1Biochemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia,
2Food Irradiation Research Department, National Centre for Radiation
Research and Technology, Atomic Energy Authority, Cairo, Egypt.
Corresponding author email: aishahghazwani@gmail.com
Article Publishing History
Received: 31/12/2021
Accepted After Revision: 24/02/2022
Diabetes mellitus is one of the most challenging metabolic pandemics that affect essential biochemical pathways in the body. The cost of Diabetes mellitus treatment and its side effects may call for an investigation on plant products as sources of treatment. Whereas, traditional medicine has proven that treatment with plant extracts is affordable, effective, and may have fewer negative effects than modern medicines. The current study aimed to investigate the renoprotective effects of senna (Cassia angustifolia) and fennel (Feoniculum vulgar) against streptozotocin-induced diabetes in albino rats. Male albino rats were used as animal models which divided into five groups, normal group (control), diabetic group (single i.p. 60 mg/kg of streptozotocin, (STZ), and 3 diabetic groups that were treated with aqueous extract of (senna (150mg/kg/day), or fennel (150mg/kg/day) and their combination) by gastric intubation for 4 weeks.
Diabetic rats exhibited a highly significant increase in the levels of blood glucose, renal enzymes and renal weight. Also, a significant increase in nitric oxide (NO), thiobarbituricacid reactive substances (TBARS) and xanthine oxidase (XO) accompanied by a significant decrease in vitamin C, catalase (CAT) and reduced glutathione (GSH) in renal tissues as compared to control group. In conclusion oral administration of senna and/or fennel extract reduces oxidative stress in renal tissue by lowering blood glucose levels, increasing plasma insulin, and restoring weight loss and levels of renal enzymes in diabetic rats. The present investigation suggested that the treatment with mix of (senna and fennel) exhibited antidiabetic activity, and had renoprotective effects in streptozotocin-induced diabetes rats.
Antioxidant, Diabetes, Senna, Fennel, Renoprotective.
Osman N. N, Al-Ahmadi A. M, Ghazwani A. H, Alhoraibi H. M, Alanbari K. H, Backer W. S. Role of Senna, Cassia angustifolia and Fennel, Foeniculum vulgare in Ameliorating Nephropathy in Diabetic Rats. Biosc.Biotech.Res.Comm. 2022;15(1).
Osman N. N, Al-Ahmadi A. M, Ghazwani A. H, Alhoraibi H. M, Alanbari K. H, Backer W. S. Role of Senna, Cassia angustifolia and Fennel, Foeniculum vulgare in Ameliorating Nephropathy in Diabetic Rats. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3N3YOIG“>https://bit.ly/3N3YOIG</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Diabetes Mellitus (DM) is one of the world’s fastest-growing health issues, which in some countries is now reaching epidemic proportions. One of the most severe and daunting health issues in the 21st century is the increasingly growing prevalence of DM worldwide. It is largely attributed to the lack of exercise, unhealthy diet, obesity and overweight as a result of life style. Over the last few decades, changing lifestyles in KSA have led significantly to the growing prevalence of DM and other chronic diseases. In many diabetes populations, the combination of rising diabetes prevalence and increasing lifespans will lead to an evolving range of types of morbidity associated with diabetes.
To date, among the diseases that must be given more and more attention is diabetes, where the statistics and predictions show worrying data. Saeedi et al. (2019) reported that the prevalence of diabetes is estimated at 9.3 % (463 million individuals) at the global level, rising to 10.2 % (578 million) by 2030 and 10.9 % (700 million) by 2045. Besides, about 1.6 million deaths are directly attributed to diabetes each year (Wong et al., 2013; Alnuaim, 2014; Naeem, 2015; WHO, 2020).
One of the features of diabetes is hyperglycemia. Chronic hyperglycemia damages nearly all types of cells in the body. A relationship has been identified between hyperglycemia, oxidative stress and various pathways that can lead to organ and tissue damage. Moreover, complications associated with hyperglycemia include stroke, nerve damage, poor vision, heart attack and renal disease. According to the Center for Control Disease and Prevention (CDC) over a third (37%) of adults diagnosed with diabetes had chronic renal disease; and fewer than (25%) with moderate to severe chronic renal disease. The renal disease of diabetes is referred to as diabetic nephropathy. It is results when diabetes damages blood vessels and other cells in the renal tissues. Over time, severe damage to these blood vessels can lead to renal disfunctionand kidney failure (Piero et al., 2015; Jangid et al., 2017; NIDDK, 2017; Giri et al., 2018; Clinic, 2019; WHO, 2020; CDC, 2020).
There are a variety of oral hypoglycemic agents are available on the market, but they can lead to a high risk of secondary failure in the long term. Therefore, safe treatment is a demand to prevent diabetes complications with minimal side effects. For several years, plant-based drugs or herbal medicines have been prescribed by conventional medical practitioners to treat different disease conditions as it is low cost and has fewer side effects. In the treatment and control of diabetes, numerous herbal medicines have proved their potential. Sweet potatoes, basil, fenugreek, turmeric, okra and bitter gourd are classified as herbal plants used in diabetes management. Moreover, senna (Cassia angustifolia) and fennel (Foeniculumvulgare) are reported to possess hypoglycemic, antioxidant and anti-inflammatory effects. Therefore, both plants have the capacity to alleviate diabetes and its related effects (Jamshidi-Kia et al., 2018; Sudhakar et al., 2020; Jani and Goswami, 2020; Rohman and Putra, 2020; Farid et al., 2020; Patle et al., 2020).
A study into the ameliorative potential of these plants on diabetes patients’ nephropathy could lead to design a safer and more effective drug. So, the current study was planned to evaluate the nephro-protective potential of both senna and fennel water extracts in streptozotocin-induced diabetes in albino rats.
MATERIAL AND METHODS
Chemicals and preparation of the herbal extracts: Streptozotocin (STZ) was obtained from Saint Louis, in Missouri, USA.The leaves of senna (Cassia angustifolia) and seeds fennel (Foeniculum vulgare) were purchased from the local traditional market in Jeddah, Saudi Arabia. The senna leaves and fennel seeds were ground with the help of a grinder and 100g of this powder was dissolved by bringing to a boil for 30 min with 400 ml of distilled water. The mixture was filtered and centrifuged at 3500 rpm for 20 min. The supernatant was stored at 4ºC prior to its drying, till usage.
Induction of Diabetes in Experimental Animals: Male albino rats (n=50) weighing about (150 to 200 g) were obtained from the Animal Experimental Unit of King Fahd Centre for Medical Research, King Abdul-Aziz University. The animals were kept in plastic cages and were fed with tap water and rat chow ad libitum in a stable environment (temperature 28 ± 2ºC, humidity 60 ± 5%) with 12-hour light and dark cycle. Animal procedures were carried out according to the instruction of the Ethics Committee, King Fahad Medical Research Centre which were in compliance with the international guidelines for proper use and care of laboratory animals.
A single intraperitoneal injection of 60 mg/kg BW of streptozotocin (STZ) was used to cause diabetes in rats(Akbarzadeh et al., 2007). STZ was freshly prepared by dissolving it in citrate buffer (0.5M, pH 4.5). Three days after STZ injection, fasting blood glucose (FBG) level was measured by using OneTouch Select Analyzer (Life Scan, Inc., UK) and rats having FGB level less than 200 mg/dL were discarded from the study.
Experimental Design: Fifty animals were randomly divided into five groups of ten rats each as details in Table (1).
Table 1. Test Animal Treatment Groups
| Groups | Treatment |
| Group 1
(Control) |
Citrate buffer (0.01M, pH 4.5) |
| Group 2
Diabetes (Dia) |
STZ (60 mg/kg Body Weight (BW), Intraperitoneal (i.p)). |
| Group 3
(Dia + Senna) |
STZ (60 mg/kg BW i.p) + Senna aqueous extract (150 mg/kg BW/ day)(Shanmugasundaram et al., 2011). |
| Group 4
(Dia + Fennel) |
STZ (60 mg/kg BW i.p) + Fennel aqueous extract (150 mg/kg BW/ day) (Sadrefozalayi and Farokhi, 2014). |
| Group 5
(Dia +Mix (Senna+Fennel)) |
STZ (60 mg/kg BW i.p) + aqueous extract of mixture from Senna (150 mg/kgBW/ day) and Fennel (150 mg/kgBW/day). |
Each aqueous extract was given by oral gavage for a period of 30 days.
At the end of the experimental period (4 weeks), rats fasted overnight before scarification. Blood samples were taken from the retro-orbital plexus of each anesthetized rat, then centrifuged at 3000 rpm for 10 minutes to separate serum. Immediately after taking a blood sample, the animals were sacrificed; the kidneys of each animal were removed, then homogenized with 0.1 M cold phosphate buffer (pH 7.4) and centrifuged at 10,000 g for 15 minutes. The supernatant was used for biochemical evaluation.
Biochemical analysis: Blood glucose levels were measured by enzymatic kits according to the protocol of Kunst (1984). Serum insulin levels were determined by solid phase enzyme-linked immune-sorbent assay using Immunospec Insulin Quantitative Test Kit (model E29-88). Determination of the creatinine and blood urea nitrogen (BUN)were measured as described by Burtis and Ashwood (2001). Uric acid was measured according to the methods of Kalekar (1947). The total protein(TP)was measured as described by Henry et al. (1957). Thiobarbituric acid reactive substances (TBARS) was used to measure lipid peroxidation and was determined according to the method of Ohkawa et al. (1979). Xanthine oxidase (XO) activity was determined using the method of Bergmeyer et al. (1974). Nitrite assay was measured as an end product for knowing nitric oxide (NO) concentration according to the method of Miranda et al. (2001). The activity of catalase(CAT) was determined using the method of Aebi (1984). The content of reduced glutathione (GSH) was measured by following the protocol of Beutler et al. (1963). Ascorbic acid (vitamin C) was determined by using commercial kits (CAT NO. AS2516).
Statistical analysis: Analysis of data was done by Statistics Package for Social Sciences (SPSS) version 20. The data was expressed as arithmetic mean and standard deviation of the mean (SD). One-way analysis of variance (ANOVA), least significant difference (LSD) equation for parametric parameters, was used to analyze the differences between groups. A p-value less than or equal 0.05 was considered significant.
RESULTS AND DISCUSSION
The mean body weight and kidneys weight of all groups were shown in Table (2). The comparisons between groups indicated that at the beginning of the experiment (zero-day), the mean values of body weight for all groups were matched to the mean value in the normal group. The data of the present study showed a significant decrease (P < 0.00) in the bodyweight of diabetic rats during the experimental period when compared to the control group. While diabetic rats treated with either senna leaves extract (150mg/kg BW), fennel seed extract (150 mg/kg BW), or their mixture gained significant weight (P < 0.00) compared to diabetic untreated rats, the effect of the mixture of both senna and fennel was more efficient than each of them only.
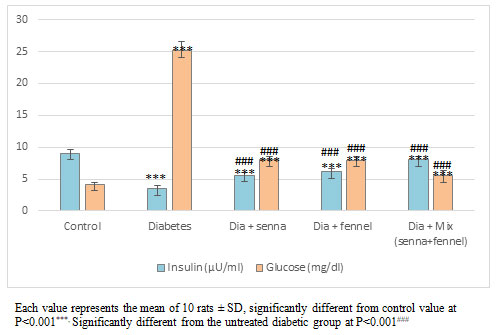
The percentage change in the body in control, diabetic, senna and/or fennel weights were 33%, -4%, 14%, 12%and 22%, respectively. In kidneys weight, the statistical analysis indicated significant variations in the mean values of the right kidney and left kidney between groups. Treatment of diabetic rats with either senna and /or fennel exhibits a significant improvement of the organ weight, although this improvement did not reach the mean values in the control group. The levels of blood glucose and insulin of normal and experimental rats are shown in Figure (1). There was a significant elevation (p=0.000) in blood glucose accompanied by a significant decreased (p=0.000) in plasma insulin in diabetic rats compared with normal rats.
Administration of senna or fennel tends to bring the parameters significantly towards the normal. The effect of the mixture of both senna and fennel was more efficient than each of them only. The Post Hoc analysis using the LSD test exhibits no significant change (p=0.912 and 0.10) between the diabetic group treated with senna and the diabetic group treated with fennel in levels of blood glucose and plasma insulin, respectively.
Table 2. Effect of aqueous extracts of senna or/ and Fennel on body and kidneys weight in diabetic rats.
| Initial weight (g) |
Final weight (g) |
Left kidney (g) |
Right kidney (g) |
|
| Control | 206.4± 6.96 | 275.2± 4.44 | 0.546± 0.093 | 0.556± 0.055 |
| Diabetes | 204.0± 6.18 | 196.1± 5.09*** | 0.957± 0.129*** | 0.996± 0.145*** |
| Dia + Senna | 200.1± 5.97 | 231.7± 4.64*** ### | 0.764± 0.061***## | 0.786± 0.077***## |
| Dia + Fennel | 205.4± 7.66 | 232.9± 4.28*** ### | 0.757± 0.062***### | 0.772± 0.057***## |
| Dia + Mix (Senna+Fennel) |
201.2± 9.41 | 257.4± 2.41*** ### | 0.639± 0.060*### | 0.695± 0.041***### |
Each value represents the mean of 10 rats ± SD.
Significantly different from control value at P<0.05*, 0.001***
Significantly different from the untreated diabetic group at P<0.01## ,0.001###
Figure 1: Effect of aqueous extracts of Senna or/ and Fennel on glucose and insulin in diabetic rats.
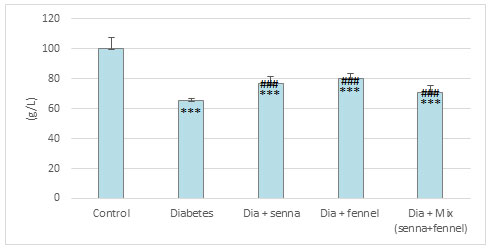
The results illustrated in Figures (2-5) revealed that STZ resulted in a significant (p<0.00) raise in the level of creatinine, uric acid and BUN accompanied by significant (p< 0.00) decline in the level of total protein in diabetic rats in comparison with normal control. The prolonged administration of diabetic rats with aqueous extract of senna and/or fennel for 30 consecutive days showed a significant (p< 0.00) decreased in the creatinine, uric acid, and BUN and significant (p<0.00) increased in TP when compared with untreated diabetic rats. The statistical analysis exhibits no significant change (p=.218 and 0.105) between the diabetic group treated with senna and the diabetic group treated with fennel in levels of BUN, TP respectively. The changes in the levels of nitric oxide and TBARS, and the activity of xanthine oxidase in renal tissues in control and experimental rats are presented in Figures (6-8).
There was a significant elevation (p=.000) in TBARS, nitric oxide and xanthine oxidase in renal tissues in diabetic rats when compared to the control group. The aqueous extracts of senna and fennel offered significant protection against alteration in the oxidative biomarkers of diabetic rats. However, the administration of aqueous extracts of the mixture of senna and fennel was more effective than senna or fennel only. No significant changes in renal TBRAS, nitric oxide and xanthine oxidase between the diabetic groups treated with either senna or fennel.
Figure 2: The levels of Creatinine in diabetic rats treated with aqueous extracts of Senna or/ and Fennel.
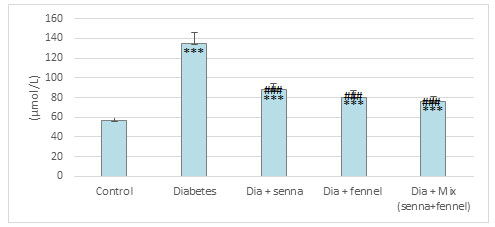
Figure 3:The level of UricAcid in diabetic rats treated with aqueous extracts of Senna or/ and Fennel.
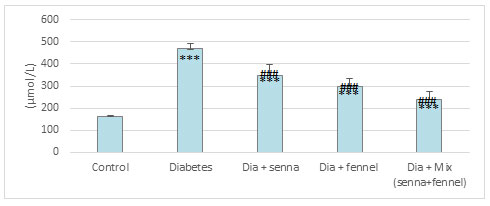
Figure 4:The levels of Blood Urea Nitrogen (BUN) in diabetic rats treated with aqueous extracts of Senna or/ and Fennel.
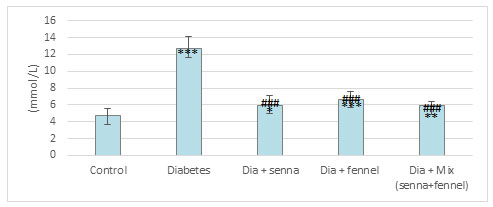
Figure 5: The levels of Total protein (TP) in diabetic rats treated with aqueous extracts of Senna or/ and Fennel.
Each value represents the mean of 10 rats ± SD, Significantly different from control value at
P<0.05* , 0.001***, Significantly different from the untreated diabetic group at P<0.001###
Figure 6: The effect of aqueous extracts of Senna or/ and Fennel on renal tissues Nitric Oxide in diabetic rats
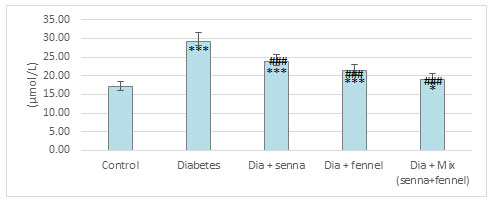
Figure 7: The effect of aqueous extracts of Senna or/ and Fennel on renal tissues TBRAS in diabetic rats
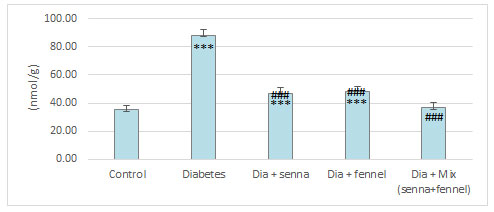
Figure 8: The effect of aqueous extract of Senna or/ and Fennel on renal tissues Xanthine Oxidase in diabetic rats
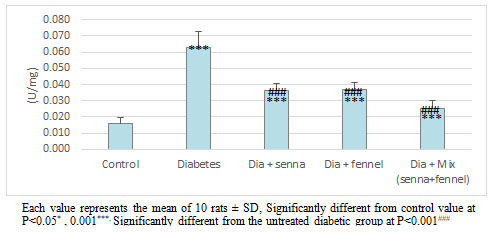
Figures 9-11 clearly illustrate the effect of senna and fennel on the antioxidant enzymes. A marked reduction was noted in the level of non-enzymatic antioxidants such as reduced glutathione, vitamin C, and the activity of enzymatic antioxidant (catalase) in the renal tissue of STZ induced diabetic rats when compared with normal rats. Administration senna and fennel or their mixture for the 30 days to STZ induced diabetic rats increased significantly (p < 0.000) the renal vitamin C, GSH levels and the activity of CATas compared with untreated diabetic rats. Moreover, the results exhibit no significant differences in renal (catalase, glutathione) between the diabetic group treated with senna and the diabetic group treated with fennel.
Figure 9:The effect of aqueous extracts of Senna or/ and Fennel onVitamin C in renal tissues of diabetic rats.
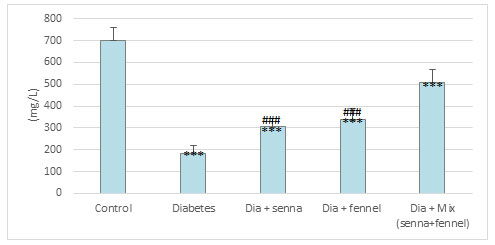
Figure 10: The effect of aqueous extracts of Senna or/ and Fennel on Catalase in renal tissues of diabetic rats.
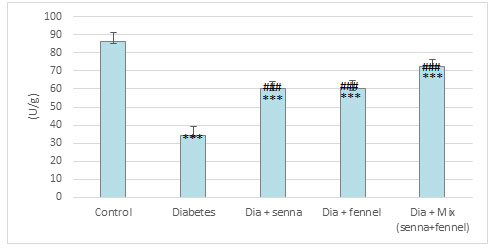
Figure 11: The effect of aqueous extracts of Senna or/ and Fennel on Glutathione in renal tissues of diabetic rats.
Despite the abundance of the market with antidiabetic drugs, the ambition still to reach natural products that can use as treatment with the least potential for side effects is an important matter. This has led to an attempt to apply the research on herbs that may be used to produce natural medicine which reduce suffering and potential fears. Therefore, an examination was made to evaluate the antidiabetic effects of the extracts of senna and fennel in diabetic rats and also study their renal protective effects. The data of the present study, showed a significantly decreased in the body weight of diabetic rats during the experimental period when compared to the control group.
The current results are consistent with the findings of Sureka et al. (2021) and Kodidela et al. (2020). This may be due to increased use of protein and fat for energy production, as diabetic rat cells may not be able to use glucose for energy production due to decreased insulin action or secretion. In addition, increased protein catabolism to provide amino acids for gluconeogenesis leads to muscle waste and weight loss(Srinivasan et al., 2014).The diabetic model rats of this study showed ameliorative in body weights after being treated with senna and/or fennel compared to the respective diabetic rat’s untreated group. This result might point out the effectiveness of the senna and fennel by their ability to enhance insulin action (Osman et al., 2017).
Moreover, the data of the present study, showed an increase in the weight of the kidney (hypertrophy) in STZ-treated animals when compared with the normal group, which might be due to autophagy failure in the kidney, where Ma et al. (2020) found that autophagy deficiency in kidney tubules led to exaggerated renal hypertrophy in STZ-induced diabetic mice. In this study, diabetes was confirmed by the disparity levels of glucose and insulin, where STZ-diabetic rats showed high levels of glucose and low levels of insulin. These levels were similar to the findings of Zhang et al. (2020) and Poitout and Robertson (2002), where they demonstrated that STZ causes pancreatic cell defects and reduces the cells’ sensitivity to insulin-triggered glucose uptake, resulting in elevated blood glucose levels. Treating diabetic rats with senna or/and fennel extract significantly reduced blood glucose levels.
Mukhtar et al. (2020), reported that diabetic rats treated with 250 mg/Kg BW of aqueous leaf extract of senna for five days showed a significant reduction in blood glucose level. Likewise, El-Ouady et al. (2020) reported that the leaves aqueous extract of fennel at a dose of 10 mg/kg BW reduced blood glucose levels in (STZ)-induced diabetic rats. This decrease in blood glucose levels is attributed to the chemical contents in senna and fennel, which contain compounds that have an antidiabetic activity such as flavonoids. Flavonoids are reported to be able to regenerate damaged pancreatic β cells so that insulin deficiency can be overcome (Chotimah et al., 2008; Alqethami and Aldhebiani, 2021; Lindawati et al., 2021).
Consequently, blood glucose levels will be kept within a safe range as long as the pancreas produces enough insulin (NIDDK, 2018). Diabetes is one of the biggest factors that increases risk for kidney disease and is the number one cause of kidney failure (NIDDK, 2017). Renal damage is evident with a decrease in total protein and an increase in creatinine, uric acid and BUN. Similar structural changes were found in the present study. These results were consistent with Dabdoub et al. (2020).The kidneys remove metabolic wastes such as urea, uric acid, creatinine, and ions in order to maintain the optimum chemical composition of the body fluids (Kishore et al., 2017; Liu et al., 2018; Elkomy et al., 2020). In the case of renal tissue’s diseases, the accumulation of those metabolites in the blood may be due to a decrease in their filtering or clearance by kidneys.
In this study, the renal damage may be due to that elevated blood pressure, where Ajayi et al., (2021) proved that there is a positive relationship between STZ-induced diabetes and high blood pressure. High blood pressure reduces the blood supply to the renal tissue through constriction and narrowing of blood vessels, which eventually damages and weakens them. If kidneys’ blood vessels are damaged, they may no longer work properly. As a result, the kidneys may stop removing wastes and extra fluid from the blood (NIDDK, 2020).
The daily administration of aqueous extract of senna and fennel for 30 days caused a significant reduction in creatinine, uric acid and BUN, as well as a significant elevation in serum total protein levels in diabetic rats when compared to the diabetic untreated group. This improvement may be attributed to the components of these herbs, where senna and fennel rich in potassium, calcium and magnesium, which are known as key minerals to help control blood pressure (Basak and Reddy, 2017; HHP, 2019; Mehra et al., 2021b).The results of the current study also showed that diabetic rats resulted in an increase in oxidative stress (NO, TBARS and XO) and lowered levels of antioxidants (GSH, vitamin C, and CAT) in tissues of kidneys.
These results have coincided with the results of Samadi et al., (2021)who found that STZ disrupted the oxidative balance in the renal tissue that was proved by the increasing marker for oxidative stress and decreasing the total antioxidant status which was an assessment by Total antioxidant capacity content. Oxidative stress results from the overproduction of reactive oxygen species (ROS) plays a critical role in the pathogenesis of diabetic complications including renal diseases. Under normal circumstances, the body’s antioxidant system can swiftly eliminate ROS, but in pathological conditions like diabetes, the excess ROS produced in the body exceeds the antioxidant system’s scavenging ability (Pal et al., 2020; Charlton et al., 2021).
Indeed, diabetes triggers oxidative stress through the production of free radicals via glucose auto-oxidation, protein glycosylation and polyol pathways (Obrosova et al., 2002). Moreover, the increase in oxidative stress due to glucose auto-oxidation may possibly inactivate or weakens the activities of enzymatic and non-enzymatic antioxidants (Giugliano et al., 1996, Saddala et al., 2013), and consequently reduces ROS clearance. Many medicinal plants are reported to possess antioxidant properties and protects from complications of diabetes. In congruence with this statement, our study showed that both the aqueous extract of senna and fennel marked significant protection by increasing the antioxidants and reducing oxidative stress.
These results have coincided with Osman et al. (2017), who found that treatment streptozotocin-induced diabetic rats with senna and/or fennel extracts lead to lowering oxidative stress evidenced by the restoration of the enzymatic antioxidative of defense system (SOD, CAT and GSH). The antioxidant scavenging activity result suggests the presence of phytochemicals with antioxidant properties in the extracts of senna and fennel. Previous studies revealed the presence of various bioactive phyto- constituents in senna and fennel plants. Both senna and fennel contain polyphenols like phenolic acids, flavonoids and tannins which are familiar to be liable for the free radical-scavenging and antioxidant activities. These polyphenols’ biological actions are thought to be owing to their redox characteristics, which can aid in the absorption and neutralization of free radicals, the quenching of singlet and triplet oxygen, and the decomposition of peroxides (Guarize et al., 2012; Daskum et al., 2020, Farag et al., 2020; Mehra et al., 2021a).
CONCLUSION
Our results suggested that the treatment with extracts of leaves of senna and the seeds of fennel exhibited significant antidiabetic and antioxidant activities in STZ-induced diabetes in albino rats. Both are attenuators of diabetes-induced alterations in body weight, blood glucose and insulin levels. Moreover, these extracts have protective effects in ameliorating diabetic nephropathy through lowering levels of abnormal renal enzymes and promoting renal antioxidants to overcome complications of the oxidative stress in them. In addition, this study supports the idea that antioxidants from several sources together could provide synergistic benefits.
Disclosure statement: No potential conflict of interest was reported by the authors.
Conflict of Interest: There is no conflict of interest
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Aebi, H. (1984). Catalase in vitro. Methods in enzymology, 105, 121-126.
Ajayi, A. M., Adedapo, A. D., Badaki, V. B., Oyagbemi, A. A. and Adedapo, A. A. (2021). Chrysophyllum albidum fruit ethanol extract ameliorates hyperglycaemia and elevated blood pressure in streptozotocin-induced diabetic rats through modulation of oxidative stress, NF-κB and PPAR-γ. Biomedicine & Pharmacotherapy, 141, 111879.
Akbarzadeh, A., Norouzian, D., Mehrabi, M., Jamshidi, S., Farhangi, A., Verdi, A. A., Mofidian, S. and Rad, B. L. (2007). Induction of diabetes by streptozotocin in rats. Indian Journal of Clinical Biochemistry, 22, 60-64.
Alnuaim, A. (2014). Rising prevalence of diabetes mellitus in saudi arabia: cause for concern and call for urgent control program. King Faisal Specialist Hospital & Research Centre.
Alqethami, A. and Aldhebiani, A. Y. (2021). Medicinal plants used in Jeddah, Saudi Arabia: phytochemical screening. Saudi Journal of Biological Sciences, 28, 805-812.
Basak, B. and Reddy, R. N. (2017). Elemental analysis of different cultivar of Senna (Cassia angustifolia) using microwave digestion and flame atomic absorption spectroscopy. National Academy Science Letters, 40, 245-248.
Bergmeyer, H. U., Gawehn, K. and Grassl, M. (1974). Enzymes as biochemical reagents. Methods ofenzymatic analysis, New York, Academic Press.
Beutler, E., Duron, O. and Kelly, B. M. (1963). Improved method for the determination of blood glutathione. The Journal of laboratory and clinical medicine, 61, 882-888.
Burtis, C. A. and Ashwood, E. R. (2001). Tietz Fundamentals of Clinical Chemistry, 5th ed, Philadelphia.
CDC (2020). National diabetes statistics report, (2020). Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services.
Charlton, A., Garzarella, J., Jandeleit-Dahm, K. A. and Jha, J. C. (2021). Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology, 10, 1-18.
Chotimah, C., Sutrisna, E. and Wahyuni, A. S. (2008). Uji penurunan kadar glukosa darah oleh ekstrak air herba jaka tuwa (Scoparia dulcis L.) pada kelinci jantan yang dibebani glukosa. Pharmacon, 9, 46-51.
Clinic, M. (2019). Diabetic nephropathy [Online]. Rochester: Mayo Clinic. [Accessed 17 June 2021].
Dabdoub, B. R., Mohammed, R. H. and Abdulhadi, H. L. (2020). Ganoderma lucidum attenuates and prevents CCl4-induced hepatic and renal damage in Sprague–Dawley Rats. Systematic Reviews in Pharmacy, 11, 1704-1709.
Daskum, A., Chessed, G., Qadeer, M. and Ling, L. (2020). Phytochemical screening, Gas Chromatography Mass Spectroscopy (GC-MS) and in vitro antiplasmodial analysis of Senna siamea leaves as antimalarial, Yobe State, Nigeria. Nigerian Journal of Parasitology, 41, 60-67.
El-Ouady, F., Lahrach, N., Ajebli, M., Haidani, A. E. and Eddouks, M. (2020). Antihyperglycemic effect of the aqueous extract of Foeniculum vulgare in normal and streptozotocin-induced diabetic rats. Cardiovascular & Haematological Disorders-Drug Targets (Formerly Current Drug Targets-Cardiovascular & Hematological Disorders), 20, 54-63.
Elkomy, A., Abdelhiee, E. Y., Fadl, S. E., Emam, M. A., Gad, F. A.-M., Sallam, A., Alarifi, S., Abdel-Daim, M. M. and Aboubakr, M. (2020). L-carnitine mitigates oxidative stress and disorganization of cytoskeleton intermediate filaments in cisplatin-induced hepato-renal toxicity in rats. Frontiers in Pharmacology, 11, 1-12.
Farag, R. S., Abdel-Latif, M. S., Abd El Baky, H. H. and Tawfeek, L. S. (2020). Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnology Reports, 28, e00536.
Farid, A., Kamel, D., Abdelwahab Montaser, S., Mohamed Ahmed, M., El Amir, M. and El Amir, A. (2020). Synergetic role of senna and fennel extracts as antioxidant, anti-inflammatory and anti-mutagenic agents in irradiated human blood lymphocyte cultures. Journal of Radiation Research and Applied Sciences, 13, 191-199.
Giri, B., Dey, S., Das, T., Sarkar, M., Banerjee, J. and Dash, S. K. (2018). Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomedicine & Pharmacotherapy, 107, 306-328.
Giugliano, D., Ceriello, A. and Paolisso, G. (1996). Oxidative stress and diabetic vascular complications. Diabetes care, 19, 257-267.
Guarize, L., da Costa, J. C., Dutra, L. B., Mendes, R. F., Lima, I. V. and Scio, E. (2012). Anti-inflammatory, laxative and intestinal motility effects of Senna macranthera leaves. Natural product research, 26, 331-343.
Henry, R., Sobel, C. and Berkman, S. (1957). Interferences with biuret methods for serum proteins use of benedict’s qualitative glucose reagent as a biuret reagent. Analytical Chemistry, 29, 1491-1495.
HHP. (2019). Key minerals to help control blood pressure[Online]. Harvard Health Publishing Available: https://www.health.harvard.edu/heart-health/key-minerals-to-help-control-blood-pressure [Accessed 27 July 2021].
Jamshidi-Kia, F., Lorigooini, Z. and Amini-Khoei, H. (2018). Medicinal plants: Past history and future perspective. Journal of herbmed pharmacology, 7, 1-7.
Jangid, H., Chaturvedi, S. and Khinchi, M. (2017). An overview on diabetis mellitus. Asian Journal of Pharmaceutical Research and Development, 1-11.
Jani, D. K. and Goswami, S. (2020). Antidiabetic activity of Cassia angustifolia Vahl. and Raphanus sativus Linn. leaf extracts. Journal of Traditional and Complementary Medicine, 10, 124.
Kalekar, H. M. (1947). Differential spectrophotometry of purine compounds by means of specific enzymes. Journal of Biological Chemistry, 167, 429-443.
Kishore, L., Kaur, N. and Singh, R. (2017). Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. journal of food and drug analysis, 25, 576-588.
Kodidela, S., Shaik, F. B., Chinta, V., Mohammad, S. A., Pasala, C., Mittameedi, C. M., Maddu, N., Wudayagiri, R. and Nallanchakravarthula, V. (2020). Possible ameliorative role of green tea on chronic alcohol mediated renal toxicity of STZ-induced diabetic rats. Clinical Nutrition Experimental, 34, 1-25.
Kunst , A. (1984). UV-methods with hexokinase and glucose-6-phosphate dehydrogenase. Methods of enzymatic analysis, 163-172.
Lindawati, N. Y., Puspitasari, D., Murtisiwi, L. and Rahmania, T. A. (2021). Correlation of Flavonoid content on Antidiabetic activity in red beans (Phaseulus vulgaris L.) and its Processed Products. Research Journal of Pharmacy and Technology, 14, 1293-1297.
Liu, H., Cai, X., Dai, L., Ma, J. and Mo, Y. (2018). Elevated uric acid levels in premenopausal female systemic lupus erythematosus patients: association with potential or existing renal damage. European Journal of Inflammation, 16, 1-6.
Ma, Z., Li, L., Livingston, M. J., Zhang, D., Mi, Q., Zhang, M., Ding, H.-F., Huo, Y., Mei, C. and Dong, Z. (2020). p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. The Journal of clinical investigation, 130, 5011-5026.
Mehra, N., Tamta, G. and Nand, V. (2021a). A review on nutritional value, phytochemical and pharmacological attributes of Foeniculum vulgare Mill. Journal of Pharmacognosy and Phytochemistry, 10, 1255-1263.
Mehra, N., Tamta, G., Nand, V. and Biswas, S. (2021b). Compositional analysis of Foeniculum vulgare seeds genotypes collected from Tarai region of Uttarakhand. 10, 268-271.
Miranda, K. M., Espey, M. G. and Wink, D. A. (2001). A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric oxide, 5, 62-71.
Mukhtar, I. G., Yakasai, B. W. and Firdausi, D. T. (2020). Hypoglycemic effect of aqueous leaf extract of Senna singueana on alloxan-induced diabetic wistar rats. Journal of Medicine in the Tropics, 22, 41-45.
Naeem, Z. (2015). Burden of Diabetes Mellitus in Saudi Arabia. International journal of health sciences, 9, V-VI.
NIDDK. (2017). Diabetic Kidney Disease[Online]. National Institute of Diabetes and Digestive and Kidney Diseases Available: https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/diabetic-kidney-disease [Accessed 18 June 2021].
NIDDk. (2018). Insulin Resistance & Prediabetes[Online]. National Institute of Diabetes and Digestive and Kidney Diseases Available: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulin-resistance [Accessed 13 August 2021].
NIDDK. (2020). High Blood Pressure & Kidney Disease[Online]. National Institute of Diabetes and Digestive and Kidney Diseases Available: https://www.niddk.nih.gov/health-information/kidney-disease/high-blood-pressure [Accessed 27 July 2021].
Obrosova, I. G., Huysen, C. V., Fathallah, L., Cao, X., Greene, D. A. and Stevens, M. J. (2002). An aldose reductase inhibitor reverses early diabetes‐induced changes in peripheral nerve function, metabolism, and antioxidative defense. The Federation of American Societies for Experimental Biology Journal, 16, 1-26.
Ohkawa, H., Ohishi, N. and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry, 95, 351-358.
Osman, N., Jambi, E. and Aseri, N. (2017). Assessment of antidiabetic and antioxidant activities of Cassia angustifolia and Feoniculum vulgare in diabetic rats. International Journal of Pharmaceutical Research & Allied Sciences, 6.
Pal, S., Rao, G. N. and Pal, A. (2020). High glucose-induced ROS accumulation is a critical regulator of ERK1/2-Akt-tuberin-mTOR signalling in RGC-5 cells. Life Sciences, 256, 1-14.
Patle, D., Vyas, M. and Khatik, G. L. (2020). A Review on natural products and herbs used in the management of diabetes. Current diabetes reviews.
Piero, M., Nzaro, G. and Njagi, J. (2015). Diabetes mellitus-a devastating metabolic disorder. Asian journal of biomedical and pharmaceutical sciences, 5, 1-7.
Poitout, V. and Robertson, R. P. (2002). Minireview: secondary β-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology, 143, 339-342.
Rohman, F. and PUTRA, W. E. (2020). The bioinformatics perspective of Foeniculum vulgare fruit’s bioactive compounds as natural anti-hyperglycemic against alpha-glucosidase. Biodiversitas Journal of Biological Diversity, 22, 79-84.
Saddala, R. R., Thopireddy, L., Ganapathi, N. and Kesireddy, S. R. (2013). Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root. Experimental and toxicologic pathology, 65, 15-19.
Sadrefozalayi, S. and Farokhi, F. (2014). Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna journal of phytomedicine, 4, 110-117.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A. A. and Ogurtsova, K. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice, 157, 107843.
Samadi, M., Aziz, S. G.-G. and Naderi, R. (2021). The effect of tropisetron on oxidative stress, SIRT1, FOXO3a, and claudin-1 in the renal tissue of STZ-induced diabetic rats. Cell Stress and Chaperones, 26, 217-227.
Shanmugasundaram, R., Devi, K., Soris, T., Maruthupandian, A. and Mohan, V. (2011). Antidiabetic, antihyperlipidaemic and antioxidant activity of Senna auriculata (L.) Roxb. leaves in alloxan induced diabetic rats. International Journal of PharmTech Research, 3, 747-56.
Srinivasan, S., Sathish, G., Jayanthi, M., Muthukumaran, J., Muruganathan, U. and Ramachandran, V. (2014). Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Molecular and cellular biochemistry, 385, 159-168.
Sudhakar, C., Mishra, V., Hemani, V., Verma, A., Jain, A., Jain, S. and Charyulu, R. N. (2020). Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends in Food Science & Technology
Sureka, C., Elango, V., Al-Ghamdi, S., Aldossari, K. K., Alsaidan, M., Geddawy, A., Abdelaziz, M. A., Mohideen, A. P. and Ramesh, T. (2021). Ameliorative property of Sesbania grandiflora on carbohydrate metabolic enzymes in the liver and kidney of streptozotocin-induced diabetic rats. Saudi Journal of Biological Sciences, 28.
WHO. (2020). Diabetes[Online]. World Health Organization. Available: https://www.who.int/news-room/fact-sheets/detail/diabetes [Accessed 16 June 2021].
Wong, E., Backholer, K., Gearon, E., Harding, J., Freak-Poli, R., Stevenson, C. and Peeters, A. (2013). Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. The lancet Diabetes & endocrinology, 1, 106-114.
Zhang, T., Jayachandran, M., Ganesan, K. and Xu, B. (2020). The Black Truffle, Tuber melanosporum (Ascomycetes), Ameliorates Hyperglycemia and Regulates Insulin Signaling Pathway in STZ-Induced Diabetic Rats. International Journal of Medicinal Mushrooms, 22, 1057-1066.


