1Department of Biotechnology, University Institute of Engineering & Technology, Kurukshetra University, Kurukshetra, Haryana, India
2Department of Biotechnology Engineering, Ambala College of Engineering & Applied Research, Devsthali, Ambala Cantt, Haryana, India
Corresponding author email: drpranayjain@gmail.com
Article Publishing History
Received: 11/02/2020
Accepted After Revision: 21/03/2020
In the present study, isolation, purification and characterization of antimicrobial compound obtained from rhizospheric soil fungus Aspergillus ibericus, was carried out in order to determine the bioactive constituents present in the metabolite which are aactually responsible for the antimicrobial potential of fungus. The fungal metabolite was preliminarily screened for its antimicrobial activity against various test microorganisms. Three solvents of different polarity, ethyl acetate, chloroform and petroleum ether were tested for the extraction of antimicrobial metabolite from culture filtrate. Quantitative analysis of antimicrobial compound was carried out by using Thin Layer Chromatography (TLC). Separation of crude extract was performed by analytical HPLC followed by purification of extract through preparative HPLC. The probable structure of bioactive compound determined by NMR spectroscopy which was found to be 7-hydroxy-3-(methoxy carbonyl)-2-methylene heptanoic acid having molecular formula C10H16O5 with molecular mass 216. The Minimum Inhibitory Concentration of bioactive fraction obtained from A. ibericus was found to be significant as 19.5µg/ml against Pseudomonas aeruginosa, Escherichia coli, Streptococus pyogenes, Candida albicans and Candida tropicalis, moderate as 625µg /ml against Staphylococcus aureus, Streptococcus mutans and weak as 1.25mg/ml against Bacillus subtilis.
Aspergillus ibericus, antimicrobial metabolite, Thin layer chromatography, Analytical and Preparative HPLC, NMR spectroscopy, Minimum Inhibitory Concentration
Geetanjali G, Jain P, Pundir R. K. Isolation, Purification and Characterization of Antimicrobial Metabolite from Aspergillus ibericus. Biosc.Biotech.Res.Comm. 2020;13(1).
Geetanjali G, Jain P, Pundir R. K. Isolation, Purification and Characterization of Antimicrobial Metabolite from Aspergillus ibericus. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/32I0IrN
Copyright © Geetanjali et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Opportunistic infection is an infection caused by microbes like bacteria, viruses, fungi, or protozoa that do not cause any disease in healthy host. These opportunists can emerge from normally present in or on human body (innocuous) or from environmentally acquired microbes. These microbes take advantage of an opportunity such as a host with impaired defense system, an altered microbiota or breached integumentary barriers (Cragg and Newman, 2001, Cabrera et al., 2020). Some of the opportunistic organisms include Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella, Clostridium difficile, Streptococcus pneumoniae, Streptococcus pyogenes etc (Enoch et al., 2006; Chen et al. 2020).
Antibiotics form the most critical field of microbial biotechnology as they are found to cure various kind of bacterial and fungal infections, but one of the problem in the fight against infectious diseases is the development of resistance to the agents used to control them. The phenomenon of resistance in clinical isolates has been known since antimicrobial drugs entered the medicine. Drug resistance, emerging and re-emerging infectious diseases has emphasized the need of search for new strains and compounds with antimicrobial potential (Zinner, 2007; Lakoh et al. 2020).
Natural sources such as bacteria, fungi and plants can be explored for new chemical entities as natural products provide a vast source of chemically diverse biologically active leads for therapeutic agents. Medicinal plants provide enormous secondary metabolites having potential to use as natural drugs in modern medicine. These bioactive secondary metabolites synthesized by medicinal plants can also strongly affect plant-associated microbial communities and their physiological functions as microorganisms live in a world of chemical signals. Surprisingly, not only the plants themselves are able to produce substances with therapeutic properties, but research continues to show that number of natural bioactive compounds are actually produced by their associated microbes (Bull and Stach, 2007; Binyamin et al., 2019).
The rhizosphere is defined as the soil zone in vicinity of plant roots, a site of high microbial activity and diversity in comparison to non-rhizosphere bulk soil. Organic compounds released by plant roots may act as basis of chemotaxis to attract some species and repel others, resulting in the existence of different communities. The microbial diversity and selection for competent microbes (for limited nutrients and space) in rhizosphere, makes it potentially an important source of natural products (Berdy, 2005; Shaikh and Mokat, 2017).
In keeping view of the above justifications, for the continuous search of new isolates from rhizosphere soil of medicinal plants, having antimicrobial activity, the present study aimed at the following objectives which included isolation of rhizosphere soil fungi Aspergillus ibericus from medicinal plant Ficus religiosa and screening for its antimicrobial activity against various test organisms. The fungus A. ibericus belongs to Order Eurotiales, Class Eurotiomycetes and Family Trichocomaceae. The morphological characteristics of fungus A. ibericus are black colony color, reverse side yellow, granular texture, biseriate sporulating structure, rough spores with maturity, conidia diameter of 5-5.5µm, conidia head diameter of 55-70 µm, colonies initiate with white hyphae and quickly form jet black conidia (Aneja, 2003). Isolated strain of A. ibercus seems to be broad spectrum in its mode of action as it inhibited the growth of all test microbes including gram-positive, gram-negative and yeasts. The work also included purification and characterization of antimicrobial metabolite and determination of minimum inhibitory concentration of antimicrobial metabolite.
MATERIAL AND METHODS
Soil samples were collected from rhizosphere of medicinal plant Peepal (Ficus religiosa) from Botanical garden, Kurukshetra University, Kurukshetra. by removing 1-1.5 inch of top soil with sterilized spatula.The serial dilution agar plate method was used for isolation of Aspergillus ibericus from soil sample (Cappucino and Sherman, 1996; Aneja, 2003). Potato dextrose agar (PDA) (CDH) for fungi was used as isolation medium. Fungal colonies appearing on their respective media were transferred to potato dextrose agar plates (one colony on each plate) at 30°C for 4-5 days. The colonies were then transferred on potato dextrose agar slants and incubated at 30°C for 4-5 days and were maintained at 4°C in a refrigerator for further studies.
The antimicrobial activity of A. ibericus was evaluated by using agar well diffusion assay (Nandhini and Selvam, 2011). Potato dextrose agar plates were inoculated with 100µl of standardized inoculum (0.5 McFarland Standard) of each test microbe (in triplicates) and was spread with sterile swabs. Wells were made into agar plates containing the test microbe inoculum. 200µl volume of extract was poured into a well of inoculated plates. Uninoculated potato dextrose broth (Hi-Media) was used as negative control. Antibiotics ciprofloxacin (antibacterial) and fluconazole (antifungal) were used as positive control. Then plates were left at room temperature for ten minutes allowing the diffusion of extract into agar. After incubation for 24 hours at 37°C, the plates were observed for inhibition zone surrounding the well containing extract. The zone of growth inhibition was measured and expressed in millimeters (mm). The mean and standard deviation of diameter of inhibition zones were calculated.
Three solvents of different polarity viz ethyl acetate, chloroform and petroleum ether were tested for the extraction of antimicrobial metabolite from culture filtrate. The filtrate was solvent extracted with each of the solvent separately, in a separating funnel taking equal volumes of filtrate and solvent (Kekuda et al., 2013).Quantitative analysis of antimicrobial compound was carried out by using Thin Layer Chromatography (TLC). The positions of different spots and solvent (distance it covered) were marked. The relative flow (Rf) value was determined (Rajalakshmi and Mahesh, 2014 with slight modifications).
Separation of crude extract by analytical HPLC (Shimadzu) was performed at CSIR- Indian Institute of Integrative Medicine, Jammu. The analytical column RP-18e chromolith with length of 100mm and internal diameter of 4.6mm with particle size of 5µm was used for separation of crude extract.All components which showed peaks in analytical HPLC were purified by preparative HPLC at CSIR- Indian Institute of Integrative Medicine, Jammu. The preparative HPLC system (Shimadzu UFLC) consisted of pump LC20AD, autosampler SIL20A HT, column oven CTI 10AS and detector PDA SPD M20A. The column Merck Semi-prep RP-18e with length of 250mm and inner diameter of 10mm with particle size of 5µm. The analysis was carried out using a gradient of water with 0.1% formic acid and acetonitrile. Flow rate 2ml/min. and column temperature 45°C was maintained.
Characterization of active component showing antimicrobial activity was performed by Proton (1H), Carbon (13C) and 2D NMR (Heteronuclear Single Quantum Coherence (HSQC), Heteronuclear Multiple Bond Coorelation (HMBC)) at CSIR-Indian Institute of Integrative Medicine, Jammu. A 400MHz Bruker spectrophotometer was used to record the NMR of antimicrobial metabolite. Chemical shift values were given in parts per million (ppm) with tetramethylsilane as internal standard. The solvent used was deuterated methanol.
MIC of antimicrobial compound against all the test microbes was determined by two-fold dilution method. In this method, two-fold serial dilution of antimicrobial metabolite was prepared by first reconstituting the metabolite (10mg/ml) in 10% dimethyl sulphoxide (DMSO). The dilutions were made in 10% DMSO to achieve a decreasing concentration range. A 200µl volume of each dilution was introduced into wells (triplicate) in nutrient agar plates already seeded with 100µl of inoculum of the test microbes. All plates were incubated at 37°C for 24 hours and were observed for the inhibition zones to know the minimum concentration of metabolite which is sufficient to inhibit growth of test microbes (Andrews, 2001; Aneja et al., 2010 with some modifications).
Statistical analysis: The data obtained from various experiments were subjected to analysis of variance (One Way ANOVA) to evaluate the significance of each parameter by estimating p-value and f-value. The level of significance was considered as p<0.05 (Pan et al., 2016).
RESULTS AND DISCUSSION
Aspergillus ibericus isolated from rhizosphere soil samples of Ficus religiosa was effective against all test microbes including four gram-positive, two gram-negative and two yeasts. Antimicrobial activity of the fungus against test microbes is shown in Table 1 in terms of zone of growth inhibition. in the current study, Aspergillus ibericus showed maximum antimicrobial activity against most of the test microbes with very strong response against B. subtilis, S. aureus, S. mutans, S. pyogenes, P. aeruginosa, E. coli and C. albicans (24mm, 25mm, 22mm, 25mm, 21mm, 22mm and 26mm, respectively) and strong response against C. tropicalis (15mm).
Table 1. Antimicrobial activity of rhizospheric soil fungus Aspergillus ibericus strain PIF2
| Fungal isolate | Zone of growth inhibition (mm)
Test microorganisms |
||||||||
| Bacteria | Yeast | ||||||||
| Gram-positive | Gram-negative | ||||||||
| Bs | Sa | Sm | Sp | Pa | Ec | Ca | Ct | ||
| PIF2 | 24.66±0.57 | 25.00±0.00 | 22.66±0.57 | 25.00±0.00 | 21.00±0.00 | 22.33±0.57 | 26.66±0.57 | 15.00±1.00 | |
| Ciprofloxacin | 24.00±0.00 | NA | 25.00±0.00 | 26.00±0.00 | 25.00±0.00 | 23.00±0.00 | ND | ND | |
| Fluconazole | ND | ND | ND | ND | ND | ND | NA | NA | |
Values are mean inhibition zone ± Standard deviation of three replicates
NA: No antimicrobial activity; ND: Not determined; Bs: Bacillus subtilis; Sa: Staphylococcus aureus; Pa: Pseudomonas aeruginosa; Ec: Escherichia coli; Sm: Streptococcus mutans; Sp: Streptococcus pyogenes; Ca: Candida albicans; Ct: Candida tropicalis
The extraction of crude bioactive metabolites from the culture filtrate by solvent extraction method is an important factor to find best solvent that have the potential to extract maximum concentration and most potent antimicrobial compounds (Haque et al., 2017). If the compounds secreted by microorganism are highly soluble in an appropriate water immiscible organic solvent, it can be easily extracted form culture broth (Haque et al., 2017; Kumar and Jadeja, 2018).
Extracellular metabolites are low molecular weight compounds that are secreted by microbial cells into a specific environment, namely the culture media (Pinu and Villas-Boas, 2017). There are some advantages of extracellular metabolite analysis over the analysis of intracellular metabolites. The separation of extracellular metabolites from microbial cells and from their intracellular metabolites can be achieved by simple techniques such as centrifugation for bacterial cells and filtration for fungi, while the extraction of intracellular metabolites from microbial cells is a complicated process (Tredwell et al., 2011; Keller, 2019). The analysis of extracellular metabolites provides invaluable information about the metabolism of different microorganisms that change in response to different environmental conditions.
The culture broth of selected strain A. ibericus was extracted with three solvents, ethyl acetate, chloroform and petroleum ether and metabolite was evaporated to dryness. Three solvents of different polarity were used to extract the active compound from filtrate. Antimicrobial activity of metabolites extracted with different solvents was measured in terms of diameter of zone of growth inhibition. Metabolite extracted with chloroform showed activity against only four test microbes mainly B. subtilis, S. aureus, S. mutans and E. coli. Compound extracted with petroleum ether showed antimicrobial activity against five test microbes B. subtilis, S. aureus, S. mutans, P. aeruginosa and C. albicans with zone of growth inhibition between 14mm and 17mm respectively.
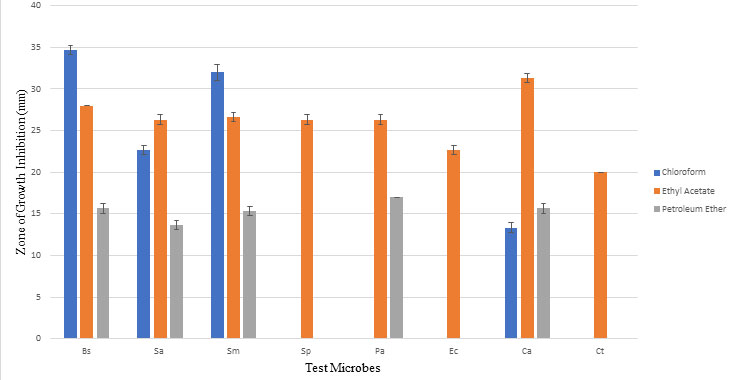
Metabolite extracted with ethyl acetate showed maximum activity against all test microbes with zone of growth inhibition ranging between 20 mm and 28mm. This suggests polar nature of antimicrobial compound extracted from culture filtrate of strain A. ibericus. One-way ANOVA analysis at 5% significance level shows calculated F value (5.87) greater than F critical value (3.46) and P value (0.000941) less than 0.05, which indicates that null hypothesis (there is no significant difference between the values) is rejected and there is significant difference between values. Table 2 and Figure 1 shows antimicrobial activity of metabolite extracted with different solvents. Figure 2 shows antimicrobial activity of metabolite extracted with different solvents against test microbes C. albicans, E. coli and S. aureus. Ethyl acetate as best solvent for extraction of antimicrobial compound from microbes was reported by many researchers (Awla et al., 2016; Ahsan et al., 2017; Haque et al., 2017; Hussaini and Gulve, 2019).
Table 2. Optimization of solvent and antimicrobial activity
| Solvent | Zone of growth inhibition (mm)
Test microorganisms |
|||||||
| Bacteria | Yeast | |||||||
| Gram-positive | Gram-negative | |||||||
| Bs | Sa | Sm | Sp | Pa | Ec | Ca | Ct | |
| Chloroform | 34.66±0.57 | 22.66±0.57 | 32.00±1.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 13.33±0.57 | 0.00±0.00 |
| Ethyl acetate | 28.00±0.00 | 26.33±0.57 | 26.66±0.57 | 26.33±0.57 | 26.33±0.57 | 22.66±0.57 | 31.33±0.57 | 20.00±0.00 |
| Petroleum ether | 15.66±0.57 | 13.66±0.57 | 15.33±0.57 | 0.00±0.00 | 17.00±0.00 | 0.00±0.00 | 15.66±0.57 | 0.00±0.00 |
Values are mean inhibition zone ± Standard deviation of three replicates
Bs: Bacillus subtilis; Sa: Staphylococcus aureus; Sm: Streptococcus mutans; Sp: Streptococcus pyogenes; Pa: Pseudomonas aeruginosa; Ec: Escherichia coli; Ca: Candida albicans; Ct: Candida tropicalis
Figure 1: Optimization of solvent and antimicrobial activity Bs: Bacillus subtilis; Sa: Staphylococcus aureus; Pa: Pseudomonas aeruginosa; Ec: Escherichia coli; Sm: Streptococcus mutans; Sp: Streptococcus pyogenes; Ca: Candida albicans; Ct: Candida tropicalis. When statistically analyzed at significance level 0.05 by One Way ANOVA, proved to be significantly different.
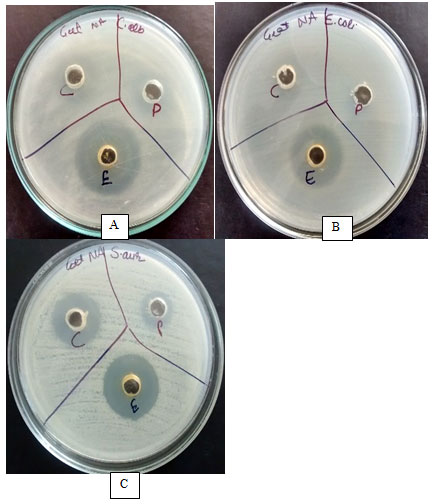
Figure 2: Optimization of solvent and antimicrobial activity against test microbes A) C. albicans, B) E. coli and C) S. aureus; C: Chloroform, P: Petroleum ether and E: Ethyl acetate.
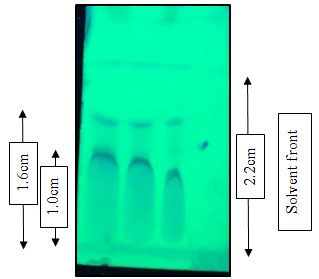
The fungal crude extract was subjected to TLC analysis for the separation of the bioactive compounds. Two fractions designated as first and second were observed when developed in dichloromethane: methanol (85:15) on silica gel TLC sheets with Rf values 0.72 and 0.45 respectively (Figure 3).
Figure 3: Thin layer chromatography: Separation of crude extract with dichloromethane and methanol (85:15)
For purification by Preparative HPLC, firstly compound was subjected to analytical HPLC. In analytical HPLC, four main peaks were observed. Peak 1 at retention time 6.129, peak 2 at 6.671, peak 3 at 12.124 and peak 4 at 16.856 was observed with 4.435%, 18.554%, 24.929%, 52.081% area respectively. All four peak fractions were collected in pure form by preparative HPLC. El-Naggar et al. (2001) purified antimicrobial compound produced by Streptomyces violates using analytical and preparative high-performance liquid chromatography (HPLC). Both analytical and preparative HPLC had been performed for purification for antifungal compounds produced by Lactobacillus plantarum IMAU10014 by Wang et al. (2012). Analytical HPLC and Preparative HPLC had been performed for purification of ent-pimara-8(14),15-diene from engineered Aspergillus nidulans by Bromann et al. (2014). Alshaibani et al. (2016) performed analytical and preparative HPLC techniques for purification of active compounds from Streptomyces sp. SUK 25 with antimethicillin-resistant Staphylococcus aureus activity. Preparative HPLC had been performed for purification of antimicrobial substances from endophytic actinomycetes by Sunaryanto and Mahsunah (2013). Reis et al. (2018) characterized secondary metabolites from endophytic fungi Nodulisporium sp. isolated from the medicinal plant Mikania laevigata through high performance liquid chromatography coupled with mass spectrometry.
Antimicrobial activity of four fractions was analyzed against test microbes and compound showing peak 4 at retention time of 16.856 and with major fraction 52.081% showed antimicrobial activity against all test microbes.NMR spectroscopy had been performed for verification of structure of active compound obtained by purification from engineered Aspergillus nidulans by Bromann et al. (2014). Alshaibani et al. (2016) performed 1D and 2D NMR for characterization of active compounds from Streptomyces sp. SUK 25 with antimethicillin-resistant Staphylococcus aureus activity.
Recently, Fan et al. (2020) characterized the structures of the compounds produced by seaweed derived fungus Pyrenochaetospsis sp. by extensive NMR. The structures of the compounds were elucidated by extensive NMR, H In the present study, purified fraction showing antimicrobial activity was analyzed by NMR spectroscopy (Pretsch et al., 2009). The probable structure of compound was found to be 7-hydroxy-3-(methoxy carbonyl)-2-methylene heptanoic acid having molecular formula C10H16O5 with molecular mass 216.MIC is defined as the lowest concentration of an antimicrobial agent which, under defined in vitro conditions, prevents the appearance of visible growth of a microorganism within a defined period of time. Extract from the isolate should be pure enough to fully characterize the activity of an antimicrobial compound (Lihan et al., 2014, Pelo et al. 2020).
The cut-off value for MIC are significant when MIC ≤ 100µg/ml, moderate in range of 100µg/ml-625µg/ml and weak when MIC ≥ 625µg/ ml. (Kuete, 2010) In the present study, MIC of bioactive fraction obtained from A. ibericus was found significant as 19.5µg/ml against P. aeruginosa, E. coli, S. pyogenes, C. albicans, C. tropicalis, moderate as 625µg /ml against S. aureus, S. mutans and weak as 1.25mg/ml against B. subtilis and also compared to selected antibiotics fluconazole (showed no activity against any test microbe at any concentration) and streptomycin (120µg for P. aeruginosa, 5µg for S. mutans, S. pyogenes, E. coli, 0.1µg for S. aureus and B. subtilis). When compared with selected antibiotics, bioactive fraction showed significant MIC value of 3.9µg against P. aeruginosa, E. coli, S. pyogenes, C. albicans, C. tropicalis whereas fluconazole showed no activity and streptomycin showed value of 120µg against P. aeruginosa, 5µg against E. coli, 5µg against S. pyogenes and no activity against two yeasts.
CONCLUSION
It may be concluded that fungus Aspergillus ibericus isolated from rhizosphere soil of medicinal plant Ficus religiosa is a promising source of antimicrobial metabolite. The research work shows rhizospheric soil of medicinal plants is a rich source of clinically important microorganisms. The antimicrobial metabolite produced by the fungal isolate A. ibericus was further purified and characterized. Antimicrobial metabolite obtained from A. ibericus is effective against test microbes gram-positive bacteria, B. subtilis, S. aureus, S. mutans and S. pyogenes, gram-negative bacteria, P. aeruginosa and E. coli and yeasts such as C. albicans and C. tropicalis. The antimicrobial metabolite is thus broad spectrum in nature. It may also be suggested that further research is needed to determine the cytotoxicity and in vivo efficacy against opportunistic pathogens before it is used for commercialization purpose.
ACKNOWLEDGEMENT
The authors are grateful to Hon’ble Vice-Chancellor, Kurukshetra University, Kurukshetra and the management and staff of CSIR-IIIM Jammu for providing necessary infrastructural facilities to carry out the research work.
Conflict of Interest: The authors declare that there is no conflict of interests.
REFERENCES
Ahsan T, J Chen, X Zhao, M Irfan and Y Wu (2017) Extraction and Identification of Bioactive Compounds (Eicosane and Dibutyl phthalate) Produced by Streptomyces Strain KX852460 for the Biological Control of Rhizoctonia solani AG-3 Strain KX852461 to Control Target Spot Disease in Tobacco Leaf. AMB Express Vol 7 Page 54.
Alshaibani MM, J Jalil, NM Sidik, R Edrada-Ebel and NM Zin (2016) Isolation and Characterization of Cyclo-(tryptophanylprolyl) and Chloramphenicol from Streptomyces sp. SUK 25 with Antimethicillin-Resistant Staphylococcus aureus Activity. Drug Design, Development and Therapy Vol 10 Pages 1817-1827.
Andrews JM (2001) Determination of Minimum Inhibitory Concentration. The Journal of Antimicrobial Chemotherapy Vol 48 Pages 5-16.
Aneja KR (2003) Experiments in Microbiology, Plant Pathology and Biotechnology. 4th ed. New Age International (P) Publishers, New Delhi.
Aneja KR, R Joshi and C Sharma (2010) In Vitro Antimicrobial Activity of Sapindus mukorossi and Emblica officinalis Against Dental Caries Pathogens. Ethnobotanical Leaflets Vol 14 Pages 402-412.
Awla HK, J Kadir, R Othman, TS Rashid and MY Wong (2016) Bioactive Compounds Produced by Streptomyces sp. Isolate UPMRS4 and Antifungal Activity Against Pyricularia oryzae. American Journal of Plant Sciences. Vol 7 Pages 1077-1085.
Berdy J (2005) Bioactive Microbial Metabolites. The Journal of Antibiotics Vol 58 No 1 Pages 1-26.
Binyamin R, Nadeem SM, Akhtar S, Khan MY and Anjum R. (2019). Beneficial and pathogenic plant-microbe interactions- A review. Soil and Environment. Vol 38(2) Pages 11-33.
Bromann K, K Viljanen, VM Moriera, J Yli-Kauhaluoma, L Ruohonen and T Nakari-Setala (2014) Isolation and Purification of Ent-pimara-8(14),15-diene from Engineered Aspergillus nidulans by Accelerated Solvent Extraction Combined with HPLC. Analytical Methods Vol 6 Pages 1227-1234.
Bull AT and JE Stach (2007) Marine Actinobacteria: New Opportunities for Natural Product Search and Discovery. Trends in Microbiology Vol 15 No 11 Pages 491-499.
Cabrera R, Fernández-Barat L, Motos A. (2020) Molecular characterization of methicillin-resistant Staphylococcus aureus clinical strains from the endotracheal tubes of patients with nosocomial pneumonia. Antimicrobial Resistance & Infection Control. Vol 9 Page 43.
Cappucino JG and N Sherman (1996) Microbiology- A Laboratory Manual. 4th ed. The Benjamin/Cummings Publishing Company, Inc. US.
Chen Y-H, Huang K-Y A, Huang Y-C, Chi H, Lu C-Y, Chang L-Y, Ho Y-H, Chi C-Y, Liu C-C, Huang L-M, Yang TYO and Huang Y-C (2020) Prevalence and molecular characterizations of Staphylococcus aureus nasal colonization among patients in pediatric intensive care units in Taiwan. Antimicrobial Resistance & Infection Control Vol 9: Page 41.
Cragg GM and DJ Newman (2001) Medicinals for the Millennia: The Historical Record. Annals of the New York Academy of Sciences Vol 953 Pages 3-25.
El-Naggar MY, MA Hassan and WY Said (2001) Isolation and Characterization of an AntimicroAVISbial Substance Produced by Streptomyces violates. Egyptian Journal of Biology Vol 3 Pages 11-21.
Enoch DA, HA Ludlam and NM Brown (2006) Invasive Fungal Infections: A Review of Epidemiology and Management Options. Journal of Medical Microbiology Vol 55 Pages 809-818.
Fan B, Dewapriya P, Li F, Blumel M and Tasdemir D (2020) Pyrenosetins A-C, new Decalinoylspirotetramic acid derivatives isolated by bioactivity-based molecular networking from the seaweed-derived fungus Pyrenochaetopsis sp. FVE-001. Marine Drugs. Vol 18 Page 47.
Haque MA, H Imam, HK Hana, S Islam, D Ganim and Haque MR (2017) Maintenance and Optimization of Culture/Fermentation Media to Achieve Maximum Bioactive Metabolites from Marine Streptomyces sp. and their Cytotoxic Effects. Indian Journal of Geo Marine Sciences Vol 46 No 01) Pages 170-175.
Hussaini SZ and Gulve R (2019) Antimicrobial activity of ethyl acetate extract of antibiotic isolated from fresh water actinomycetes. International Journal of Research Culture Society Vol 3 No 6 Pages 56-61.
Kekuda PTR, KN Rakesh, S Junaid and N Dileep (2013) Antibacterial and Antioxidant Activities of Streptomyces species SRDP-H03 Isolated from Soil of Hosudi, Karnataka, India. Journal of Drug Delivery and Therapeutics Vol 3 No 4 Pages 47-53.
Keller NP (2019) Fungal secondary metabolism: regulation, function and drug discovery. Nature Reviews Microbiology Vol 17: Pages 167-180.
Kuete V (2010) Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Medica Vol 76 No 14 Pages 1479-1491.
Kumar RR and Jadeja VJ (2018) Characterization and partial purification of an antibacterial agent from halophilic actinomycetes Kocuria sp. strain rsk4.Bioimpacts Vol 8 No 4 Pages 253-261.
Lakoh S, Li L, Sevalie S, Guo X, Adekanmbi O, Yang G, Adebayo O, Yi Le, Coker JM, Wang S, Wang T, Sun W, Habib AG and Klein EY (2020) Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: a cross- sectional study. Antimicrobial Resistance & Infection Control. Vol 9: Page 38.
Lihan S, CS Lin, I Ahmad, FM Sinang, NK Hua and AA Sallehin (2014) Antimicrobial Producing Microbes Isolated from Soil Samples Collected from Nanga Merit Forest in Sarawak, Malaysian Borneo. European Journal of Experimental Biology Vol 4 No 1 Pages 494-501.
Nandhini SU and MM Selvam (2011) Bioactive Compounds Produced by Streptomyces strain. International Journal of Pharmacy and Pharmaceutical Sciences Vol 5 No 1 Pages 176-178.
Pan F, Z Liu, Q Chen, YW Xu, K Hou and W Wu (2016) Endophytic Fungus Strain 28 Isolated from Houttuynia cordata Possesses Wide-Spectrum Antifungal Activity. Brazilian Journal of Microbiology Vol 47 Pages 480-488.
Pelo S, Mavumengwana V and Green E (2020) Diversity and antimicrobial activity of culturable fungal endophytes in Solanum mauritianum. International Journal of Environmental Research and Public Health. Vol 17: Page 439
Pinu FR and SG Villas-Boas (2017) Extracellular Microbial Metabolomics: The State of the Art. Metabolites Vol 7 No 3 Page 43.
Pretsch E, P Buhlmann and M Badertscher (2009) Structure Determination of Organic Compounds Tables of Spectral Data. Fourth, Revised and Enlarged Edition. Springer-Verlag Berlin Heidelberg.
Rajalakshmi S and N Mahesh (2014) Production and Characterization of Bioactive Metabolites Isolated from Aspergillus terreus in Rhizosphere Soil of Medicinal Plants. International Journal of Current Microbiology and Applied Sciences Vol 3 No 6 Pages 784-798.
Reis IMA, Ribeiro FPC, Almeida PRM and Branco A (2018) Characterization of the secondary metabolites from endophytic fungus Nodulisporium sp. isolated from the medicinal plant Mikania laevigata (Asteraceae) by reversed phase high performance liquid chromatography coupled with mass spectrometric multistage. Pharmacognosy Magazine. Vol 14 No 59 Pages 495-498.
Shaikh MN and Mokat DN (2017) Bioactive metabolites of rhizospheric fungi associated with Cimbopogon citratus (DC.) Stapf. Journal of Pharmacognosy and Phytochemistry. Vol 6 No 6 Pages 2289-2293.
Sunaryanto R and AH Mahsunah (2013) Isolation, Purification, and Characterization of Antimicrobial Substances from Endophytic Actinomycetes. Makara Journal of Sciences Vol 17 No 3 Pages 87-92.
Tredwell GD, B Edwards-Jones, DJ Leak and JG Bundy (2011) The Development of Metabolomic Sampling Procedures for Pichia pastoris, and Baseline Metabolome Data. PLoS ONE. Vol 6 No 1 Page e16286.
Wang H, Y Yan, J Wang, H Zhang and W Qi (2012) Production and Characterization of Antifungal Compounds Produced by Lactobacillus plantarum IMAU10014. PLoS ONE Vol 7 No 1 Page e29452.
Zinner SH (2007) Antibiotic Use: Present and Future. New Microbiologica Vol 30 No 3 Pages 321-325.