Department of Microbiology, Marwadi University, Rajkot-Morbi Road, Rajkot, Gujarat, India.
Corresponding author email: vivek.pattani@gmail.com
Article Publishing History
Received: 18/11/2020
Accepted After Revision: 20/03/2021
Chemical fertilisers have been used intensively in recent years leading to the degradation in the quality of the soil. Diversity of microorganisms is important, as their unique features can be utilized for crop production and environment. Microorganisms are usually inhabited in all parts of the plant from the roots to the shoot and internal regions of the plants. Rhizosphere microbial variety conveys an assortment of microorganisms which offer advantageous properties to the plant environments. In the present study, an attempt has been made for the screening of bacteria for plant growth-promoting activities such as nitrogen fixation, phosphate solubilization and indole acetic acid production. Soil samples were collected from thirty-four different places of districts Junagadh, Gir Somnath, Amreli, Diu, Dwarka and Jamnagar.
Twenty soil samples were from forest region and fourteen soil samples were from the coastal region of Saurashtra. The nitrogen-fixing capability of the isolates was evaluated using Ashby’s media containing bromothymol blue. Total 57 nitrogen-fixing bacteria based on their colony morphology were isolated, of which 49 bacterial isolates were able to solubilize phosphate and 27 were able to produce indole acetic acid. Of 57 bacterial isolates, 23 isolates showed positive results for nitrogen fixation, phosphate solubilization and indole acetic acid production. Nitrogen and phosphorus are one of the major essential macronutrients for plant growth and development. Indole acetic acid serves as one on the plant hormone for growth of plants. The present study indicates 23 bacterial isolates can have the potential for plant growth-promoting bacteria and as a greater number of isolates were from forest region which also indicates the fertility of the soil.
Bacteria, Plant-Growth Promoting, Nitrogen Fixation, Phosphate Solubilization, Indole Acetic Acid.
Kaneriya J. P, Pattani V. B, Joshi K. Screening and Isolation of Plant Growth-Promoting Bacteria from Forest and Coastal Regions of Saurashtra, Gujarat. Biosc.Biotech.Res.Comm. 2021;14(1).
Kaneriya J. P, Pattani V. B, Joshi K. Screening and Isolation of Plant Growth-Promoting Bacteria from Forest and Coastal Regions of Saurashtra, Gujarat. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3rwC8pc“>https://bit.ly/3rwC8pc</a>
Copyright © Kaneriya et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Farmers are currently using chemical fertilisers to intensively supplement the basic nutrients of the soil-based plant system. The advantage of accessibility and intensive use create environmental issues of chemical fertilizers today’s agriculture. The use of chemical fertilizers does however have their advantages and drawbacks in agriculture. Hence, there’s an increasing demand for various ways to support the crop production and to maintain the nutrient within the soil environment for ecological equilibrium in an agroecosystem.
The engagement of microorganism as inoculants or for plant growth-promotion is promising and are widely accepted practices which are been employed in agriculture for the agricultural produce. Symbiotic / non-symbiotic soil bacterium that colonizes root rhizosphere of plant and promotes the expansion in terms of crop yields, (Gouda et al., 2018; Santos et al., 2019, Lebrazi et al., 2020).
Diversity of microorganisms is important, as their unique features can be utilized for crop production and environment (Costa et al., 2018). Variety of microorganism assists with developing the biological system comprises of an organism, soil, and plant. The working of this environment is significantly represented by microbial elements. Microorganisms are usually inhabited in all parts of the plant from the roots to the shoot and internal regions of the plants (Harman and Uphoff, 2019).
In all structures, most of these microorganisms help and raise the plant to live emphatically and offer significant great conditions to the plants. In all structures, the greater part of these organisms helps and elevate the plant to live soundly and offer useful focal points to the plants. Among all these plant growth-promoting bacteria undertake a significant job and are a focal situation in quality and the quantity of yield. Rhizosphere microbial variety conveys an assortment of microorganisms which offer advantageous properties to the plant environments (Thakur et al., 2020).
Plant growth-promoting effect of the PGPB is usually explained by the discharge of metabolites which directly promote the plant growth (Rilling et al., 2019). There are several ways to elucidate the activities of PGPB benefit to the host plant. PGPB have potential to supply plant growth regulators like cytokinins, indole acetic acid (IAA) and gibberellins, enhancing organic process, promote solubilization of inorganic and organic phosphate. The inoculation with PGPB strain like Azotobacter could help to scale back the utilization of nitrogen-based chemical fertilizer (Sharma et al., 2016; Roriz et al., 2020).
Plant growth‒promoting bacteria (PGPB) have been studied as a sustainable alternative to the use of chemical fertilizers to increase crop yields, and effective PGPB have been isolated from diverse plants and soil compartments. Naturally occurring bacteria, commonly found in the soil associated with the roots of plants, positively affect the growth of plants in a number of different ways (Rilling et al., 2019; Glick, 2020). This includes increases in plant yield, nutritional content, tolerance to various abiotic and biotic stresses, and the production of useful secondary metabolites. Due to its topographic state, the Saurashtra region, Gujarat India has a wide variation.
The region has a range that ranges from both forest and coastal areas to wetlands. Saurashtra region has shallow, medium black, calcareous soils with a rainfall range of 400mm to 700mm and dry sub-humid climate. Groundnut, cotton, sesamum, sugarcane, rice, pulses, jowar and bajra are major crops produced in the Saurashtra region (Gondaliya et al., 2017; Ravi and Fulekar, 2018). In the present study, an effort has been made to screen for free-living plant growth-promoting bacteria from the forest and coastal region of Saurashtra.
MATERIAL AND METHODS
Soil samples from Gir forest and Coastal areas of Saurashtra region, Gujarat were collected. Soil samples were collected from thirty-four different places of districts Junagadh, Gir Somnath, Amreli, Diu, Dwarka and Jamnagar from Saurashtra region, Gujarat India. Of the thirty-four soil samples, 20 were from forest region and 14 were from the coastal region of Saurashtra.
The sampling area for the soil was dug to a depth of about 25-30 cm and then collected and transferred to sterile polyethene bags. The soil samples for further use were stored in a refrigerator at low temperatures. For the isolation and screening of nitrogen-fixing bacteria from soil, serial dilution technique and spread plate method using Jenson agar medium was used (Sahoo et al., 2014).
In case of soil samples from coastal regions Jenson agar medium with varying salt concentration of 0.5%, 2%, 4%, 6%, 8% and 10% respectively. Different components of Jenson agar medium were weighed, dissolved in an appropriate amount of water, pH was adjusted and autoclaved at 1210C (15 psi) for 15 minutes. Ten gram of soil sample was suspended in 90 ml of sterilized distilled water blank and kept on a rotary shaker for 30 minutes so that microorganism adhered to the soil particles get dispersed uniformly into the water.
Using serial dilution technique serial dilution were made up to 10-7. From dilution of 10-5 to 10-7, 100 μl was spread on Jenson agar medium plates in triplicates. The spreaded plates were incubated at 30±1ºC till the visible colonies appeared. Individual colonies of different bacterial isolates showing different morphological features were picked up, purified by streaking on solidified Jenson agar medium plates.
The isolated colony of each isolate, colony characters were described according to Microbiology: A Laboratory Manual (Cappuccino and Welsh, 2017). Individual isolated pure colonies were picked up and maintained on Jenson agar slants for further use. They were streaked on freshly prepared nutrient agar plates and incubated for 3 days at 30±1 ºC. Gram’s staining of isolates was done according to the procedure given by (Brown and Heidi, 2015). Cell shape was also recorded. Nitrogen-fixing capability of the isolates was evaluated using Ashby’s media containing bromothymol blue (Hingole and Pathak, 2016).
Plates containing medium were prepared and streaked with different isolates in triplicates. Plates were incubated at 30±1ºC. Isolates fixing nitrogen showed growth on the medium with a change in colour from green to blue. Uninoculated plates in triplicates served as control. Different bacterial isolates were screened for their phosphate solubilizing ability by growing them on Pikovskaya agar medium (Gupta and Pandey, 2019). Fifty microlitres of two days old culture suspension of selected isolates were spotted on the solidified agar medium plates incubated at 30±2ºC for 5-6 days. The plates were examined for the production of a clear zone around the bacterial growth. As a result of acid production, isolates which used tricalcium phosphate developed a clear zone around the colony (Gupta and Pandey, 2019).
The bacterial isolates were screened for their ability to produce IAA, in the absence and presence of tryptophan. The bacterial isolates were inoculated in 5 ml Jensen’s liquid medium incubated at 30±2ºC. Cultures were centrifuged at 3000 rpm for 30 minutes. Two ml of Salkowski’s reagent and two drops of ortho-phosphoric acid was mixed with 2 ml of supernatant. The presence of the pink colour indicated the production of IAA. Further study was performed for ammonia production and other biochemical properties, such as capsule staining, indole analysis, oxidase test and catalase test, in isolates that have shown positive results for nitrogen fixation, phosphate solubilization and indole acetic acid production (James Cappuccino and Welsh, 2017; Gupta and Pandey, 2019).
RESULTS AND DISCUSSION
Screening of nitrogen-fixing bacteria from soil: The accession number given to the isolates were GFS for the isolates obtained from the soil of forest region of Saurashtra and SCS for the coastal region of Saurashtra. Different isolates were isolated based on colony characteristics like morphology, size, and shape. From all the soil samples, about 57 bacterial isolates have been isolated. Twenty-seven isolates were from the forest region and thirty were from the coastal region of Saurashtra. It was observed that out of 57 isolates 34 were Gram-negative coccobacilli,6 were Gram-negative bacilli, 10 were Gram-positive bacilli, 6 were Gram-positive coccobacilli and one was Gram-positive cocci. They were grouped based on Gram reaction and shape of the bacterial cell (Table1). Upon capsule stain of 57 isolates, 30 were capsulated and 27 were non-capsulated.
Nitrogen fixation: Nitrogen is required for the synthesis of amino acids, chlorophyll, nucleic acids, and ATP which are required for the growth and survival of plants (Chakraborty and Tribedi, 2019). All the 57 isolates indicated by growth on Ashby’s medium and turned the greenish colour of the medium to blue (Table 2). The development of blue colour was due to the production of ammonia in the medium making it alkaline (Figure 1). It has been previously observed, that Azospirillum possess high nitrogenase activity allowing for the possibility of using this bacterium as a biofertilizer to improve soil fertility for improved and efficient farming (Richard et al., 2018). However, the confirmatory test of nitrogenase activity using acetylene reduction assay (ARA) needs to be performed to establish their nitrogen-fixing capability (El-Khaled et al., 2020).
Figure 1: Green coloured plate (negative control) and Blue coloured plate which indicated the production of ammonia and nitrogen fixation.
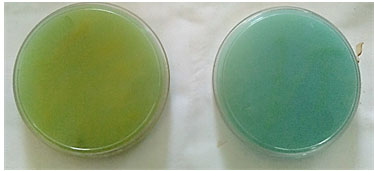
Table 1. Gram’s Stain, Morphology, and presence of capsule of all bacterial Isolates.
| Sr. | Accession no. | Gram’s stain | Morphology | Capsule | Sr. | Accession no. | Gram’s stain | Morphology | Cap-sule | |
| 1 | GFS01C1 | Negative | Cocco Bacilli | Yes | 30 | SCS01C3 | Negative | Bacilli | Yes | |
| 2 | GFS01C2 | Negative | Cocco Bacilli | No | 31 | SCS02C1 | Negative | Bacilli | Yes | |
| 3 | GFS02C1 | Negative | Cocco Bacilli | No | 32 | SCS03C1 | Positive | Cocco Bacilli | Yes | |
| 4 | GFS03C1 | Negative | Cocco Bacilli | Yes | 33 | SCS03C2 | Negative | Cocco Bacilli | No | |
| 5 | GFS04C1 | Negative | Bacilli | Yes | 34 | SCS04C1 | Positive | Bacilli | No | |
| 6 | GFS05C2 | Negative | Cocco Bacilli | Yes | 35 | SCS05C1 | Positive | Cocco Bacilli | No | |
| 7 | GFS05C1 | Negative | Cocco Bacilli | No | 36 | SCS05C2 | Positive | Cocco Bacilli | No | |
| 8 | GFS06C1 | Negative | Cocco Bacilli | Yes | 37 | SCS06C1 | Negative | Cocco Bacilli | No | |
| 9 | GFS07C1 | Positive | Bacilli | Yes | 38 | SCS07C1 | Positive | Bacilli | No | |
| 10 | GFS07C2 | Negative | Cocco Bacilli | No | 39 | SCS07C2 | Negative | Cocco Bacilli | Yes | |
| 11 | GFS08C1 | Negative | Cocco Bacilli | Yes | 40 | SCS07C3 | Negative | Cocco Bacilli | No | |
| 12 | GFS10C1 | Negative | Cocco Bacilli | Yes | 41 | SCS08C1 | Negative | Cocco Bacilli | No | |
| 13 | GFS11C1 | Positive | Bacilli | Yes | 42 | SCS09C1 | Negative | Cocco Bacilli | Yes | |
| 14 | GFS12C1 | Negative | Cocco Bacilli | No | 43 | SCS10C1 | Positive | Bacilli | Yes | |
| 15 | GFS13C1 | Positive | Cocci | No | 44 | SCS11C1 | Negative | Cocco Bacilli | Yes | |
| 16 | GFS13C2 | Negative | Cocco Bacilli | No | 45 | SCS11C2 | Negative | Bacilli | No | |
| 17 | GFS14C1 | Negative | Cocco Bacilli | No | 46 | SCS12C1 | Positive | Bacilli | No | |
| 18 | GFS15C1 | Positive | Bacilli | Yes | 47 | SCS12C2 | Negative | Cocco Bacilli | No | |
| 19 | GFS15C2 | Negative | Cocco Bacilli | Yes | 48 | SCS12C3 | Positive | Bacilli | No | |
| 20 | GFS16C1 | Positive | Bacilli | Yes | 49 | SCS12C4 | Negative | Cocco Bacilli | No | |
| 21 | GFS16C2 | Negative | Cocco Bacilli | Yes | 50 | SCS12C5 | Positive | Cocco Bacilli | Yes | |
| 22 | GFS17C1 | Negative | Cocco Bacilli | No | 51 | SCS13C1 | Negative | Bacilli | No | |
| 23 | GFS18C1 | Negative | Cocco Bacilli | No | 52 | SCS13C2 | Negative | Bacilli | Yes | |
| 24 | GFS18C2 | Negative | Cocco Bacilli | Yes | 53 | SCS13C3 | Positive | Bacilli | Yes | |
| 25 | GFS19C1 | Negative | Cocco Bacilli | Yes | 54 | SCS13C4` | Negative | Cocco Bacilli | Yes | |
| 26 | GFS19C2 | Negative | Cocco Bacilli | No | 55 | SCS13C1 | Negative | Cocco Bacilli | No | |
| 27 | GFS20C1 | Negative | Cocco Bacilli | Yes | 56 | SCS13C2 | Negative | Cocco Bacilli | No | |
| 28 | SCS01C1 | Negative | Cocco Bacilli | Yes | 57 | SCS13C3 | Positive | Cocco Bacilli | Yes | |
| 29 | SCS01C2 | Positive | Cocco Bacilli | Yes |
Phosphate solubilization: Phosphorus is one of the major essential macronutrients for plant growth and development. However, the concentration of soluble P in the soil is very low (Zhu et al., 2011). The use of phosphate solubilizer bacteria as inoculants will increase P intake by plant and cultivation at the same time (Olanrewaju et al., 2017). Of the 57 bacterial isolates, 49 isolates solubilized tri-calcium phosphate as indicated by the production of clearance zone around the bacterial colony on Pikovskaya’s agar medium plates (Table 2).
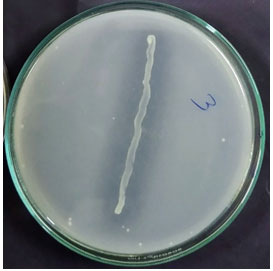
Solubilization of tri-calcium phosphate requires either acid production or chelate formation by the bacterium in the medium. Probably other isolates did not produce acid insufficient amount or chelate to solubilize tri-calcium phosphate in the medium (Figure 2). Several studies showed that PGP bacteria were responsible for solubilizing the insoluble P. It was also reported that excretion of organic acids was one of the most important factors in phosphate solubilization (Hemambika et al., 2013; Alori et al., 2017; Pérez-Rodriguez et al., 2020).
Table 2. Plant Growth Promoting activities of the bacterial isolates.
| Sr. | Accession no. | Nitrogen fixation | Phosphate Solubilization | Indole Acetic Acid | Sr. | Accession no. | Nitrogen fixation | Phosphate Solubilization | Indole Acetic Acid | |
| 1 | GFS01C1 | Positive | Positive | Positive | 30 | SCS01C3 | Positive | Positive | Negative | |
| 2 | GFS01C2 | Positive | Positive | Negative | 31 | SCS02C1 | Positive | Positive | Negative | |
| 3 | GFS02C1 | Positive | Negative | Negative | 32 | SCS03C1 | Positive | Positive | Positive | |
| 4 | GFS03C1 | Positive | Positive | Positive | 33 | SCS03C2 | Positive | Positive | Positive | |
| 5 | GFS04C1 | Positive | Positive | Positive | 34 | SCS04C1 | Positive | Positive | Positive | |
| 6 | GFS05C2 | Positive | Positive | Positive | 35 | SCS05C1 | Positive | Positive | Negative | |
| 7 | GFS05C1 | Positive | Positive | Negative | 36 | SCS05C2 | Positive | Positive | Negative | |
| 8 | GFS06C1 | Positive | Negative | Negative | 37 | SCS06C1 | Positive | Positive | Negative | |
| 9 | GFS07C1 | Positive | Positive | Positive | 38 | SCS07C1 | Positive | Negative | Negative | |
| 10 | GFS07C2 | Positive | Negative | Positive | 39 | SCS07C2 | Positive | Positive | Positive | |
| 11 | GFS08C1 | Positive | Positive | Positive | 40 | SCS07C3 | Positive | Positive | Positive | |
| 12 | GFS10C1 | Positive | Positive | Positive | 41 | SCS08C1 | Positive | Negative | Negative | |
| 13 | GFS11C1 | Positive | Positive | Positive | 42 | SCS09C1 | Positive | Positive | Negative | |
| 14 | GFS12C1 | Positive | Positive | Positive | 43 | SCS10C1 | Positive | Positive | Negative | |
| 15 | GFS13C1 | Positive | Positive | Positive | 44 | SCS11C1 | Positive | Positive | Negative | |
| 16 | GFS13C2 | Positive | Negative | Negative | 45 | SCS11C2 | Positive | Positive | Negative | |
| 17 | GFS14C1 | Positive | Negative | Negative | 46 | SCS12C1 | Positive | Positive | Positive | |
| 18 | GFS15C1 | Positive | Negative | Positive | 47 | SCS12C2 | Positive | Positive | Positive | |
| 19 | GFS15C2 | Positive | Positive | Positive | 48 | SCS12C3 | Positive | Negative | Positive | |
| 20 | GFS16C1 | Positive | Positive | Positive | 49 | SCS12C4 | Positive | Negative | Positive | |
| 21 | GFS16C2 | Positive | Positive | Positive | 50 | SCS12C5 | Positive | Positive | Positive | |
| 22 | GFS17C1 | Positive | Positive | Negative | 51 | SCS13C1 | Positive | Negative | Negative | |
| 23 | GFS18C1 | Positive | Positive | Negative | 52 | SCS13C2 | Positive | Positive | Negative | |
| 24 | GFS18C2 | Positive | Negative | Negative | 53 | SCS13C3 | Positive | Positive | Negative | |
| 25 | GFS19C1 | Positive | Negative | Negative | 54 | SCS13C4 | Positive | Positive | Negative | |
| 26 | GFS19C2 | Positive | Positive | Positive | 55 | SCS13C1 | Positive | Positive | Negative | |
| 27 | GFS20C1 | Positive | Positive | Negative | 56 | SCS13C2 | Positive | Positive | Negative | |
| 28 | SCS01C1 | Positive | Positive | Positive | 57 | SCS13C3 | Positive | Negative | Negative | |
| 29 | SCS01C2 | Positive | Positive | Negative |
Figure 2: Solubilization of phosphate as seen around the bacterial isolate.
Production of Indole Acetic Acid: Out of 57 bacterial isolates 27 produced IAA from tryptophan (Table 2). These broth cultures containing tryptophan showed red colouration on the addition of Salkowski reagent. Indole acetic acid production is characteristic of the production of plant growth promoters.Bacterial IAA contributes to the growth of the lateral and adventitious root lead and triggers the bacterial proliferation of roots by exuding the root in order to increase their absorption of minerals and nutrients (Glick, 2010). In previous studies it has been indicated that IAA-producing rhizobacteria could be harnessed to improve plant growth ( Das et al., 2019; Lebrazi et al., 2020).
Biochemical tests: All the 57 isolates were able to produce ammonia, oxidase and catalase.
Table 3. List of bacterial isolates which showed plant promoting properties nitrogen fixation, phosphate solubilization & indole acetic acid production.
| Sr. | Accession no. | Nitrogen fixation | Phosphate Solubilization | Indole Acetic Acid | Sr. | Accession no. | Nitrogen fixation | Phosphate Solubilization | Indole Acetic Acid | |
| 1 | GFS01C1 | Positive | Positive | Positive | 13 | GFS16C2 | Positive | Positive | Positive | |
| 2 | GFS03C1 | Positive | Positive | Positive | 14 | GFS19C2 | Positive | Positive | Positive | |
| 3 | GFS04C1 | Positive | Positive | Positive | 15 | SCS01C1 | Positive | Positive | Positive | |
| 4 | GFS05C2 | Positive | Positive | Positive | 16 | SCS03C1 | Positive | Positive | Positive | |
| 5 | GFS07C1 | Positive | Positive | Positive | 17 | SCS03C2 | Positive | Positive | Positive | |
| 6 | GFS08C1 | Positive | Positive | Positive | 18 | SCS04C1 | Positive | Positive | Positive | |
| 7 | GFS10C1 | Positive | Positive | Positive | 19 | SCS07C2 | Positive | Positive | Positive | |
| 8 | GFS11C1 | Positive | Positive | Positive | 20 | SCS07C3 | Positive | Positive | Positive | |
| 9 | GFS12C1 | Positive | Positive | Positive | 21 | SCS12C1 | Positive | Positive | Positive | |
| 10 | GFS13C1 | Positive | Positive | Positive | 22 | SCS12C2 | Positive | Positive | Positive | |
| 11 | GFS15C2 | Positive | Positive | Positive | 23 | SCS12C5 | Positive | Positive | Positive | |
| 12 | GFS16C1 | Positive | Positive | Positive |
Isolates tabulated in the Table 3 can act as potential plant growth promoting bacteria. Of the 23 isolates tabulated in Table 3, 14 are from forest region and 9 were from coastal region of the Saurashtra region. These isolates have potential for biofertilizers which can be useful in agricultural practices.
CONCLUSION
In conclusion the present study attempt was made to isolate plant growth promoting bacteria which could be harnessed to improve plant growth. Nitrogen fixing bacteria, and phosphate solubilizing bacteria and Indole acetic acid producing bacteria were isolated. So it can be stated that presence of growth promoting bacteria are responsible for the beneficial effects on plant growth and they can be used as potential biofertilizers. However quantitative analysis of the above parameters can help us to better understand the efficiency of the bacterial isolates.
Conflict of Interest : The authors declare no conflict of interest among themselves.
REFERENCES
Alori, E. T., Glick, B. R. and Babalola, O. O. (2017). Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. 8.
Brown, A. and Heidi, S. (2015). Benson’s Microbiological Applications: Laboratory Manual in General Microbiology, New York, McGraw-Hill Education.
Chakraborty, P. and Tribedi, P. J. F. m. (2019). Functional diversity performs a key role in the isolation of nitrogen-fixing and phosphate-solubilizing bacteria from soil. 64, 461-470.
Costa, O. Y., Raaijmakers, J. M. and Kuramae, E. E. (2018). Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Frontiers in microbiology, 9, 1636.
Das, S., Nurunnabi, T. R., Parveen, R., Mou, A. N., Islam, M. E., Islam, K. M. D. and Rahman, S. J. I. J. C. M. A. S. (2019). Isolation and Characterization of Indole Acetic Acid Producing Bacteria from Rhizosphere Soil and their Effect on Seed Germination. 8, 1237-1245.
El-Khaled, Y. C., Roth, F., Radecker, N., Kharbatia, N. M., Jones, B., Voolstra, C. R. and Wild, C. (2020). Simultaneous measurements of dinitrogen fixation and denitrification associated with coral reef substrates: advantages and limitations of a combined acetylene assay.
Glick, B. R. (2010). Using soil bacteria to facilitate phytoremediation. Biotechnology Advances, 28, 367-374.
Glick, B. R. (2020). Introduction to plant growth-promoting bacteria. Beneficial plant-bacterial interactions. Springer,1-37.
Gondaliya, V., Bansal, R. K. and Shaikh, A. (2017). Diversification of agricultural crops to adapt to climate change: A case study of Gujarat. Indian Journal of Economics Development, 13, 174-180.
Gouda, S., Kerry, R. G., Das, G., Paramithiotis, S., Shin, H.-S. and Patra, J. K. (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological research, 206, 131-140.
Gupta, S. and Pandey, S. (2019). ACC Deaminase Producing Bacteria With Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Frontiers in Microbiology, 10.
Harman, G. E. and Uphoff, N. (2019). Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica, 2019.
Hemambika, B., Balasubramanian, V., Rajesh Kannan, V. and Arthur James, R. (2013). Screening of Chromium-Resistant Bacteria for Plant Growth-Promoting Activities. Soil and Sediment Contamination: An International Journal, 22, 717-736.
Hingole, S. S. and Pathak, A. P. (2016). Isolation of halotolerant Plant growth promoting Klebsiella pneumoniae from Tuppa, Nanded, Maharashtra. International Journal of Innovative Biological Research, 5, 6.
James Cappuccino and Welsh, C. (2017). Microbiology: A Laboratory Manual, Pearson Education.
Lebrazi, S., Fadil, M., Chraibi, M., Fikri-Benbrahim, K. J. J. o. G. E. and Biotechnology (2020). Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. 18, 1-10.
Olanrewaju, O. S., Glick, B. R. and Babalola, O. O. (2017). Mechanisms of action of plant growth promoting bacteria. World Journal of Microbiology and Biotechnology, 33, 197.
Pérez-Rodriguez, M. M., Piccoli, P., Anzuay, M. S., Baraldi, R., Neri, L., Taurian, T., Lobato Ureche, M. A., Segura, D. M. and Cohen, A. C. (2020). Native bacteria isolated from roots and rhizosphere of Solanum lycopersicum L. increase tomato seedling growth under a reduced fertilization regime. Scientific Reports, 10, 15642.
Ravi, R. K. and Fulekar, M. (2018). A review on seasonal agriculture pattern and agrochemicals utilisation in different regions of Gujarat state, India. International Journal of Biology Research, 3, 158-163.
Richard, P. O., Adekanmbi, A. O. and Ogunjobi, A. A. J. A. J. o. M. R. (2018). Screening of bacteria isolated from the rhizosphere of maize plant (Zea mays L.) for ammonia production and nitrogen fixation. 12, 829-834.
Rilling, J., Acuña, J., Nannipieri, P., Cassan, F., Maruyama, F. and Jorquera, M. (2019). Current opinion and perspectives on the methods for tracking and monitoring plant growth‒promoting bacteria. Soil Biology Biochemistry, 130, 205-219.
Roriz, M., Carvalho, S. M., Castro, P. M. and Vasconcelos, M. W. (2020). Legume Biofortification and the Role of Plant Growth-Promoting Bacteria in a Sustainable Agricultural Era. Agronomy, 10, 435.
Sahoo, R. K., Ansari, M. W., Dangar, T. K., Mohanty, S. and Tuteja, N. (2014). Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma, 251, 511-523.
Santos, M. S., Nogueira, M. A. and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express, 9, 205.
Sharma, P., Kumawat, K. and Kaur, S. (2016). Plant Growth Promoting Rhizobacteria in Nutrient Enrichment: Current Perspectives. Biofortification of Food Crops. Springer,263-289.
Thakur, N., Kaur, S., Tomar, P., Thakur, S. and Yadav, A. N. (2020). Microbial biopesticides: current status and advancement for sustainable agriculture and environment. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier,243-282.
Zhu, F., Qu, L., Hong, X. and Sun, X. (2011). Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evidence-Based Complementary Alternative Medicine, 2011.


