Department of Microbiology, Chaudhary Charan Singh University, Meerut, India.
Corresponding author email:: narayanumeshwar17@gmail.com
Article Publishing History
Received: 24/06/2022
Accepted After Revision: 15/09/2022
Resistance has been reducing the effectiveness of antibiotics for the past few decades. Researchers are constantly investigating new herbal medicines which can be a better option as well. Aqueous and ethanol extraction method were followed to obtain clove buds and neem twig (datum) extracts. The obtained extracts were tested against selected bacteria using the well diffusion method and broth dilution method to assess the antimicrobial activities. The obtained data were recorded as MIC50 MIC80 and MBC (minimum bactericidal concentration). The tremendous antibacterial activities in extract of Azadirachta indica and Syzygium aromaticum were observed highest in 6.25 mg/mL of ethanol extraction method. It is concluded that neem and clove showed tremendous antimicrobial activities and both have been found quite effective in oral health even today.
Antagonistic Activity, Cariogenic Bacteria, Herbal Extract, MBC and MIC50.
Narayan U, Garg A. P. Potential Impact of Azadirachta Indica and Syzygium Aromaticum on Growth and Development of Significant Cultivable Oral Bacterial Flora. Biosc.Biotech.Res.Comm. 2022;15(3).
Narayan U, Garg A.P. Potential Impact of Azadirachta Indica and Syzygium Aromaticum on Growth and Development of Significant Cultivable Oral Bacterial Flora. Biosc.Biotech.Res.Comm. 2022;15(3). Available from: <a href=”https://bit.ly/3KQ6Wfn“>https://bit.ly/3KQ6Wfn</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Oral bacterial flora is found as both good and bad bacteria. Some behaves as commensals as they help maintain oral eco balance such as Lactobacillus acidophilus. Although they are also cariogenic bacteria. In addition, some behaves as opportunistic pathogens like Streptococcus mutans which is the most cariogenic bacteria. In fact, opportunistic pathogenic bacteria cause dental diseases such as dental caries, periodontal disease, endodontic disease and pyogenic infection. A variety of microbial flora found in oral cavity is cultivable and non-cultivable. Predominant cultivable microbial flora has been studied in this research. Among all the microorganisms present in the mouth, bacteria are the most predominant as they include both aerobic and anaerobic bacteria (Baveza 2022).
Antibiotics have been used for long to cure infections. But medical science, is facing a major challenge of resistance to antibiotics by pathogens that has a deep impact on health of our society. Resistance against antibiotics have drawn the attention of researchers to develop an approach for patient care. At present, researchers are finding it difficult to prevent resistance in pathogens and are constantly trying to get rid of this problem whose advance antibiotic is being developed.
Apart from this, researchers are paying attention to new approaches along with the new antibiotic source, herbal medicine is also being seen as a better option. Herbal medicines are derived from plants and they have been used for centuries. Uses of herbal medicine were common in developing countries. Traditionally, diseases were also treated and prevented by these medicines due to lack of advance medical science facility (Treicher 2021).
Herbal medicines have a such chemical compounds that interfere the function of various pathogens including alkaloids, tannins, glycosides, steroids, volatile oils, fixed oils, resins, phenols and flavonoids that are commonly used in medicine and other application which are obtained from their specific parts of plants such as leaves, buds, fruits, seeds, barks and roots (Gupta et al. 2012). As per report of WHO, 80% people of developing country rely on traditional medicine for their primary health and in 85% medicine, herbal medicines are used (Wang 2020; Treicher 2021).
The trust on herbal medicine is increasing gradually. It means a big part of world’s population rely on herbal medicine (Wang 2020). The great diversity of plants represents different sources and type of herbal medicine on broad level. This information grants us a significant role plants as sources of herbal medicine for new herbal medicine in the world. Although, development of resistance diminishes the effect of antibiotics since last few decades. Recently researchers are constantly engaged in the screening and discovery of new herbal medicine.
That specify the significant role of herbal plants in new herbal medicines (Treicher 2021). Clove (Syzygium aromaticum) is the aromatic buds of flower of a tree. It belongs to the family Myrtaceae, widely used as spices and home remedies. It has been reported that clove has antimicrobial activities against numerous types of oral bacteria like Streptococcus mutans (Gupta and Prakash 2021).
Azadirachta indica is commonly known as “Indian neem” and belongs to family Maleacae. Almost whole tree has medicinal properties. Different parts of neem are used as herbal medicine such leaf, bark fruits seeds and root. The neem is used since ancient time as home remedies. Still, it is known as “village dispensary”. In (1992), U.S. National academy of science published a report to confer information about medicinal properties of neem. (Kumar and Parmar 1995; Biswas et al. 2002).
There are various ingredients such as ß-sitosterol, quercetin and polyphenolic flavonoids, in fresh leave are found in neem as well as seeds containing azadirachtin and gedunin that have antibacterial and antifungal properties (Sarmiento et al. 2011). It has been observed that recently, researchers are trying to further upgrade the information about the medicinal properties of neem. It also has been observed that neem has antimicrobial properties against cariogenic and pyogenic bacteria (Dhaniya 2011).
Additionally, Rajasekaran (2008) revealed medicinal properties of neem as antiviral, antifungal, antibacterial, antiseptic, antiulcer and antipyretic (Gupta and Prakash 2021). Neem and clove have been shown to have a strong antimicrobial effect, which scientists have also confirmed in their research paper. In addition, it has beneficial for human health. Apart from this, it was also observed that people who used routinely are less susceptible for dental disease (Tasanarong and Gupta 2021).
MATERIAL AND METHODS
Bacterial strains were used in this study including Streptococcus mutans (MTCC 497), Streptococcus mitis (MTCC 482), Streptococcus salivarius (MTCC 412), Lactobacillus acidophilus (MTCC 384), Staphylococcus aureus (MTCC96), Staphylococcus epidermidis (SMCMB 1084), Veillonella rogosae (SMCMB 1121), Bacteroides fragilis (SMCMB 1181), Fusobacterium nucleatum (SMCMB 1096), Micrococcus luteus (MTCC106), Pseudomonas aeruginosa (MTCC2581. All strains were revived on nutrient agar medium (NAM) and Lactobacillus acidophilus on De Man, Rogosa and Sharpe (MRS) agar medium to obtain fresh culture.
All ingredients were weighed and mixed in distilled water. It was placed it on heated magnetic stirrer for homogenous suspension. Before pH adjustment, the medium was mixed by gently stirring then adjusted the pH by digital pH meter (Systronics, μ system 361) as required. Medium was sterilized in autoclave at 121℃ for 15 minutes and 15 ibs. It was cooled to about 50℃. Approximately 15 mL of medium was poured in each sterile polystyrene disposable Petri dish aseptically under laminar air flow chamber, then allowed to cool to solidify. If the culture medium was not to be used at that time, then it was placed in refrigerator at 4℃ until used.
Antibacterial susceptibility was done by Disc Diffusion, broth dilution and well diffusion method. Disc diffusion method was followed as per guideline of Clinical Laboratory Standards Institute (CLSI). Muller-Hinton agar medium supplemented with 2% glucose was prepared and adjusted pH at 7.2. Fresh test culture was used for investigation of antimicrobial activities and further, prepared inoculum of 2, 5 x 103 CFU/Ml as compare to McFarland density. 100 µl of inoculum was inoculated on Muller-Hinton Agar medium following spread culture technique.
The desired antibiotic disc was placed on culture medium with the help of forceps aseptically. Agar well diffusion method worked on same as that of disc diffusion method. Agar well diffusion method was preferred for investigation of antimicrobial activity of extract of chewing product against isolated oral organisms. 100 µl of inoculum of 2.5 x 103 CFU/mL was inoculated on Muller-Hinton Agar medium following spread culture technique. It was then allowed to absorb inoculum on surface of medium for 4-5 minutes in laminar air flow chamber (LAF).
Wells were cut as per the requirement at equidistance using 6 mm sterile Cork borer. 1250 mg/Ml Stock solution of all chewing extract was used. DMSO was used as solvent for dissolving extract of chewing products. 50 µl of test extract of chewing product was filled in pre-cut wells of 6 mm in diameter on MHA plate which was already inoculated with desired concentration of organism. Inoculated petri plate was incubated at 37℃ for 24-48 hrs.
Zone of inhibition was measured in mm of diameter by Hi Antibiotic Zone Scale- C PW297 (Hi- Media, Mumbai). Data was recorded as zone of inhibition in mm of average of two independent replicates. It was carried out for investigation of minimum amount able to inhibit growth of test organism. A set of 12 test tube with cap was used. Muller-Hinton broth was used as medium.
In first test tube, 2 mL of double strength medium was taken, which containing 200 mg of herbal extract. In next 2 to 11 test tube, 1 mL of double strength medium was taken in each test tube. Distilled water was taken in 12th test tube as negative control. Now first test tube was properly mixed using micropipette 5 to 6 time for uniform distribution. I mL of the suspension was transferred from the first to the second test tube and again mixed thoroughly and then from the second to the third.
This was continued for up to 10 test tubes. I mL of 5 × 103 CFU/mL was inoculated into each test tube. It now showed the expected density of 2.5 × 103 cfu/ml. Test tube 11 was used as a positive control, containing double-strength medium and 1 mL of inoculum was added. Now final volume of each test was 2 mL. All test tube was placed at 37℃ in BOD incubator for overnight to 48 hours. Turbidity of each test tube was measured by spectrophotometer.
Based on optical density (OD), inhibitory point was marked and 50 µl of suspension from this marked test tube was spreaded on culture medium with the help of sterile glass spreader. Incubated at 37℃ for 24 h and counted the appeared colonies on culture medium. CFU (Colony Forming Unit) was calculated for each marked and control test tube. This data was used for determination of MIC50, MIC80 and MBC. MIC50 shows a 50% reduction in growth as compared to the positive control and MIC80 shows an 80% growth reduction compared to the positive control.
MBC (minimum bactericidal concentration) were shown to be completely decreased in growth at the particular concentration compared to the positive control. For the comparative evaluation of the DD and BMD methods, Mean and MIC ranges were calculated for each genus species combination. The diameter of zones of inhibition (in mm) surrounding the antimicrobial disc at 24 and 48 h of incubation was plotted against their respective BMD MICs read after 24 hours and 48 hours of inoculation in the form of Scatterplot. Stoical analyses were made using SPSS software package.
RESULTS AND DISCUSSION
Table 1. Antimicrobial activity of selected extract of Azadirachta indica (neem twig) against isolated significant oral fungal flora by agar well diffusion (AWD) method with broth dilution method (BMD) (given each data is an average of two independent replicates).
| Test bacterial isolates | Zone of inhibition
By well diffusion method (diameter in mm) |
Growth inhibition in percent detected by Broth Dilution Method(BDM) at different concentration (mg/mL) | |||||||||
| 100 | 50 | 25 | 12.5 | 6.25 | 3.125 | 1.562 | 0.781 | 0.390 | 0.195 | ||
| S.mutans | 27 | Ngo | Ngo | Ngo | Ngo | MBC | 83 | 52⃰ | 21 | 15 | Nam |
| S,mitis | 26 | Ngo | Ngo | Ngo | Ngo | MBC | 82 | 50⃰ | 20 | 10 | Nam |
| S.salivarius | 27 | Ngo | Ngo | Ngo | Ngo | MBC | 82 | 48⃰ | 18 | Nam | Nam |
| S.aureus | 26 | Ngo | Ngo | Ngo | Ngo | MBC | 80 | 49⃰ | 18 | Nam | Nam |
| S.epidermidis | 22 | Ngo | Ngo | Ngo | MBC | 78 | 49⃰ | 20 | Nam | Nam | Nam |
| L.acidophilus | 22 | Ngo | Ngo | Ngo | MBC | 80 | 50⃰ | 20 | Nam | Nam | Nam |
| V.rogosae | 21 | Ngo | Ngo | Ngo | MBC | 78 | 49⃰ | 18 | Nam | Nam | Nam |
| B.fragilis | 23 | Ngo | Ngo | Ngo | MBC | 82 | 51⃰ | 21 | Nam | Nam | Nam |
| F.nucleatum | 25 | Ngo | Ngo | Ngo | Ngo | MBC | 80 | 50⃰ | 16 | Nam | Nam |
| M.luteus) | 23 | Ngo | Ngo | Ngo | MBC | 80 | 49⃰ | 18 | n.am. | Nam | Nam |
| P.aerogenosa | 28 | Ngo | Ngo | Ngo | Ngo | Ngo | MBC | 82 | 50 | 15 | Nam |
Note: – mm= millimetre, n.g.o.=no growth observed, n.am.=no antimicrobial activity, MBC= Minimum Bactericidal Concentration, MIC50 =Minimum Inhibitory Concentration that inhibit approximately 50% growth.
Table 2. Antimicrobial activity of selected extract of Syzygium aromaticum (clove buds) against isolated dominant oral fungal flora by agar well diffusion method and broth dilution method (given each data is an average of two independent replicates).
| Oral isolates | Zone of inhibition- well diffusion method
(diameter in mms) |
Inhibition of growth in percent determined by Broth Dilution Method (BDM) at different concentration .(mg/mL) | |||||||||
| 100 | 50 | 25 | 12.5 | 6.25 | 3125 | 1.562 | 0.781 | 0.390 | 0.195 | ||
| S.mutans | 29 | Ngo | Ngo | Ngo | Ngo | MBC | 83 | 52⃰ | 25 | 15 | Nam |
| S,mitis | 26 | Ngo | Ngo | Ngo | MBC | 80 | 49⃰ | 18 | Nam | Nam | Nam |
| S.salivarius | 27 | Ngo | Ngo | Ngo | MBC | 82 | 52⃰ | 20 | 15 | Nam | Nam |
| S.aureus | 24 | Ngo | Ngo | Ngo | MBC | 80 | 52⃰ | 20 | 10 | Nam | Nam |
| S.epidermidis | 23 | Ngo | Ngo | Ngo | MBC | 78 | 50⃰ | 18 | Nam | Nam | Nam |
| L. acidophilus | 18 | Ngo | Ngo | MBC | 82 | 52⃰ | 20 | Nam | Nam | Nam | Nam |
| V.rogosae | 17 | Ngo | Ngo | MBC | 80 | 50⃰ | 20 | Nam | Nam | Nam | Nam |
| B.fragilis | 23 | Ngo | Ngo | Ngo | MBC | 80 | 48⃰ | 18 | Nam | Nam | Nam |
| F.nucleatum | 21 | Ngo | Ngo | MBC | 83 | 50⃰ | 20 | Nam | Nam | Nam | Nam |
| M.luteus | 22 | Ngo | Ngo | MBC | 82 | 52⃰ | 20 | Nam | Nam | Nam | Nam |
| P.aerogenosa | 24 | Ngo | Ngo | Ngo | MBC | 80 | 50⃰ | 20 | 10 | Nam | Nam |
Note: – mm= millimetre, Ngo. =no growth observed, n.am.=no antimicrobial activity, MBC= Minimum Bactericidal Concentration, (*) =MIC50 (Minimum Inhibitory Concentration that inhibit approximately 50% growth)
Figure 1: Comparison of Clove, neem, antibacterial activity with Amoxillin, Ofloxacin
and Azithromycin antibiotic against S. mutans
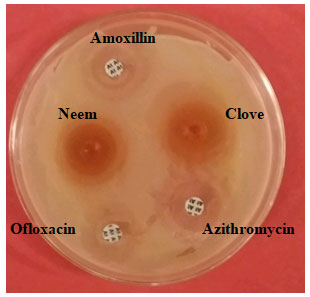
Figure 2: Antibacterial activity of extract of clove and neem against
S. aureus, using agar well diffusion method
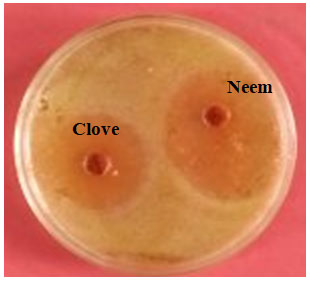
Figure 3: Antimicrobial activity of extract of neem and clove against
isolated L. acidophilus, using well agar diffusion method
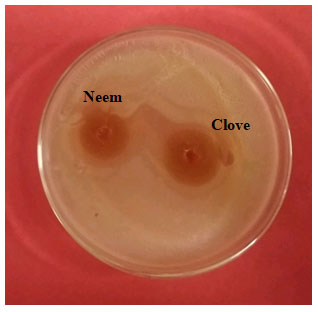
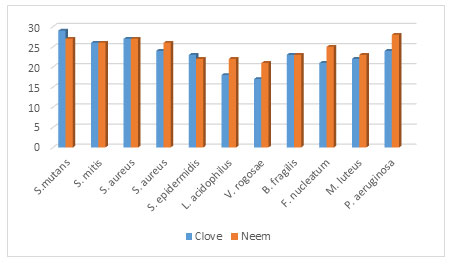
Figure 4: Antimicrobial activities of clove and neem extract against isolated significant oral bacteria
Tooth decay was reported as the most common disease in the oral cavity. Streptococcus mutans and Lactobacillus acidophilus were observed to be the most common cariogenic bacteria involved in dental caries (Wang 2020). Azadirachta indica and Syzygium aromaticum were investigated for their antimicrobial activities, Current investigation revealed a strong antibacterial activity (Wang 2020; Marina 2022).
Also, it was observed that ethanolic extraction method was much better than aqueous extraction. However, many researchers claim that the constituents of neem like β-bourbonene, β-copaene, β-caryophyllene, β-cadinene, Neophytadiene have antimicrobial activity It also further animal testing should be done (Marina 2022). The present study show that the herbal products used in these studies represent better alternate of antibiotics Herbal products decrease the burden of drug. resistance.
These are beneficial as home remedies. Zone of inhibition were observed in mm of diameter around wells containing herbal chewing extract used in this studies. Several researchers also confirmed earlier about their medicinal properties of Azadirachta indica (neem) (Marina 2022). Tasanarong (2021) reported that extract of neem has strong antimicrobial against most of oral bacteria (Tasanarong 2021; Marina 2022).
Antimicrobial activities were showed by extract of neem against Streptococcus mutans (27 mm) (figure 1.0) Streptococcus mitis (26 mm), Streptococcus salivarius (27 mm (figure 2.0), Staphylococcus aureus (26 mm), (figure 3.0) Streptococcus epidermidis (22 mm), Lactobacillus acidophilus (22 mm) (figure 3.0), Veillonella rogosae (21 mm), Bactericides fragilis (23 mm), Fusobacterium nucleatum (25 mm). Micrococcus luteus (23 mm), Pseudomonas aeruginosa (28 mm) as well as MIC50 clove were recorded1.562 mg/mL for S. mutans, 3.125 mg/mL for S. mitis, S. salivarius,
S. aureus, S. epidermidis, B. fragilis and p. aeruginosa along with MIC50 for L. acidophilus, V. rogosae, F. nucleatum and M. luteus were observed 6.25 mg/mL. 6.25 mg/mL. Additionally, MBC was also calculated for all isolates used in this study. MBC for S. mutans was recorded 6.25 mg/mL and for S. mitis, S. salivarius, S. aureus S. epidermidis, B. fragilis and P. aeruginosa 12.5 mg/mL, respectively. As well as for L. acidophilus, V. rogosae, F. nucleatum and M. luteus, were observed 25 mg/mL (table 1.0 and figure 4.0).
Recently several researchers studied on effect of extract of clove on cultivable oral microbial flora. Gupta (2021) revealed that clove have strong antimicrobial activity against Streptococcus mutans. Present study also claims similar study as earlier investigated. Antimicrobial activities were showed by extract of clove like Streptococcus mutans (29 mm), (figure 4.0), Streptococcus mitis (26 mm), Streptococcus salivarius (27 mm), Staphylococcus aureus (24 mm), (figure 2.0) Staphylococcus epidermidis (23 mm), Lactobacillus acidophilus (18 mm) (figure 3.0), Veillonella rogosae (18 mm), Bactericides fragilis (17 mm), Fusobacterium nucleatum (21 mm).
Micrococcus luteus (22 mm), Pseudomonas aeruginosa (24 mm). MIC50 were observed as 1.562 mg/mL for Streptococcus mutans, 3.125 mg/mL for Streptococcus mitis, Streptococcus salivarius, Staphylococcus aureus, Staphylococcus epidermidis, Bactericides fragilis, Pseudomonas aeruginosa respectively. 6.25 mg/mL for Lactobacillus acidophilus, Veillonella rogosae Fusobacterium nucleatum and Micrococcus luteus. Minimum Bactericidal Concentration (MBC). Gupta and Prakash (2021) revealed that Syzygium aromaticum (clove) have antimicrobial activity due to their eugenol derivatives in vitro (figure 4.0) (Gupta 2021).
CONCLUSION
The findings of the present studies have confirmed antimicrobial activity of clove and neem against microbial flora. Clove and neem are known for ages for the antimicrobial activities against wide level of microorganisms. These are beneficial when microbes are getting resistant for antibiotic.
REFERENCES
Ahn, K., (2017). The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Reports 50 (3) pp. 111-116.
Alexa, V. T., Atena I., Galuscan,1., et al. (2019). Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils against Streptococcus mutans from the Oral Cavity. National Center for Biotechnology Information, NLM, 24(22): 4043
Al-Hashmi, Z.S. and Hossain., M.A. (2016). Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pacific Science Review A: Natural Science and Engineering, 18 (2) (2016), pp. 128-131.
Baveza C. P. (2022). Oral Microbiology, Textbook of Microbiology, 6, 369-375
Bull M, Plummer S, Marchesi J, et al. (2013). The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbial Lett.349:77–87.
Dagli, M., Dagli, Q. I., Rashad, R., S. et al. (2015). Essential oils, their therapeutic properties, and implication in dentistry: A review, J Int Soc Prev Community Dent. 5(5): 335–340.
Gao, L., Xu, T., Huang, G. C. et al. (2018). Oral microbiomes: more and more importance in oral cavity and whole body, National Center for Biotechnology Information, NLM, 9(5): 488–500.
Gupta, A., Ansari, S., Gupta, S., et al. (2019). Therapeutics role of neem and it bioactive constituents in disease prevention and treatment, International Journal of Biological Macromolecules 8(3), 680–691.
Gupta C. and Prakash D., (2021). Comparative study of the Antimicrobial Activity of Clove oil and Clove Extract on Oral Pathogens, Dent. Open j, 7(1): 12-15. Doi: 10.17140/DOJ-7-144
Hu, Q., Gerhard, H., Upadhyaya, I., Venkitanarayanan, K., et al. (2016). Antimicrobial eugenol nano emulsion prepared by gum arabic and lecithin and evaluation of drying technologies. International Journal of Biological Macromolecules, 87,130–140.
Kumar KP, Yadav A, Srivastava S, et al. (2018). Recent trends in Indian traditional herbs Syzygium aromaticum and health benefits. Pharmaco. Phytochemical. 1(1): 6-17.
Lapinska B., Szram A., Zarzycka B. et al., (2020). An in vitro Study on the Antimicrobial Properties of Essential Oil Modified Resin Composite against Oral Pathogens, Journal materials, 19 (10):3390
Palombo E.A., (2011). Traditional medicinal plant extracts and natural products with activity against oral Bacteria: Potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med.680354.
Purkait S., Bhattacharya A., Bag A., et al. (2020). Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination, Arch Microbiol. 202(6):1439-1448.
Marina R., Wylie and Merelle, D.S. (2022). The Antimicrobial Potential of the Neem tree Azadirachta indica, Frontiers pharmacology Sec. Ethno pharmacology, doi.org/10.3389/fphar.2022.891535
Mirpour,M., Gholizadeh Z., Siahmazgi A., et al. (2015) Antibacterial activity of clove, gall nut methanolic and ethanolic extracts on Streptococcus mutans PTCC 1683 and Streptococcus salivarius PTCC 1448, J Oral Biol Craniofac Res. 5(1): 7–10.
Packia C.J., Sowmia N., Viveka S., et al. (2012). The inhibiting effect of Azadirachta indica against dental pathogens. Asian J. Plant Sci Res.; 2: 6–10.
Qiao Hu, Zhou, M. and Wei, S. (2018). Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field, J. of food science, 83:1476-1483.
Reddy R. R. (2013). Antimicrobial activity of Azadirachta Indica (neem) leaf, bark and seed extracts. Int. J. Res. Phytochem. Pharma. 3(1), 1-4
Saleem, S. G, Hussain, M. A. and Bukhari S.N. (2018). A comprehensive review of phytochemical profile, bioactive for pharmaceuticals, and pharmacological attributes of Azadirachta indica, Phytotherapy Research, 32(7) pp. 1241-1272.
Sheng J and Marquis R.E. (2006). Enhanced acid resistance of oral Streptococci at lethal pH values associated with acid-tolerant catabolism and with ATP synthaseact vity. FEMS Microbiol Lett 262:93–8.
Shewale, S and Rathod, V.K. (2018). Extraction of total phenolic content from Azadirachta indica or (neem) leaves: kinetics study. Prep Biochem Biotechnol.;48:312–20.
Tasanarong T., Patntirapong and Aupaphong V. (2021). The inhibitory effect of a novel neem paste against cariogenic bacteria, Journal of Clinical and Experimental dentistry, 13(11): e1083–e1088.
Treicher, L. M. (2021). Exploring the Future of infectious Disease Treatment in a Post- Antibiotic Era: A Comparative Review of Alternative Therapeutics. J.Glob. Antimicrobial. Resist 24, 285-295.doi:10. 1016.ijar.2020.12.025
Wang, C. H., Heish, Y.H., Kao C.Y. et al. (2020) Defeating Antibiotic Resistant Bacteria: Exploring Alternative Therapies for a Post- Antibiotic Era. International Journal Molecular Science 21(3). doi; 10.3390/ijms2103061


