1Master of PlantBbreeding’s, Istanbul, Turkey
2Department of Environmental Sciences and Engineering, Ardabil Branch,
Islamic Azad University, Ardabil, Iran
3Young Researchers Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran.
Corresponding author email: khayatnezhad@gmail.com
Article Publishing History
Received: 26/10/2020
Accepted After Revision: 09/12/2020
This study investigated the specific changes in nitrate reductase (NR) activity through a range of experiments with different salt treatments. Even under desirable normal conditions, higher plants reply on nitrate as the main source of nitrogen for obtaining optimal growth. Although plants can use various forms of nitrogen, its reduced form, ammonia, is preferable for plant growth as it is directly incorporable into organic compounds. This reduction is made possible via a series of catalysis reactions in which two enzymes, including nitrogenase and nitrite reductase, play the main roles in the conversion of nitrate to ammonia. The observations indicated that low-applied NaCl concentrations triggered higher enzyme activity in both Dapoli 3 and HR374 varieties; a low concentration could be described as about 8mM in both the varieties and, specifically, about 40% in HR374.
Despite the observed indirect relation between NaCl concentrations in varieties and enzyme activity levels, an increase in NaCl concentration also had a negative (inhibitory) impact on enzyme activity, as it respectively declined by 50% and 40% in Dapoli 3 HR374 after applying 150 mM of NaCl by irrigation. The study presoaked the seeds in different NaCl concentrations before germination and applied a stage of irrigation with three different concentrations of NaCl (8, 3, and 80 mM). The observations indicated that enzyme activity (EA) slightly improved in the extract collected from the leaves. Also, the study did not detect a significant association between a short-time drought and EA; however, as the drought duration increased to eight days, Line2542 and Line958 respectively represented a 45% and 36% EA decline.
Eleusine Coracana, Nitrate Reductase, Saline, Drought, Presowing Salt Stress
Karasakal A, Khayatnezhad M, Gholamin R. The Effect of Saline, Drought, and Presowing Salt Stress on Nitrate Reductase Activity in Varieties of Eleusine coracana (Gaertn). Biosc.Biotech.Res.Comm. 2020;13(4).
Karasakal A, Khayatnezhad M, Gholamin R. The Effect of Saline, Drought, and Presowing Salt Stress on Nitrate Reductase Activity in Varieties of Eleusine coracana (Gaertn). Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/2Jli887
Copyright © Karasakal et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The catalysis reaction leading to the reduction of N03‑ to NH4 + includes two stages in which the two enzymes mentioned above are involved. Two electrons allow for reducing NO3‑ to N02‑ in the first stage by NR:N03‑ + 2H + + 2e ‑ ‑‑‑‑‑‑ > N02‑ + H2O. Having positive effects on plant growth, NR is also regarded as the leading enzyme of the pathway, involved in the incorporation of nitrate to amino acids (Campbell 1985, Gholamin and Khayatnezhad 2012). Many research studies report a significant positive correlation between NR activity and plant growth (Zieserl, Rivenbark et al. 1963). With prosthetic groups containing flavin adenine dinucleotide (FAD), haem, and molybdenum, nitrate reductase is considered as an intricate protein, with a large structure and a heavy molecular weight, which consists of two subunit proteins each near 115 kD (Gholamin and Khayatnezhad 2020).
Evans and Nason (Evans and Nason 1953), for the first time, discovered NR cotyledons of some soybean varieties, and ever since, other research studies have detected this enzyme in all parts of the plant (Gholamin and Khayatnezhad 2020). Research concerning nitrate reductase faces two challenging issues regarding the NR localization and regulation, as based on the results of many studies, a few researchers speculate it is detectable in the cytoplasmic space (Suzuki, Gadal et al. 1981).Vaughn et al. (Vaughn, Duke et al. 1984) examined the fixed tissue selection of soybean cotyledons in a series of immunofluorescence studies and confirmed the cytoplasmic localization of NR. Yet, NR is present in chloroplasts based on the results of relevant research confirm (Losada and Guerrero 1979, Gholamin and Khayatnezhad 2020, Gholamin and Khayatnezhad 2020).
Kamachi et al. 1987 conducted an immunogold localization study recently, examining spinach chloroplasts and they concluded that NR is specific to chloroplasts (Kamachi, Amemiya et al. 1987). On the other hand, some research has provided evidence suggesting plastids in roots are the points where the NR is localized (Layzell 1990). Proposedly, NR has a two-site ping pong model the reaction mechanism (Campbell 1985, Khayatnezhad 2012, Khayatnezhad and Gholamin 2012). The first acceptor, FAD, receives the two electrons donated by the reduced pyridine nucleotide, which pass through cyt b557 site to Mo cofactor site, where they are donated to N03, which gets reduced to NO2.
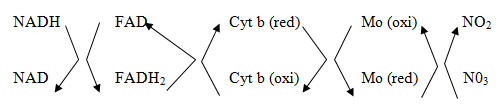
Research studies commonly describe NADH as the agent responsible for reducing the activity of NR (Oaks, Wallace et al. 1972). The results of two research studies and suggest that the function of NR is also associable with NADPH FMNH2, and FADH2, and reduced benzyl and methyl viologens (Prakash, Singh et al. 1984).Alterations in the physiological properties and the environmental conditions can effectively adjust NR activity and impact the production and degradation in higher plants (Guerrero, Vega et al. 1981, Khayatnezhad and Gholamin 2012), some of which include changes in light conditions, sources of nitrogen and energy, vital mineral substances, water-induced stress, temperature fluctuations, PGRs, plant age and metabolic inhibitors (Naik, Abrol et al. 1982). Supply of substrate, coenzymes, and energy are the main determinants of NR activity.Nitrate reductase is highly responsive to substrate (Somers, Kuo et al. 1983) with potential quick turnovers (Oaks, Wallace et al. 1972). The balance between enzyme production and turnover is generally an important maintaining factor of NR levels (Lee and Stewart 1979). May research studies have confirmed the necessity of N03‑ as a substrate for NR induction (Rajasekhar, Gowri et al. 1988, Khayatnezhad and Gholamin 2020).
Also, alterations in light conditions are extremely influential on NR induction (Kapoor, Prakash et al. 1987). The observations in relevant research identify two other agents for regulating NR activity, including NH4 and amino acids (Guerrero, Vega et al. 1981), as the end product of N03‑ reduction (NH4 +) is a presumed suppressor of NR activity. Conversely, some scholars associate NH4+ with the stimulation of NR activity (Smith and Thompson 1971, Khayatnezhad and Gholamin 2020).
Since NR is a molybdoflavo protein, the presence of Mo, as an essential NR activity stimulating factor is considered universal. As Mo is found in the structure of the NR molecule, plants with Mo deficiency represent lower NR activity compared to those provided with external sources of Mo, in which Nr activity quickly increases (Afridi and Hewitt 1964). It has been confirmed that saline soil conditions impair the nitrogen assimilation process (Demboski 1999). Based on the results of relevant research indicate that nitrate reduction is believed to be highly affected by external stress conditions. The research by Smirnoff et al., (Smirnoff, Winslow et al. 1985) proposed two main influential factors on NR activity: nitrate availability or protein synthesis, stating any factor affecting those can also influence NR activity. Various factors are proved influential on NR activity, including soil wet content, salinity, and bacterial nitrification.
The findings of a vast range of research represent that medium saline content is a suppressor of NR activity (Lal and Bhardwaj 1987). Rakova et al. (Rakova, Klyshev et al. 1978) studied the effects of salinity on NR activity and structure, finding that salinity conditions cause structural disintegration of this enzyme’s molecules; two main factors are involved for this disintegration, including the dissociation of FAD and Mo from the apoprotein respectively in leaves and roots. Furthermore, many other relevant studies confirm the positive association between salinity and increased NR activity. For instance, the study by Boucaud (BOUCAUD 1972) identified salinity as an indirect factor for increasing NR activity, claiming the effect originates from the modification of CO2 assimilation and protein synthesis by salt.
Another relevant study attributed increased NR activity under salinity conditions to higher osmotic potential enhanced by salinity, describing NR activity as specific to different saline conditions (treatments). They found that while a lower NaHCO3 concentration had positive effects, a higher concentration limited the NR activity. In a similar study, Sharma and Purohit (1986) discovered that NaCl and NaF had a limiting effect on NR activity Joshi (Joshi 1987), though, observed the stimulating effect of NaCl and the inhibitory effect of Na2SO4 .
MATERIAL AND METHODS
Extraction and Assay buffer: 0.1M Tris ‑ HC1 (pH 7.5’), KN03 0.1M, Sulphanilamide 1% in 1 N HCl, 1N (1‑naphthyl ethylenediamine dihydrochloride 0.1%. In nitrate-to-nitrite reduction reaction, NR plays the accelerating role (catalyst). The study used sulphanilamide as an amino compound coupling with NEDD in the presence of NO2 to synthesize an azo dye. The initial NO2 concentration corresponds with the amount of azo dye. The study arranged two groups of control and treatment cultivars (Dapoli 3 and HR374) and harvested and chopped the leaves and used their known weight as an enzyme source.
The study utilized the method proposed by Klepper et al. (1971) to assay the in vivo NR activity. The amount of assay mixture in the experiment was firstly 2.0 ml containing 0.1M Tris- HCL buffer pH 7.5, 0.005 M KNO3, and 50 mg of fresh plant material. The experiment put the assay mixture in an incubator for 20 minutes at 370C and terminated the reaction using 1.0 ml of sulphanilamide as an additive after adding NEDD and measured the wavelength of the resulting pink color at 525nm. Finally, the study used the nitrite standard curve to estimate nitrite amounts. The unit of expressing EA was: mg NO2–/hour/gm fresh tissue.
RESULTS AND DISCUSSION
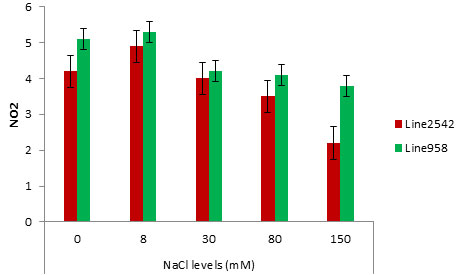
Figure 1 illustrates the data concerning NR activity levels in E. coracana leaves under a treatment regiment with varying NaCl concentrations of sodium chloride. As clearly presented in Fig.1, a low (i.e. 8mM ) NaCl concentration results in a higher EA in both varieties of in the study. On the other hand, though, this observation also indicated that increasing NaCl concentration will harm EZ, as it was observed EA respectively declined by 50% and 40% in Dapoli 3 and HR374 under a regiment of 150mM of NaCl. When the study presoaked the seeds in different NaCl concentrations before germination and applied a stage of irrigation with three different concentrations of NaCl (8, 3, and 80 mM). The observations indicated that enzyme activity (EA) slightly improved in the extract taken from the leaves. As depicted in Fig.2, however, an increase in the NaCl concentration to 150mM led to a 25% decrease in EA, representing the relevantly positive effect of a presowing stage on the increasing plants’ tolerance to salinity.
Figure 1: Interaction NACL levels on Nitrate activity
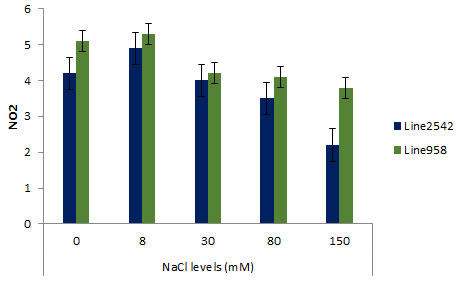
Figure 2 illustrates a summary of NR activity under the effect of drought in Line2542 and Line958, indicating non-significance of short-time drought stress; however, the NR activity in Line2542 and Line958 respectively declined by 45% and 36% following an eight-day drought stress application.
Figure 2: Effect of Pre Sowing salt on Nitrate activity
Observations indicate the although a direct increasing role is not attributable to NaCl in terms of NR induction and activity, its ability to modify CO2 assimilation and protein synthesis can indirectly result in the mentioned effects. In two similar studies, over a decade, on Salsola foetida, Austwenfeld (Austenfeld 1974) found a positive association between low NaCl concentration levels and NR activity. Moreover, they found that alterations in NR activity might have caused fluctuations in the protein content since NR is known for limiting the rate in the whole nitrate assimilation. Joshi, in a study concerning Cajanus cajan, found that a similar association between a gradual increase in NR activity and the increasing NaCl concentrations in the soil, attributing the increased activity to the increased osmotic potential as a result of higher NaCl concentrations (Joshi 1984). Several studies, though, claim that salinity can limit NR activity (Sankhla and Huber 1975), suggesting the disintegration of NR under saline conditions in roots of pea and rice (Rakova, Klyshev et al. 1978).
REFERENCES
Afridi, M. and E. Hewitt (1964). “The inducible formation and stability of nitrate reductase in higher plants: I. Effects of nitrate and molybdenum on enzyme activity in cauliflower (Brassica oleracea var. Botrytis). Journal of Experimental Botany 15(2): 251-271.
Austenfeld, F.-A. (1974). Der Einfluß des NaCl und anderer Alkalisalze auf die Nitratreduktaseaktivität von Salicornia europaea L.Zeitschrift für Pflanzenphysiologie 71(4): 288-296.
Boucaud J. (1972). Effets du NaCl sur ľactivité de la nitrate réductase chez deux variétés halophiles: Suaeda maritima var. macrocar pa et var. flexilis. Physiologia Plantarum 27(1): 37-42.
Campbell, W. H. (1985). The biochemistry of higher plant nitrate reductase.
Demboski, J. R. (1999). Molecular systematics and biogeography of long-tailed shrews (Insectivora: Sorex) and northern flying squirrels (Rodentia: Glaucomys).
Evans, H. J. and A. Nason (1953). Pyridine nucleotide-nitrate reductase from extracts of higher plants.” Plant Physiology 28(2): 233.
Gholamin, R. and M. Khayatnezhad (2012). Effect of different levels of manganese fertilizer and drought stress on yield and agronomic use efficiency of fertilizer in durum wheat in Ardabil. Journal of Food, Agriculture & Environment 10(2 part 3): 1326-1328.
Gholamin, R. and M. Khayatnezhad (2020). Assessment of the Correlation between Chlorophyll Content and Drought Resistance in Corn Cultivars (Zea mays).” Helix 10(05): 93-97.
Gholamin, R. and M. Khayatnezhad (2020). The Effect of Dry Season Stretch on Chlorophyll Content and RWC of Wheat Genotypes (Triticum durum L.). Biosc.Biotech.Res.Comm 13(4).
Gholamin, R. and M. Khayatnezhad (2020). Study of Bread Wheat Genotype Physiological and Biochemical Responses to Drought Stress.Helix 10(05): 87-92.
Gholamin, R. and M. Khayatnezhad (2020). The Study of Path Analysis for Durum wheat (Triticum durum Desf.) Yield Components.Biosc.Biotech.Res.Comm 13(4).
Guerrero, M. G., J. M. Vega and M. Losada (1981). The assimilatory nitrate-reducing system and its regulation.Annual Review of Plant Physiology 32(1): 169-204.
Joshi, S. (1987). Effect of soil salinity on nitrogen metabolism in Cajanus cajan L. Indian J. Plant Physiol 30: 223-225.
Joshi, S. S. (1984). Effect of salinity stress on organic and mineral constituents in the leaves of pigeonpea (Cajanus cajan L. var. C-11) Plant and soil 82(1): 69-76.
Kamachi, K., Y. Amemiya, N. Ogura and H. Nakagawa (1987). “Immuno-gold localization of nitrate reductase in spinach (Spinacia oleracea) leaves.” Plant and cell physiology 28(2): 333-338.
Kapoor, H. C., S. Prakash and T. Madaan (1987). Regulation of in vivo nitrate reductase activity in cotton (Gossypium hirsutum) leaves in light and dark and the possible role of cytokinin.Indian journal of biochemistry & biophysics 24(6): 326.
Khayatnezhad, M. (2012). Evaluation of the reaction of durum wheat genotypes (Triticum durum Desf.) to drought conditions using various stress tolerance indices. African Journal of Microbiology Research 6(20): 4315-4323.
Khayatnezhad, M. and R. Gholamin (2012). The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). African Journal of Microbiology Research 6(12): 2844-2848.
Khayatnezhad, M. and R. Gholamin (2012). Effect of nitrogen fertilizer levels on different planting remobilization of dry matter of durum wheat varieties Seimareh African Journal of Microbiology Research 6(7): 1534-1539.
Khayatnezhad, M. and R. Gholamin (2020). A Modern Equation for Determining the Dry-spell Resistance of Crops to Identify Suitable Seeds for the Breeding Program Using Modified Stress Tolerance Index (MSTI) Biosc.Biotech.Res.Comm 13(4).
Khayatnezhad, M. and R. Gholamin (2020). Study of Durum Wheat Genotypes’ Response to Drought Stress Conditions.Helix 10(05): 98-103.
Lal, R. and S. Bhardwaj (1987). Studies on nitrogen metabolism of salinity stressed field pea (Pisum sativum, Linn. var. arvensis) Indian Journal of Plant Physiology 30(2): 165-169.
Layzell, D. B. (1990). N2 fixation, NO3-reduction and NH4+ assimilation. Plant physiology, biochemistry and molecular biology, Longman Scientific and Technical Harlow: 389-406.
Lee, J. A. and G. R. Stewart (1979). Ecological aspects of nitrogen assimilation. Advances in botanical research, Elsevier. 6: 1-43.
Losada, M. and M. Guerrero (1979). The photosynthetic reduction of nitrate and its regulation.” Photosynthesis in relation to model systems. Elsevier, Amsterdam: 365-408.
Naik, M., Y. Abrol, T. Nair and C. Ramarao (1982). Nitrate assimilation-its regulation and relationship to reduced nitrogen in higher plants.Phytochemistry 21(3): 495-504.
Oaks, A., W. Wallace and D. Stevens (1972). Synthesis and turnover of nitrate reductase in corn roots.Plant Physiology 50(6): 649-654.
Prakash, S., P. Singh, S. Sawhney and M. Naik (1984). Regulation of nitrate assimilation in plants in light and dark.Plant science letters 34(1-2): 25-34.
Rajasekhar, V., G. Gowri and W. H. Campbell (1988). Phytochrome-mediated light regulation of nitrate reductase expression in squash cotyledons. Plant physiology 88(2): 242-244.
Rakova, N., L. Klyshev and B. Kasymbekov (1978). Effect of sodium salts on activity of nitrogen metabolism enzymes isolated from plants with different salt resistance [Maize, rice, cotton, peas, kidney beans, Salicornia herbaceae Soviet Plant Physiology.
Sankhla, N. and W. Huber (1975). Effect of salt and abscisic acid on in vivo activity of nitrate reductase in seedlings of Phaseolus aconitifolius.Zeitschrift für Pflanzenphysiologie 76(5): 467-470.
Smirnoff, N., M. Winslow and G. Stewart (1985). Nitrate Reductase Acivity in Leaves of Barley (Hordeum vulgare) and Durum Wheat (Triticum durum) during Field and Rapidly Applied Water Deficits. Journal of experimental botany 36(8): 1200-1208.
Smith, F. and J. F. Thompson (1971). Regulation of nitrate reductase in excised barley roots. Plant physiology 48(2): 219-223.
Somers, D. A., T.-M. Kuo, A. Kleinhofs, R. L. Warner and A. Oaks (1983). Synthesis and degradation of barley nitrate reductase. Plant Physiology 72(4): 949-952.
Suzuki, A., P. Gadal and A. Oaks (1981). Intracellular distribution of enzymes associated with nitrogen assimilation in roots.Planta 151(5): 457-461.
Vaughn, K. C., S. O. Duke and E. A. Funkhouser (1984). Immunochemical characterization and localization of nitrate reductase in norflurazon‐treated soybean cotyledons.” Physiologia Plantarum 62(3): 481-484.
Zieserl, J. F., W. Rivenbark and R. Hageman (1963). Nitrate Reductase Activity, Protein Content, and Yield of Four Maize Hybrids at Varying Plant Populations 1 Crop Science 3(1): 27-32.