1PhD Research scholar, Department of Botany, Bishop Heber College, Trichy
2Associate Professor and Head, Department of Botany, Bishop Heber College, Trichy
3The Rapinat Herbarium and Centre for Molecular Systematics, St. Joseph’s College, Trichy
(Affiliated to Bharathidasan University, Tiruchirappalli – 24.)
Corresponding author email: steffyclarefcc@gmail.com
Article Publishing History
Received: 04/10/2019
Accepted After Revision: 21/12/2019
The present study aims to find out the presence of phytochemicals and evaluate the anti diabetic and anti-inflammatory activities of Premna rajendranii.Anti diabetic assay was evaluated using α- amylase inhibitory assay by DNSA method, anti-inflammatory activity was tested using the method of Mizushima et al with simple modifications and the methods of Peach and Tracey (1956), Gibbs (1974), Harborne (1984), Trease and Evans (1985), Edeoga et al.,(2005), Khandewal (2008), Kokate et al.,(2001), Sofowara (2009) and Tiwari et al.,(2011) were used to identify the nature of phytochemical constituents present in Premna rajendranii.Anti diabetic analysis of methanolic leaf extract of Premna rajendranii showed optimal activity when compared to standard drug. Maximum inhibition showed at 100µg (79.3%) and minimum inhibition showed at 25 µg (44.82%). Anti-inflammatory activity of methanolic leaf extract of P.rajendranii has shown high inhibition than standard at 50µl i.e. 33.88±0.01. Phytochemical screening shows the presence of Alkaloids, Carbohydrates, Flavonoids, Steroids, Glycosides, Phenols, Proteins, Tannins, Saponins, and Terpenoids in the leaf of Premna rajendranii.Anti diabetic and anti-inflammatory analysis showed the presence of bioactive compounds and the medicinal values of Premna rajendranii.
Premna rajendranii, Anti Diabetic, Anti-inflammatory, Phytochemical Analysis
Francis S, Gideon V. A, Britto J, Mariyaraj J. In vitro Phytochemical Profiling of Anti Diabetic and Anti Inflammatory Activities of Premna rajendranii - An Endemic Species. Biosc.Biotech.Res.Comm. 2019;12(4).
Francis S, Gideon V. A, Britto J, Mariyaraj J. In vitro Phytochemical Profiling of Anti Diabetic and Anti Inflammatory Activities of Premna rajendranii – An Endemic Species. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2PeHox4
Copyright © Francis et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Plants have an important role in the day to day life of mankind. Each and every plant contains phytocompounds either with medicinal properties or poisonous properties. Scientists and researchers are more focused on medicinal plants for the benefit of mankind. According to them each of the medicinal plant is a source of drug candidate. From ancient times India is considered a rich repository of medicinal plants and Indian traditional systems of medicine such as Ayurveda, Siddha and folk medicine and well known. Genus Premna has an indispensable value in Indian health care practice. Species of Premna are known for the preparation of famous Ayurvedic formulation Dashamoolaarishtam (Joshi et al., 2017).
Premna’s medicinal properties have been used in Indian traditional system of medicine especially for diarrhoea, stomach and hepatic disorders. The various biological activities including antioxidant, antibacterial, anti-inflammatory, cytotoxic and heapatoprotective have been displayed both at extract and pure compound level (Rekha, 2015). During the taxonomic revision of Indian Verbenaceae, Rajendran and Daniel (2002) recognized 31 species and 6 varieties of Premna. Recently, PrabhuKumar et al. (2013) reported the discovery of a new species Premna rajendranii from Western Ghats (Chinnar of Kerala and Madukkarai of Tamilnadu). Apart from this, a research team comprising Robi, Augustin, Sasidharan and Udayan (2013) rediscovered an endemic and rare species Premna paucinervis (C. B. Clarke) Gamble from the Vagamon hills along South Western Ghats of Kerala after a lapse of 140 years of its original type collection by R.H. Beddome (1872) from the Anamalayas in the Western Ghats (Tamilnadu) (Bose, 2014). Premna included earlier in the family Verbenaceae, was recently transferred to the Lamiaceae family based on molecular data (A.P.G. IV. 2009; Francis et al., 2019).
MATERIALS AND METHODS
Collection and authentication
The plant material for our investigation was collected from the scrub jungles of the Madukkarai Hills, Coimbatore District in Tamilnadu, and authenticated by Dr. S. John Britto S.J, at the Rapinat Herbarium and Centre for Molecular Systematics, St. Joseph’s College (Autonomous), Tiruchirappalli. The voucher Specimen (RHT 68887) was deposited in the Rapinat Herbarium.
Extraction: The leaves were shade dried and powdered using mechanical grinder. The powder sample was stored in an air tight container and the portion of the powder was taken in test tube and solvent (Methanol) was added to it such that plant powder soaked in it and shaken well. The solution then filtered with the help of muslin cloth and filtered extract was taken and used for antidiabetic and anti-inflammatory analysis.
Preliminary Phytochemical Analysis
Test’s for Alkaloids: Mayer’s Test
To a few ml of filtrate, one or two drops of Mayer’s reagent were added by the side of the test tube. A white creamy precipitate indicated the test as positive.
Wagner’s Test
To a few ml of filtrate, few drops of Wagner’s reagent were added by the side of the test tube. A reddish brown precipitate confirmed the test as positive.
Hager’s Test
To a few ml of filtrate 1 or 2 ml of Hager’s reagent (Saturated aqueous Solution of picric acid) was added. A prominent yellow precipitate indicated the test as positive. (Kokate et. al., 2001).
Test for flavonoids:Pews Test: To 2-3ml extract, was added zinc powder in a test tube, followed by drop wise addition of conc. HCL. Formation of purple red or cherry colour indicated the presence of flavonoids (Peach et.al., 1956).
Lead acetate test
1ml extract was treated with 1ml 10% lead acetate (Pb(OAc)4) solution. Formation of yellow colour precipitate indicated the presence of flavonoids.
Alkaline reagent test
To 2ml test solution, sodium hydroxide solution was added to give a yellow or red colour (Khandewal et.al. 2008).
Conc.H2SO4 test
5ml of dilute ammonia solution was added to the extract followed by conc.H2SO4. Yellow colour indicated the presence of flavonoids.
Tests for Phenolic Compounds and Tannins:Ferric Chloride Test: The extract (50 mg) was dissolved in 5 ml of distilled water. To this few drops of neutral 5% Ferric Chloride solution was added. A dark green colour indicated the presence of phenolic compounds.
Potassium dichromate test
To the extract add 5% potassium dichromate solution. Positive result was confirmed by a formation of brown precipitate (for phenol).
Lead Acetate Test
The extract (50 mg) was dissolved in distilled water and to this 3 ml of 10% Lead Acetate solution was added. A bulky white precipitate indicates the presence of phenolic compounds (Treare et.al., 1985).
Braymer’s Test
To 2 ml extract, added 2 ml H2O and followed with 2-3 drops of FeCl3 (5%). Green precipitate proved presence of tannins.
Tests for Saponins: Foam Test: To 1ml of extract, add 2ml of distilled water and shaken vigorously and allowed to stand for 10 min. There is the development of foam on the surface of the mixture. Then shake for 10 minutes, it indicates the presence of saponins ( Khandewal et.al.,2008).
NaHCO3 Test
To extract a drop of sodium bicarbonate was added. The mixture was shaken vigorously and kept for 3 min. A honey comb like froth was formed and it showed the presence of saponins.
Tests for Glycosides: Keller Kiliani Test (Test for cardiac glycoside): To 2 ml extract, was added 1 ml glacial acetic acid, one drop 5% FeCl3 and 1 ml conc. H2SO4. A brown ring of the interface indicated the presence of cardiac glycosides (Kokate et.al., 2001; Khandewal et.al., 2008).
Glycoside Test
To small amount of extract, was added 1 ml water and shake well. Then aqueous solution of NaOH was added. Yellow color appeared that indicated the presence of glycosides (Treare et.al., 1985).
Molisch’s Test
To 1ml of extract, 2drops of Molisch’s reagent was added in a test tube and 2ml of con. H2SO4 was added carefully keeping the test tube slightly curved. Formation of violet ring at the junction indicated the presence of glycosides (Khandewal et.al., 2008).
Tests for Carbohydrates:Molish’s Test: To 2 ml of filtrate two drops of alcoholic solution of α- napthol was added, the mixture was shaken well and 1 ml of con. H2SO4 was added slowly along the sides of the test tube and allowed to stand. A violet ring indicated the presence of carbohydrates.
Benedict’s test
To 0.5 ml of filtrate, 1 ml of Benedict’s reagent was added. The mixture was heated on a boiling water bath for 2 mins. A characteristic coloured precipitate indicated the presence of sugar.
Test for Terpenoids:Salkowski’s Test: 2 ml of chloroform and 1 ml of conc. H2SO4 was added to 1 ml of extract and observed for reddish brown color that indicated the presence of terpenoids.
Tests for quinones
1ml of extract was treated with alcoholic potassium hydroxide solution. Quinines give coloration ranging from red to blue.
Test for sterols: Salkowski’s Test: To 2 ml of extract, was added 2 ml chloroform and 2 ml conc. H2SO4 from the side of the test tube. Chloroform layer appeared red and acid layer showed greenish yellow fluorescence indicated the presence of sterols (Khandewal et.al., 2008).
Tests for Proteins and Amino Acids:Biuret Test: An aliquot of 2 ml of filtrate was heated with 1 drop of 2 % CuSO4 solution. To this 1 ml of ethanol (95%) was added, followed by excess of KOH Pellets. Pink colour in the ethanolic layers indicated the presence of proteins.
Conc. H2SO4 Test
2 ml extract was treated with few drops of conc. H2SO4. Formation of white precipitate indicated the presence of proteins.
Xantho proteins Test
2 ml extract was treated with few drops of conc. HNO3 and NH3 solution. Formation of reddish orange precipitate indicated the presence of xantho proteins.
Anti-Diabetic Assay (Α- Amylase Inhibitory Assay) By Dnsa Method
α-amylase was dissolved in phosphate buffer saline (PBS, 0.02 mol/L, pH6.8) at a concentration of 0.1 mg/ml. Various concentrations of sample solutions (0.25 ml) were mixed with α-amylase solution (0.25 mL) and incubated at 37 °C for 5 min. Then the reaction was initiated by adding 0.5 mL 1.0% (w/v) starch substrate solution to the incubation medium. After incubation at 37 °C for 3 min, the reaction was stopped by adding 0.5 ml DNS reagent (1% Dinitrosalicylic acid, 0.05% Na2SO3 and 1% NaOH solution) to the reaction mixture and boiling at 100 °C for 5 min. After cooling to room temperature, the absorbance (Abs) at 540 nm was recorded by a spectrophotometer. The inhibition percentage was calculated by the following equation:
Percentage Inhibition = [(Abs1 − Abs2)/Abs1] × 100
Where, Abs1= control and Abs2= sample.
Anti-Inflammatory Activity
Method of Mizushima et al was followed with simple modifications. The reaction mixture (0.5 ml) consisted of 0.45 ml bovine serum albumin (3% aqueous solution) and varying concentration of compound (25, 50, 100,200μg/ml of final volume), pH was adjusted to 6.3 using small amount of 1N hydrochloric acid. The samples were incubated at 37ºC for 20 min and then heated at 80ºC for 2min. After cooling the samples, 2.5 ml phosphate buffer saline (pH 6.3) was added to each tube. The absorbance was measured using spectrophotometer at 660nm.The percentage inhibition of protein denaturation was calculated as follows:
Percentage of inhibition = [(Abs Control – Abs Sample) / Abs control)] x100
RESULTS AND DISCUSSION
The result of phytochemical profiling of leaves of P.rajendranii indicates the presence of Alkaloids, Flavonoids, Phenols, Tannins, Glycosides, Carbohydrates, Terpenoids, Quinones, Sterols and Proteins. High concentrations of bioactive compounds were found in methanolic, ethanolic, aqueous, acetone extracts while very low concentrations in chloroform extract. Alkaloids, Flavonoids and Glycosides were mainly seen in most of the samples except chloroform extraction (Table 1).
Table: 1.1 Preliminary phytochemical analyses in Premna rajendranii (leaf)
| Test | Chloroform | Acetone | Ethanol | Methanol | Aqueous | |
| Alkaloids | Wager’s | + | + | ++ | +++ | ++ |
| Hager’s | _ | + | ++ | +++ | ++ | |
| Mayer’s | _ | + | ++ | +++ | + | |
| Flavonoids | Pew’s | + | + | + | ++ | _ |
| Lead Acetate | + | +++ | +++ | +++ | +++ | |
| NaOH | _ | +++ | +++ | +++ | +++ | |
| Con.H2SO4 | + | +++ | +++ | +++ | ++ | |
| Phenol & tannin | FeCl3 | + | + | + | ++ | + |
| K2Cr2O7 | + | + | + | + | + | |
| Lead Acetate | _ | _ | + | ++ | + | |
| Braymers | + | _ | + | + | + | |
| Saponins | Foam | _ | _ | _ | _ | _ |
| NaHCO3 | _ | _ | _ | _ | _ | |
| Glycosides | Keller kiiani | _ | +++ | +++ | +++ | +++ |
| Glycosides | _ | +++ | +++ | +++ | +++ | |
| Molish | _ | ++ | +++ | +++ | ++ | |
| Carbohydrates | Molish | _ | + | ++ | ++ | ++ |
| Benedicts | _ | _ | + | + | _ | |
| Terpenoids | Salkowskis | _ | + | + | ++ | + |
| Quinones | Quinones | _ | _ | _ | ++ | +++ |
| Sterols | Salkowskis | _ | _ | _ | _ | _ |
| Keller kiiani | _ | + | + | ++ | ++ | |
| Protein | Biuret | _ | + | + | + | + |
Diabetes mellitus is one of the major metabolic disorders (Keerthana et al., 2013), and is characterized by high blood glucose levels. According to World Health Organization, 180 million people are currently suffering from DM and the rate is rapidly growing. There are many effective medicines for DM but with serious side effects. Recently researchers are in the experiments to find effective medicine for DM without any side effect. More than 1200 plants have a potential antidiabetic capacity (Modak et. al., 2007; Bailes, 2002; Chitturi and George, 2002; Kesari et. al., 2007,Prabhu et al 2018).
Methanolic leaf extract of P.rajendranii was investigated for in vitro antidiabetic activity with respect to inhibition of α-amylase. The concentrations of samples used for testing the inhibitory activity are 25, 50, 75 and 100 (μg). The inhibition activity of test samples showed optimum activity when compared to commercial drug at different concentrations. The maximum inhibition showed at 100 μg concentration was 79.3% and minimum inhibition showed at 25 μg concentration was 44.82%. IC50 value of sample (288.8) is higher than standard drug (246.7). Acarbose was used as standard drug (Table 2, Fig: 1).
Table 2: Antidiabetic Activity of Methanolic leaf extract of Premna rajendranii
| SL.NO: | Concentration in
(μg) |
% of inhibition | |
| Sample | Standard | ||
| 1 | 25 | 44.82±0.5 | 61.7±0.01 |
| 2 | 50 | 61.37±0.6 | 67.3±0.01 |
| 3 | 75 | 64.82±0.4 | 70.9±0.01 |
| 4 | 100 | 79.3±0.7 | 87.4±0.21 |
| IC50 value | 288.8 | 246.7 | |
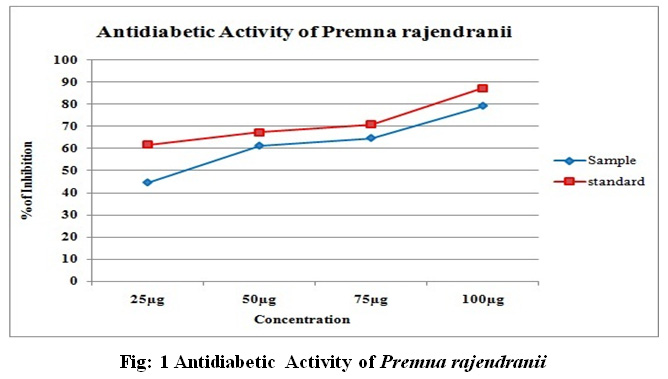 |
Figure 1: Antidiabetic Activity of Premna rajendranii |
Protein denaturation is the major cause of Inflammation (Mizushima and Kobayashi, 1968) and it is a response to negative stimuli including injury, infection (Lumeng and Saltiel, 2011). Basically it was meant to destroy invading microorganisms, inactivate toxins, repairing and healing of injuries which might lead to life threatening hypersensitivity reactions. Inflammation is the major cause for several diseases including neurological, cardiovascular, intestinal, dental and renal disorders and also linked to diabetics, ageing, obesity, multiple sclerosis, pancreatitis, cancer (Sakat et al., 2010; Burns et al., 2001; Kuek et al., 2006; Grivennikov et al.,2010; Jenny,2012; Hoque et al.,2012; Marchant et al., Wyss- Coray et al.,2012). Prescribed medicines for inflammation are mainly non steroidal anti- inflammatory drugs. These drugs have certain side effects causing gastric bleeding, ulceration, renal failure. Medicinal plants have a prolonged history of use as a remedy for inflammation (Insel, 1996; Rang et al., 2007). Premna serratifolia and Premna tomentosa have been reported to possess anti-inflammatory activity (Alam et al., 1993; Habtemariam et al., 2015).
Methanolic leaf extract of P.rajendranii has shown high inhibition than standard at 50µl i.e. 33.88±0.01. The inhibition activity of other test samples (25µl, 100µl, 200µl) showed moderate activity when compared to standard drug. Diclofenac was used as the standard drug.
Table 3: Anti-inflammatory Activity of Premna rajendranii
| SL.NO: | Concentration in (μg) | % of inhibition | |
| Sample | Standard | ||
| 1 | 25 | 6.88±0.02 | 9.79±0.01 |
| 2 | 50 | 33.88±0.01 | 13.54±0.02 |
| 3 | 100 | 34.98±0.03 | 44.86±0.05 |
| 4 | 200 | 36.08±0.1 | 87.78±0.01 |
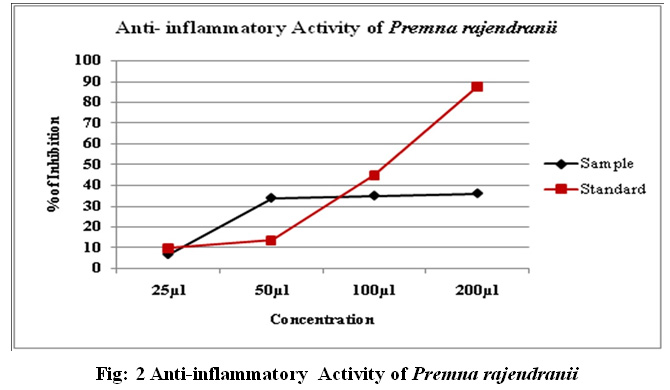 |
Figure 2: Anti-inflammatory Activity of Premna rajendranii |
CONCLUSION
Our study demonstrates that the P.rajendranii acts as an antidiabetic and anti-inflammatory agent. The antidiabetic and anti-inflammatory activities may be due to the presence of various bioactive compounds especially flavonoids. More purification needs to be done and further research on P.rajendranii is necessary for isolating bioactive compounds and their mode of action.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. S. John Britto S.J., Director, The Rapinat Herbarium and Centre for Molecular Systematics, Tiruchirappalli, and Dr. Anand Gideon for valuable support and providing Laboratory facilities.
REFERENCES
Alam, M., Joy, S., Susan, T. and Ali, S.U., (1993). Anti-inflammatory activity of Premna tomentosa Willd. In albino rats. Ancient science of life, 13(1-2), p.185.
Angiosperm Phylogeny Group, (2009). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society, 161(2), pp.105-121.
Bailes, B.K., (2002). Diabetes mellitus and its chronic complications. AORN Journal, 76(2), pp.265-282.
Burns, J., Gardner, P.T., Matthews, D., Duthie, G.G., Lean, J. and Crozier, A., (2001). Extraction of phenolics and changes in antioxidant activity of red wines during vinification. Journal of Agricultural and Food Chemistry, 49(12), pp.5797-5808.
Chitturi, S. and George, J., (2002). Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. In Seminars in liver disease (Vol. 22, No. 02, pp. 169-184). Copyright© 2002 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel.:+ 1 (212) 584-4662.
Francis, S., Gideon, V.A., Britto, S.J., Dessy, V.J., (2019). A Review on Two Endemic Species of Genus Premna and their Conservational Importance, Journal of Drug Delivery and Therapeutics, 9(4- s), pp.666-669.
Grivennikov, S.I., Greten, F.R. and Karin, M., (2010). Immunity, inflammation, and cancer. Cell, 140(6), pp.883-899.
Jenny, N.S., (2012). Inflammation in aging: cause, effect, or both?. Discovery medicine, 13(73), pp.451-460.
Joshi, V.K., Joshi, A. and Dhiman, K.S., (2017). The Ayurvedic Pharmacopoeia of India, development and perspectives. Journal of ethnopharmacology, 197, pp.32-38.
Habtemariam, S. and Varghese, G.K., (2015). A Novel Diterpene Skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5‐methyl‐10‐demethyl‐abietane‐type diterpene from Premna serratifolia. Phytotherapy Research, 29(1), pp.80-85.
Hoque, R., Malik, A., Gorelick, F. and Mehal, W., (2012). The sterile inflammatory response in acute pancreatitis. Pancreas, 41(3), p.353.
Indrianingsih, A.W., Tachibana, S. and Itoh, K., (2015). In vitro evaluation of antioxidant and α-Glucosidase inhibitory assay of several tropical and subtropical plants. Procedia Environmental Sciences, 28, pp.639-648.
Insel, P. A (1996). Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. The Pharmacological Basics of Therapeutics, 9, pp. 617-657.
Keerthana, G., Kalaivani, M.K. and Sumathy, A., (2013). In-vitro alpha amylase inhibitory and anti- oxidant activities of ethanolic leaf extract of Croton bonplandianum. Asian Journal of Pharmaceutical and Clinical Research, 6(4), pp. 32-36.
Kesari, A.N., Kesari, S., Singh, S.K., Gupta, R.K. and Watal, G., (2007). Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. Journal of Ethnopharmacology, 112(2), pp.305-311.
Khandelwal, K.R., (2008). Practical Pharmacognosy Nirali Prakashan. Pune, India, 19, pp.49-70.
Kokate, C.K., Purohit, A.P. and Gokhale, S.B. (2001), Carbohydrate and derived Products, drugs containing glycosides, drugs containing tannins, lipids and protein alkaloids. Text Book of Pharmacognosy, Nirali Prakashan, Pune.
Kuek, A., Hazleman, B.L., Gaston, J.H. and Östör, A.J., (2006). Successful treatment of refractory polyarticular juvenile idiopathic arthritis with rituximab. Rheumatology. 45(11), pp.1448-9.
Kumar, K.M.P., Sunilkumar, T., Sreeraj, V., Thomas, B., Balachandran, I. and Antony, V.T., (2013). A new species of the genus Premna L. (Lamiaceae) from Western Ghats of India. Webbia, 68(2), pp.127-131.
Lumeng, C.N. and Saltiel, A.R., (2011). Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation, 121(6), pp.2111-2117.
Marchant, D.J., Boyd, J.H., Lin, D.C., Granville, D.J., Garmaroudi, F.S. and McManus, B.M., (2012). Inflammation in myocardial diseases. Circulation Research, 110(1), pp.126-144.
Mizushima, Y. and Kobayashi, M., (1968). Interaction of anti‐inflammatory drugs with serum proteins, especially with some biologically active proteins. Journal of Pharmacy and Pharmacology, 20(3), pp.169-173.
Modak, M., Dixit, P., Londhe, J., Ghaskadbi, S. and Devasagayam, T.P.A., (2007). Recent advances in Indian herbal drug research guest editor: Thomas Paul Asir Devasagayam Indian herbs and herbal drugs used for the treatment of diabetes. Journal of clinical Biochemistry and Nutrition, 40(3), pp.163-173.
Peach, K., Tracey, M.V., (1956). Modern methods of plant analysis. Berlin: Springer Verlag
Prabhu, S., Vinodhini, S., Elanchezhiyan, C. and Rajeswari, D., (2018). Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin‐induced diabetic rats. Journal of Diabetes, 10(1), pp.28-42.
Rang, H.P., Dale, M.M., Ritter, J.M., Moore, P.K., (2007). Pharmacology. Elsevier publication, Churchill Livingstone, 6, pp.226-232.
Rekha, K.., Richa, P.K., Babu, S. and Rao, M., (2015). A phytochemistry of the genus Premna: a review. International Journal of Pharmaceutical and Chemical Sciences, 4(3), pp.317-25.
Sakat, S., Juvekar, A.R. and Gambhire, M.N. (2010). In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. International Journal of Pharmacy and Pharmaceutical Science. 2(1), pp.146-155.
Treare, G.E, Evans, W.C. (1985), Pharmacognosy 17th edn. Bahiv Tinal, London, 149.
Wyss-Coray, T. and Rogers, J., (2012). Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine, 2(1), p.a006346.


