stress.
Mondal P, Chatterjee A, Garai P, Mukherjee A, Saha N. C. Therapeutic Effects of Metronidazole Benzoate in Combination With Melatonin in Diplomonad Parasite Infection on Anabas testudineus. Biosc.Biotech.Res.Comm. 2020;13(4).
Mondal P, Chatterjee A, Garai P, Mukherjee A, Saha N. C. Therapeutic Effects of Metronidazole Benzoate in Combination With Melatonin in Diplomonad Parasite Infection on Anabas testudineus. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/38duFof
Copyright © Mondal et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The spread of infectious diseases in intensive fish farming is of major concern because it causes huge loss annually for the fish culture industry. Protozoan parasites are among the most common cause of fish disease in the culture system than any other fish parasites (Lom and Dyková, 1992; Abowei, Briyai, and Bassey, 2011). The diplomonad flagellate protozoa of the Hexamitadae family are generally intestinal parasites of fish and have been associated with medium to severe mortalities in hatcheries (Roberts and Shepherd, 1974). The information about encystment in this species is scanty. It has been proposed that cyst-like structures confer long term survival to some protozoa species outside the host body. It becomes pathogenic when the number of parasites is very high in both salmonids and tropical aquarium fish (Lloyd and Williams, 2014).
Reports suggest that massive numbers of these protozoa cause high mortalities in hatchery fingerlings (Woo and Poynton, 1995; Uzmann, Paulik, and Hayduk, 2011). These parasites can potentially damage the host tissue and induce systemic infections. Some species are associated with the hole in the head diseases in cichlids. The typical characteristic of this disease is the appearance of parasite filled ulcerative lesions in the head region (Williams et al., 2011; Lloyd and Williams, 2014; Amesberger-Freitag et al., 2019). Histopathological analysis showed the formation of granulomatous tissue and lesions in the kidney, liver, and spleen of Atlantic salmon with systemic infections of flagellated protozoa. Reports suggested that flagellate parasites create problems with nutrition by consuming essential nutrients by disintegrating the epithelium of the intestine (Gratzek, 1988).
These parasitic infections are most frequently found in young carp and also some aquarium and various marine fish. The infected fish becomes weak, listless, anorexic, and emaciated so that the head appears large with respect to the body in the advanced stage of infection. Fishes show loss of equilibrium in highly infected conditions and they swim on their side (Lom and Dyková, 1992). Populations with acute infection exhibit high death rate over a very short period because of the rapid increase in the number of the parasite causes damage to the intestinal epithelium. The trout and other salmonids, when infected with these parasites, the effects commonly observed are anemia, weight loss, dark body color, intestinal anomaly, and excessive mucus secretion. Blood also ooze out from infected intestine because of intestinal hemorrhage and hepatic disintegration may also be found (Buchmann and Bresciani, 1997; Williams et al., 2011).
Severe parasitic infection in fish promotes the oxidative stress in the body of fish (Garcia Sampaio et al., 2008). Elevated oxidative stress can cause direct tissue damage and may decrease the performance and growth of a natural fish population (Stumbo, Goater, and Hontela, 2012). Metronidazole benzoate (MB) is the most common drug, widely used to treat the protozoan infection in fish farms and hatcheries. MB works better in partially reduced condition, and thus effective against anaerobic bacteria and protozoans. Melatonin is an indolamine present in the natural physiological system. It is well documented that exogenous melatonin can stimulate antioxidant enzymes and reduce the oxidative stress in fish (Mondal et al., 2017). But there is no previous report on the application of melatonin in combination with other drugs to treat the parasite-infected fish or hole in the head disease in Anabastes tudineus. These reasons prompted us to test the therapeutic potentiality of the antioxidant melatonin alone or combined with MB to treat the parasitic infection in the fish population.
In our study, we assessed the toxicity and therapeutic efficacy of MB in combination with melatonin and demonstrated that combination therapy of MB with melatonin effectively controlled the parasitic infection and promoted recovery from the hole in the head disease in Anabas testudineus. MB is used as the most common drug used in the treatment of parasitic infection and melatonin is a stimulating agent of antioxidative enzymes. So, we observed the combined therapy significantly increased the antioxidative enzymes and reduced the oxidative stress of the parasitic infected fish body. After treatment by MB in combination with melatonin, the blood cell count was also increased compared to the diseased condition which supports the normal body homeostasis. Liver histopathology was also improved after treatment and new skin recovered the scar area. Therefore, MB in combination with melatonin can be used as a curing measure against parasitic infection to reduce the mortality and promote good health conditions of cultured fish.
MATERIAL AND METHODS
Chemicals: Metronidazole benzoate (MB) was purchased from J.B Chemicals and Pharmaceuticals Ltd (India). All other chemicals used in this study were purchased from Sigma–Aldrich Chemical Co (USA).
Animals: Adult specimens of Anabas testudineus L. (Climbing perch, Koi) (36 ± 2.20 g body weight, 10.1 ± 1.31 cm total length) a freshwater food fish present throughout the Indian subcontinent were collected from a local hatchery and were kept for 4 weeks in tap water in a large cemented tank for acclimatization. During this period fish became infected severely by the flagellated protozoan parasites. The infected fish developed a wound in the head region which subsequently developed a hole in the head (Figure. 3). Other physiological symptoms are observed like dark body colour, slow growth, faecal pseudocasts, and anorexia. The fishes were taken to the laboratory for further study and managed according to the standard protocol (Portaluppi, Smolensky, and Touitou, 2010).
LC50 estimation: Before testing the therapeutic potency, 24 h median lethal concentrations (96 h LC50) of metronidazole benzoate was estimated by Probit analysis (Finney, 1971). Adult fishes were exposed to metronidazole benzoate treated water at different concentrations. Ten fishes were randomly assigned for each aquarium containing metronidazole benzoate treated water, prepared in tap water (having temperature 25 ±2.5°C, pH 7.1 ±0.3, free CO2 24.7 ±2.4 mg l-1, dissolved oxygen 5.2 ± 0.5 mg l-1, total alkalinity 132 ± 12.1 mg l-1 as CaCO3, hardness 125 ± 3.2 mg l-1 as CaCO3). Two different control groups were created, control A for natural healthy fish and control B for diseased fish.
The range-finding tests were performed to estimate the concentrations range of the experimental solution which varied from 100, 200, 300, 400, 500, and 600 mg l-1 metronidazole benzoate in tap water. The 24 h acute toxicity (confidence limits 95%) of the above composition to Anabas testudineus was estimated using statistical software, Finney Probit program. The percent fish mortality was subjected to statistical tests with the help of SPSS16 and Grpahpad prism to determine the significant variations among the mean mortality of test animals at different concentrations of the above composition. The protocols for all the biological assays and the physicochemical parameters of the test water were determined by following the methods of APHA (2012).
Experimental design: The experiments were performed in 5 glass aquaria filled with tap water. After confirming the LC50 value of metronidazole benzoate, we selected two doses below the LC50 value in comparison with two control groups and one group only for melatonin treatment. The fishes were not fed for 24 h before the commencement of the test. Identical groups of ten fishes were kept in five separate aquaria containing 6 l of plain tap water as control (negative control, containing healthy fish; 0.7% NaCl); diseased (positive control, containing infected fish; 0.7% NaCl); melatonin (100 pg l-1 of melatonin; 0.7% NaCl); experiment 1 (100 mg l-1 of metronidazole benzoate and 100 pg l-1 of melatonin; 0.7% NaCl) and experiment 2 (200 mg l-1 of metronidazole benzoate and 100 pg l-1 of melatonin; 0.7% NaCl). After 36 h of incubation in natural aerations, physiological observations were performed. Liver samples were collected after 3 days by sacrificing the fish to study the liver histopathology. Oxidative stress parameters and antioxidant activity were biochemically estimated.
Histopathology of liver tissue: To study the histopathology, liver specimens were collected from each fish and then fixed with 10% neutral buffered formalin. Tissue samples were processed routinely for paraffin sections of 4–5 μm thickness, stained with hematoxylin and eosin (Khalil et al., 2007).
Differential count of blood cells: The blood was used for the estimation of red blood cells or erythrocyte and white blood cells or leucocytes counts. Erythrocyte and leucocytes were counted by the method of Rusia and Sood (1992) using a haemocytometer.
Enzymatic antioxidative agents: Superoxide dismutase activity: Superoxide dismutase (SOD) activity was measured according to the protocol described previously (Ewing and Janero, 1995). 0.2 g of liver tissue sample was taken and homogenized in 2 ml cold homogenization buffer solution. The suspension was then centrifuged at 14000 rpm for 30 minutes at 4ºC. The supernatant was collected for the enzyme assay. 100 μl of the extract was taken for analysis. Blank samples were covered to protect them from light whereas control samples were exposed to light. Then, they were illuminated for 10 minutes. The absorbance was measured at 560 nm with reference to the blank. One unit of SOD activity is defined as that amount of protein (in mg) causing a 50% inhibition of the photoreduction.
Catalase activity: The catalase (CAT) activity was estimated by spectrophotometric method (Aebi, 1984). 40 µl of the hepatic supernatant was taken in a cuvette and rapidly mixed with H2O2 phosphate buffer. Absorbance at 240 nm was measured using Beckman Coulter, DU 730, Life Science UV-VIS spectrophotometer up to 120 seconds at 15 seconds intervals.
Glutathione peroxidase (GPx) activity: GPx activity was measured by using the spectrophotometric method described by Castro (2008). Orthophenyl diphosphate (OPD) which is the substrate of GPx was used in this assay. Serial dilutions of OPD were made in phosphate citrate buffer (pH 5.0). 1 ml of each OPD serial dilution was mixed with 100 µl of hepatic supernatant sample and then 0.9 ml of 0.013% H2O2 was added and incubated at room temperature for 30 minutes. Absorbance was measured at 492 nm with reference to the blank. The change of absorbance value 1.0 under the assay condition is equivalent to the one unit of the enzyme action.
Determination of the level of malondialdehyde: The liver homogenates were centrifuged at 3000 g for 15 minutes and the supernatants were collected for the estimation of malondialdehyde (MDA) level by thiobarbituric acid (TBA) reactive assay with minor modifications (Draper and Hadley, 1990). Then 1 ml of the sample was taken and heated in a water bath at boiling temperature with TBA reagent (20% trichloroacetic acid, 0.5% TBA, and 2.5 N HCl; 2 ml) for 20 minutes. After cooling down the reaction mixture was centrifuged at 500 g for 10 minutes and the pellet was removed. The absorbance was measured at 532 nm. The MDA equivalents were calculated by using an extinction coefficient of 1.56 × 105/Mcm.
Statistical analysis: The data variable in a particular sampling period was expressed as mean SEM of the individuals (n =10). As all data sets passed the normality test (p < 0.05), the differences in the values of each experimental group (natural control, diseased control, melatonin treated, and two different concentrations of metronidazole benzoate and melatonin treatment) were calculated by one-way ANOVA test. F-values indicated the significance. The means were compared by using a post hoc Duncan’s multiplerange test, with p< 0.05 taken as the statistically significant threshold. In addition, a correlation coefficient test was performed to study the correlations between the profiles of different antioxidative agents, and MDA (any 2 variables at a time) in the liver of each control and experimental group. A linear regression analysis was performed for expressing the dependence of a response variable on an independent (predictor) variable. p < 0.05 level was considered as a statistically significant value in each case.
RESULTS AND DISCUSSION
Metronidazole benzoate is widely used in veterinary medicine as well as in aquaculture to prevent contamination by many virulent protozoan organisms. To investigate how metronidazole benzoate in combination with melatonin cause different degrees of stress, life parameters like acute toxicity, disease control, oxidative stress with antioxidant enzyme activities, and recovery from parasitic infection was assessed.
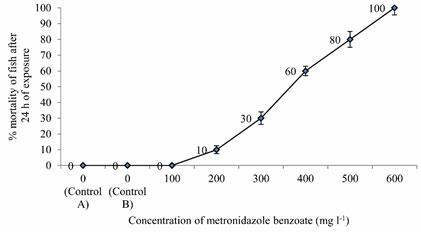
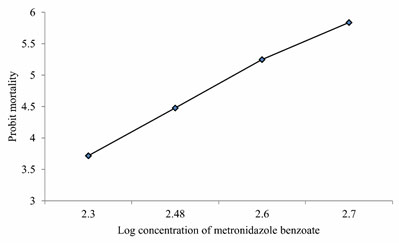
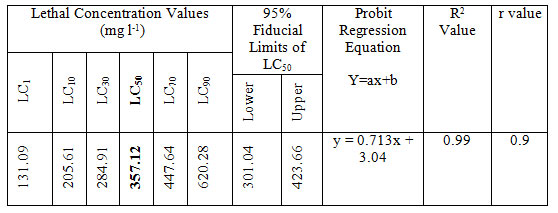
LC50 values of MB on Anabas testudineus: Before starting treatment, we estimated the LC50 dose of MB to determine the therapeutic dose. This one was the first attempt to determine the LC50 of this common medicine used in a wide range of species from mammals to fish. LC50 values for MB of 24 hours of incubation was 357.12 mg l-1 (Table 1, 2.). Probit mortality analysis is also shown in Figure 1; 2.
Figure 1: Percent Mortality of Anabas testudineus against different concentrations of metronidazole benzoate after 24 h of exposure. (p < 0.05).
Figure 2: Probit mortality of Anabas testudineus against different log concentrations of metronidazole benzoate after 24 h of exposure. (p < 0.05).
Table 1. Mean values of % mortality of Anabas testudineus exposed to different (0-600 mg l-1) concentrations of metronidazole benzoate dissolved in 0.7% saline after 24 h of exposure. Mean values within columns indicated by different superscript letters (a-f) and are significantly different (one way ANOVA at 5% level).
| Concentration (mg l-1) | % mortality of fish exposed to MB at 24 hour of exposure |
| 0
(Control A) |
0 ± 0a |
| 0
(Control B) |
0 ± 0a |
| 100 | 0 ± 0a |
| 200 | 10 ± 2.5b |
| 300 | 30 ± 4c |
| 400 | 60 ± 3d |
| 500 | 80 ± 5e |
| 600 | 100 ± 4.5f |
Table 2. LC value (LC50 with 95% confidence limits, regression equation, R2 and r values) of MB after 24 h of exposure of Anabas testudineus.
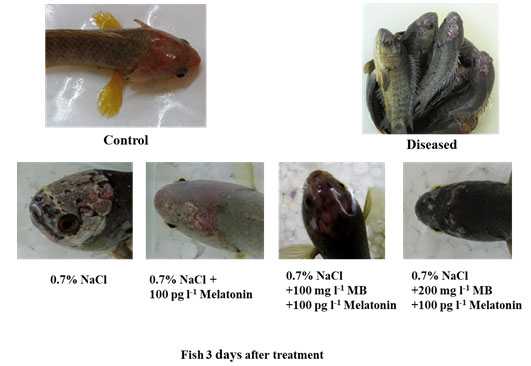
Physical observations: We found that the MB in combination with melatonin repairs the wound created by the infection of the protozoan parasite very rapidly as soon as 48 to 72 hours. After 36 hours of metronidazole benzoate (different doses) in combination with melatonin treatment, we found a great reduction of the scar area and new skin reformed. After 72 hours, the scars disappeared and no lesion mark was visible. The fish became dark in color with the restoration of their healthy movement and balance (Figure. 3.).
Figure 3: Phenotypic observation of normal control, disease control and treated groups.
Different combinations of treatment showing various levels of scar recovery in parasite-infected fish Anabas testudineus. Highest head scar recovery found in MB (200 mg l-1) in combination with melatonin treated group.
The present study provides strong evidence for MB in combination with melatonin having a good recovery capacity against the diplomonad parasite infection in the head region of the tropical fish Anabas testudineus. Before this study, no such reports were showing rapid recovery from a parasitic infection of the Hexamitadae family in A. testudineus or any other fish species. MB can solely cure this protozoan infection but it usually takes a longer duration. On the other hand, we have seen that melatonin also has the curing capacity to some extent, but it was less effective than MB when it is applied alone. However, their combination is highly effective to cure. Higher concentration (but less than LC50) of MB has better curing and repairing the property. This may help in reducing mortality in the hatchery or the stocking of fish.
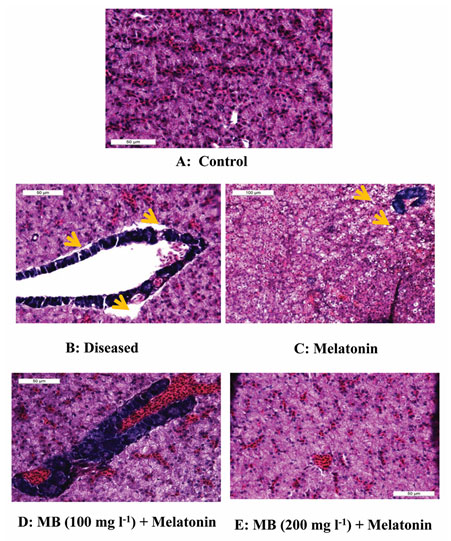
Histopathological observation of liver: The histopathological examination of all liver tissues collected from fish exposed to a different combination of MB and Melatonin showed a variation in structure. However, the most affected pathological lesions were observed in the diseased control group showed major alterations of architectures with hepatocyte degeneration and vascular dilation. Gradual improvements in the histopathology were observed in the only melatonin treatment group, but the highest recovery of the architecture of the liver was found in the combination of MB and melatonin treatment groups after 3 days (Figure. 4.).
Figure 4: Histopathology of liver tissue from the control, diseased and treated groups.
Photomicrograph of liver tissue (A) control showing normal histology, (B) diseased showing highly damaged condition like big space present between hepatic tissue and pancreas tissue and also hepato-pancreas showing vacuolation, (C) melatonin treated demonstrated slight recovery in tissue structure but some vacuolation also present, (D) MB (100mgl-1) + melatonin and (E) MB (200mgl-1) + melatonin liver of Anabas testudineus showing highly recovered structure like control. Pancreas (deep blue structure), vacuolation (yellow arrow), blood congestion (within the hepato-pancreas in image D.). Stained with H & E.
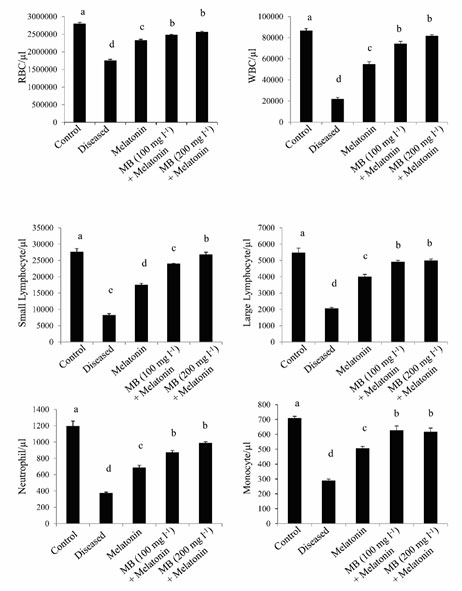
Differential blood cell counts: The blood cell counts among different experimental groups showed significant variations. Red Blood Cells (RBC) count was significantly less in the diseased group whereas the highest RBC count was observed in the healthy control group and different treatment groups like only melatonin, 100 mg l-1 MB + melatonin, and 200 mg l-1 MB + melatonin, which showed a gradual increase in cell counts. This data indicates that heavy infection of this parasite causing nutritional deficiency, which resulted in less RBC count in the diseased fish (Gratzek, 1988). The increase in RBC counts may be due to the combined effect of MB and melatonin on hematopoiesis. Similar patterns were found in different White Blood Cell (WBC) count, showing least in disease control and highest in the healthy control and treated groups (Figure. 5). An increase in WBC gives an indication of immunostimulation which may help the fish to maintain body homeostasis recovery from infection. Further research is needed to prove this hypothesis.
Figure 5: Differential blood cells counts in the control, diseased and treated groups.
Histogram showing the mean values (±SEM, in vertical bars) of the differential count in blood cells of Anabas testudineus in different experimental groups following the control, diseased or melatonin solutions (melatonin treated at the dosage of 100 pg l-1) and different doses of MB (100 or 200 mgl-1) in combination with melatonin. The alphabets on the error bars indicate significant (p < 0.05) differences in the values of a particular variable after one-way ANOVA and Duncan’s multiple range test (DMRT) thereafter. The same alphabets indicate no significant change in the values of a particular variable.
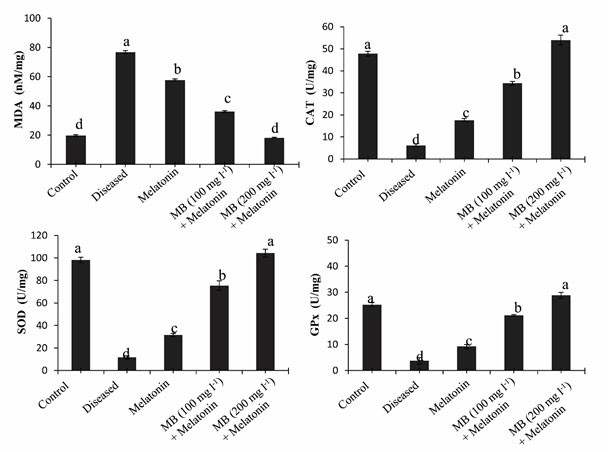
Malondialdehyde (MDA) level in the Liver: Parasitic infection can induce oxidative stress in the host tissue. Therefore, we found that the parasitic infection significantly increased lipid peroxidation which is considered as a marker of oxidative stress. Our experiment showed that high levels of lipid peroxidation product MDA present in the liver of diseased fish, which demonstrate that individuals may have a high level of oxidative stress due to parasitic infection. The MDA level decreased gradually after treatment with only melatonin, 100 mg l-1 MB + melatonin, and 200 mg l-1 MB + melatonin groups (Figure. 6.). This result signifies that treatment with MB with melatonin decreased the oxidative stress in the liver tissue of fish (Mondal et al., 2017).
Activity of antioxidative enzymes in the liver: Generation of a large number of reactive oxygen species occur during the parasitic infection of fish, causes elevated oxidative stress. CAT, SOD, and GPx form the main enzymatic defense system against the harmful effects of free radicals. In this study, we found a significant decrease in antioxidative enzymes in the diseased condition that support the ill health of fish. But after treatment by MB in combination with melatonin, the level of antioxidative enzymes (CAT, SOD, and GPx) significantly increased, which promotes good health of the fish (Figure. 6.). Melatonin is a potent free radical scavenger, may decrease the free-radical damage in the liver tissue to activate the major antioxidative enzymes like CAT, SOD, and GPx which metabolize free radicals to reduce oxidative stress in vivo (Tan et al., 1993).
Figure 6: Antioxidant enzyme activities of MDA, CAT, SOD and GPx in the hepatic tissue of control, diseased and treated groups.
Diagrammatic presentation of the mean values (±SEM, in vertical bars) of the levels of oxidative stress marker MDA and different enzymatic antioxidant (CAT, SOD, and GPx) in the liver of Anabas testudineus in the different experimental group following control, diseased or melatonin solutions (melatonin treated at the dosage of 100 pg l-1) and in different doses of MB (100 mg l-1 and 200 mg l-1) in combination with melatonin. The alphabets on the error bars indicate significant (p < 0.05) differences in the values of a particular variable after one-way ANOVA and Duncan’s multiple range test (DMRT) thereafter. The same alphabets indicate no significant change in the values of a particular variable. MDA, Malondialdehyde; CAT, catalase; GPx, glutathione peroxidase; SOD, superoxide dismutase.
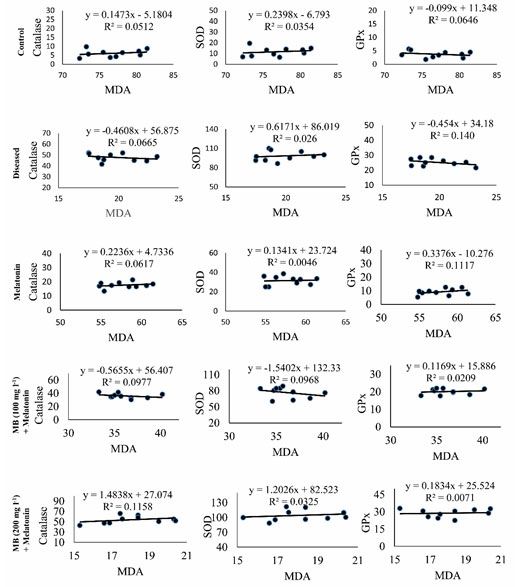
Correlation between MDA and antioxidant enzymes: Correlation coefficient analysis of the data revealed a significant (p< 0.001) negative correlation between the hepatic levels of MDA and the activity of CAT, SOD, and GPx in the liver of control, diseased and MB with melatonin-treated fish (Table. 3.). However, a significant positive correlation was found among the values between CAT, SOD, and GPx (Table. 3.). The results of the correlation coefficient analysis fitted with the findings on linear regression analysis (Figure. 7.).
Figure 7: Regression analysis between MDA concentrations and the values of CAT or SOD or GPx in the hepatic tissue of control, diseased and treated groups.
Scatter plots representing the results of single regression analysis between hepatic MDA concentrations and the values of CAT or SOD or GPx in the liver of Anabas testudineus in the different experimental group following control, diseased, melatonin solutions (100 pg l-1) or different doses of MB (100 mg l-1 and 200 mg l-1)in combination with melatonine. R2 denotes goodness of fit. CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Table 3. Values of “r” revealed from simple correlation coefficient analysis of the values on MDA (intra-cellular stress marker) or different redox parameters (any two variables at a time) in the same liver of control, diseased or treated fish Anabas testudineus.*p < 0.001; **p < 0.05; and ***p < 0.01.Abbreviations: CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
| ORRELATION | MDA | CAT | SOD | |
| Control | CAT | -0.971*** | ||
| SOD | -0.944*** | +0.973*** | ||
| GPx | -0.967*** | +0.984*** | +0.993*** | |
| Diseased | CAT | -0.911*** | ||
| SOD | -0.868** | +0.975*** | ||
| GPx | -0.966*** | +0.938*** | +0.890*** | |
| Melatonin | CAT | -0.920*** | ||
| SOD | -0.964*** | +0.901*** | ||
| GPx | -0.977*** | +0.936*** | +0.963*** | |
| Melatonin+ MB(100 mg l-1) | CAT | -0.902*** | ||
| SOD | -0.952*** | +0.889*** | ||
| GPx | -0.936*** | +0.926*** | +0.986*** | |
| Melatonin+ MB(200 mg l-1) | CAT | -0.973*** | ||
| SOD | -0.928*** | +0.975*** | ||
| GPx | -0.969*** | +0.955*** | +0.908*** | |
CONCLUSION
This study concludes that MB in combination with melatonin, may minimize the free-radical damage and ultimately lead to improving the fish’s health quality. Obtained data are consistent with earlier findings on carp in which direct stimulatory effects of melatonin on the enzymatic and nonenzymatic antioxidants were suggested. Notably, the data presented in this study demonstrate the physiological actions of melatonin and MB in reducing oxidative stress in the liver and accelerating the wound healing in parasite-infected fish. The findings from this study show the therapeutic effect of MB in combination with melatonin to reduce oxidative stress and to cure the parasitic infection of the Hexamitadae family. Our findings might help the aquaculture industry to control the protozoan infection specifically the most mortality causing protozoan parasite of the Hexamitadae family. This may be used as a curing measure to control the infection and reduce mortality of cultured fish but further research is required to make this protocol perfect.
ACKNOWLEDGEMENTS
We are thankful to the Department of Zoology, The University of Burdwan for providing the infrastructural facilities and necessary financial support and Dr. Anirban Ash for identifying the disease and providing us his laboratory for the same.
Conflict of Interest: The authors have no conflict of interest to declare.
Data Availability Statement: The data that support the findings of this study are available from the authors upon reasonable request.
REFERENCES
Abowei, J. F. N., Briyai, O. F. and Bassey, S. E. (2011). A Review of Some Basic Parasite Diseases in Culture Fisheries Flagellids, Dinoflagellides and Ichthyophthriasis, Ichtyobodiasis, Coccidiosis Trichodiniasis, Heminthiasis, Hirudinea Infestation, Crustacean Parsite and Ciliates. British Journal of Pharmacology and Toxicology 2(5): 213-226.
Aebi, H. (1984). [13] Catalase in Vitro. Methods in Enzymology 105: 121-126
Amesberger-Freitag, A. Tichy, A. EI-Matbouli, M. and Lewisch, E. (2019). Hole-in-the-head disease in discus fish, Symphysodon (Heckel, 1840): Is it a consequence of a dietary Ca/P imbalance?. Journal of Fish Diseases 42(8): 1133-1142.
Buchmann, K. and Bresciani, J. (1997). Parasitic infections in pond-reared rainbow trout Oncorhynchus mykiss in Denmark. Disease of Aquatic Organisms. Inter-Research, 28(2): 125–138.
Draper, H. H. and Hadley, M. (1990). Malondialdehyde determination as index of lipid Peroxidation. Methods in Enzymology 186: 421-431.
DX Tan, LD Chen, B Poeggeler, LC Manchester, RJ Reiter, D.X. Tan, L.D. Chen, B. Poeggler, L.C. and Manchester, R. J. R. (1993). Melatonin: A potent endogenous hydroxyl radical scavenger. Journal of Pineal Research 1:57–60.
Ewing, J. F. and Janero, D. R. (1995). Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Analytical Biochemistry 232(2): 243-248.
Finney, D. J. (1971) Probit Analysis, Cambridge University Press, Journal of Pharmaceutical Sciences 60(9): 1432.
Gratzek, J. B. (1988). Tropical fish medicine. Parasites associated with ornamental fish. The Veterinary clinics of North America. Small animal practice. Elsevier, 18(2): 375–399.
Khalil, W. K. B. Mahmoud, M. A. Zahran, M. M. and Mahrous, K. F. (2007). A sub-acute study of metronidazole toxicity assessed in Egyptian Tilapia zillii. Journal of Applied Toxicology 27(4): 380-390.
Lloyd, D. and Williams, C. F. (2014). Comparative biochemistry of Giardia, Hexamita and Spironucleus: Enigmatic diplomonads. Molecular and Biochemical Parasitology 197(1-2): 43–49.
Lom, J. and Dyková, I. (1992). Protozoan parasites of fishes. Elsevier Science Publishers.
Mondal, P. Hasan, K. N. Pal, P. K. and Maitra, S. K. (2017). Influences of exogenous melatonin on the oocyte growth and oxidative status of ovary during different reproductive phases of an annual cycle in carp Catla catla. Theriogenology. Elsevier Inc 87: 349–359.
Portaluppi, F., Smolensky, M. H. and Touitou, Y. (2010). Ethics and methods for biological rhythm research on animals and human beings. Chronobiology International 27(9-10): 1911-1929.
Roberts, R. J. and Shepherd, C. J. (1974). Handbook of trout and salmon diseases. Fishing News.
Sampaio, F. G. Boijink, C. L. Oba, E. T. Bichara, L .R. Santos, D. Kalinin, A. L. and Rantin, F. T. (2008). Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comparative Biochemistry and Physiology – C Toxicology and Pharmacology. Elsevier Inc., 147(1): 43–51.
Stumbo, A. D., Goater, C. P. and Hontela, A. (2012). Parasite-induced oxidative stress in liver tissue of fathead minnows exposed to trematode cercariae. Parasitology 139(12): 1666-1671.
Uzmann, J. R., Paulik, G. J. and Hayduk, S. H. (2011). Experimental Hexamitiasis in Juvenile Coho Salmon (Oncorhynchus kisutch) and Steelhead Trout (Salmo gairdneri). Changed publisher: Wiley. Taylor & Francis Group 94(1): 53-61.
Williams, C. F. LIoyd, D. Poynton, S. L. Jorgensen, A. Millet, C. O. and Cable, J. (2011). Spironucleus species: Economically-important fish pathogens and enigmatic single-celled eukaryotes. Journal of Aquaculture Research and Development S2-002(2): 1-13.
Woo, P. T. K. and Poynton, S. L. (1995). Diplomonadida, Kinetoplastida and Amoebida (Phylum Sarcomastigophora). CAB International.