1Department of Pharmacology and Therapeutics, Faculty of Basic Medical
Sciences, Delta State University, Abraka, Delta State, Nigeria
2Department of Pharmacology and Therapeutics, College of Medicine and Health
Sciences, Afe-Babalola University, Ado-Ekiti, Nigeria
Corresponding author email: tonyeduviere@yahoo.com
Article Publishing History
Received: 28/10/2021
Accepted After Revision: 23/12/2021
Although the implication of calcium signalling in the aetiology of anxiety remains elusive, drugs modulating calcium (like calcium channel blockers) have been discovered to be some worth beneficial as treatment option for anxiety related disorders. This study was therefore undertaken to assess probable ameliorative potential of verapamil against manic-like (stereotype behaviour) and anxiety-like symptoms in mice exposed to sleep deprivation. Mice were allotted into five treatment groups (n=5): group 1 and 2 received 10 mL/kg distilled water, groups 3 and 4 verapamil (25 and 50 mg/kg) while group 5 received astaxanthin (50 mg/kg) which served as the reference drug. Treatment was for 7 days and animals were sleep-deprived on the final 72 hours. Various behavioural tests to determine degree of stereotypical behaviour and locomotor activity were carried out. Anxiety test was done via the aid of a light/dark box and plus maze while stereotype behaviour was assess utilizing an open field box.
Oxidative stress parameters; malondialdehyde and glutathione were assessed. Histopathological perturbations in the caudate putamen were also recorded. Data were subjected to ANOVA at α0.05. The results obtained suggest that verapamil significantly suppressed stereotyped behaviour and reduced the incidence of manic-like behaviour which was induced by paradoxical sleep deprivation. Verapamil also significantly restored antioxidant levels and protected against loss of caudate neurons. In conclusion, verapamil ameliorates manic-like symptoms and anxiety in mice derived of sleep, while protecting brain neurons against oxidative stress damage induced by sleep deprivation.
Anxiety, Calcium channel blockers, Neuroprotection, Stereotype behaviour, Verapamil.
Eduviere A.T, Edje K.E, Otomewo L.O, Chukwuka J.C, Olayinka J.N, Akinluyi E.T, Adeoluwa O.A. Pharmacological Effect of Verapamil on Brain Oxidative Parameters and Mania-Like Behaviour in Paradoxical Sleep Deprived Mice. Biosc.Biotech.Res.Comm. 2021;14(4).
Eduviere A.T, Edje K.E, Otomewo L.O, Chukwuka J.C, Olayinka J.N, Akinluyi E.T, Adeoluwa O.A. Pharmacological Effect of Verapamil on Brain Oxidative Parameters and Mania-Like Behaviour in Paradoxical Sleep Deprived Mice. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3HaKbQZ“>https://bit.ly/3HaKbQZ</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
Sleep is considered the body’s own way of replenishment and restoration. Optimally, adults require at least minimum nine (9) hours of uninterrupted sleep each night. Rapid eye movement (REM) sleep, is an essential part of the sleep-wake process characterized by rapid side-to-side movements of the closed eyes, atonia, arousal and low amplitude mixed-frequency brain waves, relatively similar to those experienced during the waking state (Colavito et al. 2013). The REM sleep is an indispensable physiological occurrence of which its chronic deprivation can result in distinct behavioural and cognitive alterations (Hirshkowitz et al. 2015; Gonzalez-Castañeda et al. 2016; Ben-Azu et al. 2020). Studies have posited that anxiety is usually associated with substantive loss of REM sleep, aggressive behaviour, memory deficit, including oxidative stress.
Therefore, paradoxical sleep deprivation (PSD) is considered a good animal model for inducing mania-like behaviour, as it stimulates some aspects of a manic episode, such as hyperactivity, hypersexuality and aggressive behaviour (Martins et al. 2008; Benedetti et al. 2008; Mathangi et al. 2012). Apart from PSD, there is also extensive subjective evidence that stressful experience and other psychosocial factors contribute to the initiation and frequency of manic phases (Koyuncu et al. 2019; Baker et al. 2019; Ben-Azu et al. 2020). Mania which is a distinctive period of aberrantly and sustained elevated, energetic, irritable or elated mood, lasting for a week or any period until hospitalization is necessary (Paul and Allan 2012; Dailey and Saadabadi 2021).
Mania is the most distinguished stage of bipolar condition which is a foremost cause of disability, stigma, and cognitive impairment (Martinez-Aran et al. 2004; De Miranda et al. 2020). The possible causes of mania include; high levels of stress, alterations in sleep pattern or insomnia, use of recreational drugs or alcohol, seasonal changes, child birth or could be even due to a major significant change in the life of an individual such as going through a divorce. The indicators of mania may consist of increased talkativeness or activity, loss of appetite, disinhibition, decreased need for sleep, reckless behaviour or grandiosity, which causes severe mood distortion which affects normal functioning of the individual in daily living, psychomotor agitation, hyper-sexuality, and increased reward-seeking behaviours (American Psychiatric Association 2000).
The most generally used medications for mania/mania-like behaviour include; lithium, some anticonvulsants (valproate, carbamazepine), atypical antipsychotics (quetiapine, olanzapine, risperidone, ziprasidone, aripiprazole, clozapine), standard antipsychotics (e.g., haloperidol, chlorpromazine), and benzodiazepines (e.g., lorazepam, clonazepam) (Eduard and Sanchez-Moreno 2008; Dailey and Saadabadi 2021).
These medications might be hindered by side effects (antidepressants), habituation and tolerance (benzodiazepines), or inefficacy in some anxiety disorders (buspirone) (Goldstein 1985). Therefore, medications with probable theoretical efficacy and low side effect profile are sought after. Calcium channel blockers (CCBs) seem to meet these criteria. The CCBs, are more generally used as treatment options for cardiovascular disorders (cardiac arrhythmias, angina pectoralis, and hypertension) (Das and Plow 2010).
However, the discovery that CCBs crosses the blood-brain barrier and act directly on neuronal cells by binding to numerous brain regions suggesting that they might offer some beneficial effects as treatment options for neuropsychopathological disorders and even reduce anxiety (Urien, et al. 1987; Umukoro et al. 2010). Verapamil is among the most studied CCBs, and has shown positive activity in neurological related disorders (Umukoro et al. 2010; Dailey and Saadabadi 2021).
Dubovsky (1994) emphasized that intracellular calcium (Ca2+) plays a fundamental role in various physiologic processes, namely; action potential, release of neurotransmitters, and functioning of receptors implicated in neuronal periodicity and mood disorders. He pointed out that different antimanic drugs have calcium antagonist properties. Although the precise mechanism of CCBs in these conditions remains unknown, the interference of these drug class with calcium metabolism is a key mechanism of their action in mental disorders (McCarty et al. 2021).
Since a recent longitudinal study has posited that (i) insomnia and other associated sleep glitches worsen before a manic episode, (ii) lack of sleep can trigger mania, and (iii) that sleep problems also adversely affect mood and contribute to relapse, we decided to induce mania in mice by the process of PSD employing the platform over water model in this research while investigating the possible ameliorative potential of verapamil against such manic-like symptoms. Note that PSD is not able to cause mice to exhibit bipolar disorder, but does induce a mania-like behaviour (McCarty et al. 2021).
MATERIAL AND METHODS
Male mice (Albino Swiss) weighing 22.0±2.0g, used for this study were procured from the Animal House, College of Health Sciences of Delta State University, Abraka and were held in rectangular plastic cages at ambient temperature. They were fed with balanced rodent diet and had unrestricted access to water ad libitum. Animal handling was done according to internationally established guidelines on animal care and handling with necessary approval from the Ethical Committee of the University. Mice were individually suspended on grids (2.5 cm apart) over water in a rectangular thermoplastic cage (1.2 cm beneath the grid). Although this design predominantly targets REM sleep, a significant loss of non-REM sleep, accompanied by a significant amount of stress is unavoidable. At the expiration of the 3-day sleep deprivation period, the outcome of sleep restrictions on motor function and manic-like symptoms was assessed between 9:00 am and 12:00 noon.
The rodent sleep deprivation model was chosen to mimic circadian rhythm changes associated with mania. This model offers significant relevance as regards face validity since sleep loss is a notable feature of mania, and can therefore trigger this disorder in patients (Wehr 1992; Mansell and Pedley 2008; McClung 2011). Moreover, sleep deprivation induces several manic like behaviours, such as insomnia, hyperactivity, aggressive actions toward other animals, hyper sexuality (an increase in mounting behaviour), and stereotypy (sniffing and rearing) (Hicks et al. 1979; Fratta et al. 1987; Gessa et al. 1995; Abrial et al. 2015). These behaviours were measured in the animals and all data were presented as mean ± standard error of mean of the grouped mice. The result was analysed using ANOVA and post hoc tests (Student’s Newman–Keuls) were utilized to determine the source of main significance for every test, which was set at α0.05 (De Miranda et al. 2020).
RESULTS AND DISCUSSION
Verapamil on mania-like behaviour, motor activity and anxiety in sleep-deprived mice: The behavioural test involved the utilization of open field chamber test which revealed that sleep deprived mouse exhibited behavioural patterns similar to manic symptoms similar to bipolar disorder. There was an observed increase in hyperactivity (increased number of lines crossed) and stereotype behaviour (rearing and grooming). These results are in tandem with former studies, which indicated that REM sleep restrictions can induce manic-like behaviour in mice (Malkoff-Schwartz et al. 1998; McClung, 2007; Salvadore et al. 2010).
Previous studies also demonstrated that PSD induces hyperactivity (increased locomotor activity), which treatment with lithium attenuated (Benedetti et al. 2008; Armani et al. 2012; De Miranda et al. 2020). As obtainable in Table 1 and Figure 1 below, sleep deprivation significantly increased manic-like behaviour in PSD mice. However, the results obtained revealed that verapamil, at both doses, caused a significant reversal in hyperactivity and the various stereotype behaviours. Thus, indicating the probable use of verapamil in the reduction or attenuation of manic like symptoms.
Table 1. Effect of verapamil on manic-like behaviour induced by paradoxical sleep deprivation in mice
| Treatment | Grooming
Frequency |
Rearing Frequency | Number of lines crossed |
| VEH 10 mL/kg | 10.67±0.95 | 19.83±2.09 | 143.70±8.19 |
| VEH 10 mL/kg + SD | 18.50±0.85# | 37.67±2.08# | 213.50±10.22# |
| VPL 25 mg/kg + SD | 13.00±0.77* | 27.50±1.61* | 161.70±8.39* |
| VPL 50 mg/kg + SD | 10.83±0.79* | 20.67±1.05* | 146.00±12.88* |
| AXT 50 mg/kg+ SD | 14.33±0.84* | 27.67±2.93* | 185.50±4.87* |
Figure 1: Effect of verapamil on motor coordination in mice subjected to paradoxical sleep deprivation.
The tests for anxiety were conducted using a light/dark box and plus maze. Sleep deprived animals spent longer period in the dark box compared to animals that were not sleep deprived. In the plus maze, the sleep deprived mice expended much time in the closed arm than open arm; signifying classic indication of anxiety. However significant variations were observed when verapamil was administered – the mice spent lesser period in the dark box and more in the light box compared to with vehicle plus sleep deprived group, and for the plus maze, the mice spent significant time in open arm than closed arm, thus signifying that verapamil could possibly be used to attenuate the ministrations of anxiety associated with sleep deprivation (Table 2 and Table 3).
Table 2: Effect of verapamil on anxiety in sleep deprived mice using the light/dark box
| Treatment | Duration of mice exploration (per 5 min) | |
| Light Compartment (s) | Dark compartment (s) | |
| VEH 10 mL/kg | 155.50±7.06 | 141.20±6.65 |
| VEH 10 mL/kg + SD | 100.20±5.87# | 199.80±5.87# |
| VPL 25 mg/kg + SD | 139.80±4.83* | 158.80±5.03* |
| VPL 50 mg/kg + SD | 148.20±6.25* | 147.20±6.44* |
| AXT 50 mg/kg+ SD | 122.00±6.86* | 178.00±6.86* |
Table 3: Effect of verapamil on anxiety in sleep deprived mice using the plus maze
| Treatment | Exploration of mice (per 5 min) | |||
| Open arm frequency | Closed arm frequency | Open arm duration (s) | Closed arm duration (s) | |
| VEH 10 mL/kg | 4.33±0.33 | 6.17±0.87 | 105.50±8.61 | 194.50±8.61 |
| VEH 10 mL/kg + SD | 1.83±0.31# | 12.33±0.80# | 43.67±6.74# | 256.30±6.74# |
| VPL 25 mg/kg + SD | 3.12±0.31* | 9.10±0.54* | 83.58±7.64* | 215.20±7.83* |
| VPL 50 mg/kg + SD | 3.38±0.45* | 8.42±0.65* | 94.33±7.21* | 207.20±6.87* |
| AXT 50 mg/kg+ SD | 3.00±0.37* | 9.16±0.70* | 73.00±8.76* | 227.00±8.76* |
Verapamil on brain oxidative stress in sleep-deprived mice: In the present study, alteration of pro-oxidants and antioxidant enzymes were seen alongside the behavioural alteration. Marked reduction in glutathione levels was observed (Figure 3) alongside increase in malondialdehyde levels (Figure 2).
Figure 2: Effect of verapamil on brain malondialdehyde levels
in mice subjected to sleep deprivation
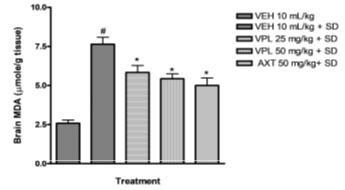
Figure 3: Effect of verapamil on brain glutathione levels in mice
subjected to sleep deprivation.
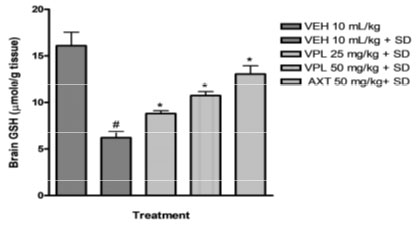
This agrees with previous findings which reported increased products of inflammation, lipid peroxidation, including alterations of the major antioxidant enzymes in bipolar disorder (BD) subjects particularly within the mania spectrum (Kuloglu et al. 2002; Ranjekar et al. 2003; Ozcan et al. 2004). In addition, animal studies also have earlier shown that PSD increases brain lipid peroxidation (Kumar and Garg 2008; Garg and Kumar 2008). In the group that received verapamil, a reversal in the decrease of glutathione levels was seen, and a decreased malondialdehyde levels was observed further indicating the probable use of verapamil in the reversal of manic-like symptoms induced by sleep deprivation (De Miranda et al. 2020).
It is known that the brain is highly prone to oxidative damage due to high rate of oxygen utilization and reduced antioxidant defense systems and the depletion of antioxidants action in discrete parts of the brain following PSD is an indication of free radical generation which could cause neuroinflammation (Tabet et al. 2000; De Miranda et al. 2020). Furthermore, PSD alters membrane fluidity, calcium ion (Ca2+), concentration, gene expression, and enhances metabolic rate (Ramanathan et al. 2002; Singh et al. 2008; Mathangi et al. 2012; Ben-Azu et al. 2020).
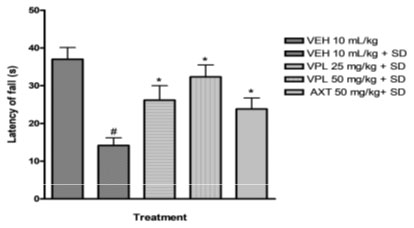
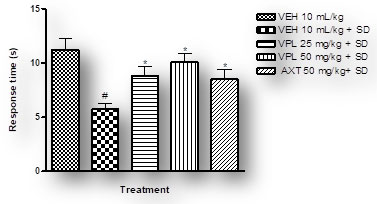
Verapamil on pain and nociception in sleep-deprived mice: Results obtained indicate that verapamil produced an anti-nociceptive effect by reducing the pain threshold and increasing reaction time of PSD mice as recorded by the two tests; hot plate (Figure 4) and tail flick (Figure 5). This gives an indication that verapamil may be useful in the mitigation of pain in patients that experiencing sleep difficulties. This also supports previous studies which showed that PSD contributes to hyperalgesia. It is, thus, logical to infer that PSD aggravates chronic pain symptoms in patients (Smith et al. 2000). Also, patients with pain disorders normally suffer anxiety due to the strain associated with living with pain. Note that the effect of verapamil on pain differs depending on dose, route of administration and pain test utilised (Tamaddonfard et al. 2014).
Supporting, sleep loss increases the experience of pain (Lautenbacher et al. 2006). Casual manipulations in experimental animals have revealed that sleep restrictions heightens nocicepton. While, clinical trials have established that complete, partial, or selective sleep restriction (deprivation) increases pain, thus lowering pain thresholds (Lentz et al. 1999; Roehrs et al. 2006; Schuh-Hofer 2013; Schrimpf et al. 2015; Faraut et al. 2015). Despite this literary evidence, the principal brain mechanism at the core of the impact of sleep deficits on nociception remains unknown (Stroemel-Scheder et al. 2020).
Figure 4: Effect of verapamil on reaction to nociception in mice
subjected to sleep deprivation using the hot plate
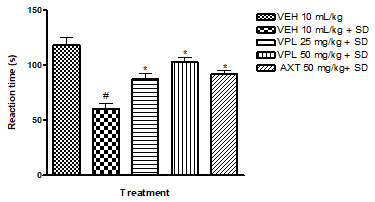
Figure 5: Effect of verapamil on response to nociception in mice subjected
to sleep deprivation utilizing the tail flick test
Verapamil on caudate putamen neurons of sleep-deprived mice: In order to further evaluate the effect of verapamil on manic-like symptoms, histology of the caudate putamen was done after sacrificing on the 7th day. The Hematoxylin and Eosin (H&E) staining of the caudate putamen (as seen on slides in Figure 6) revealed that PSD increased the magnitude of caudate putamen neuronal damage and subsequently decreased viable neuronal population in mice brains (Figure 7). In contrast, verapamil treatment revealed that there was a reduction in the extent of neuronal damage and also an increase of viable neuronal cells in PSD mice.
Figure 6: Photomicrograph of the caudate putamen of mice
after paradoxical sleep deprivation
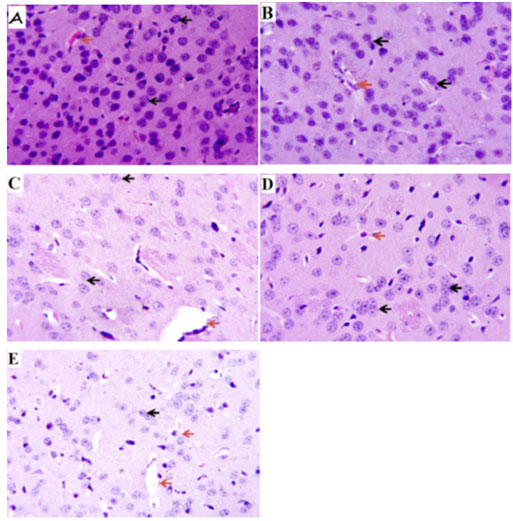
Figure 7: Effect of verapamil on viable caudate putamen
neurons in mice after subjection to sleep deprivation
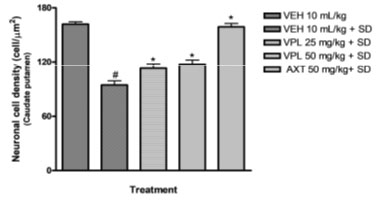
Counts were based on neuronal nuclei present in three (3) squares per slide, using the pre-calibrated Image J software.
CONCLUSION
The finding of the present experiment suggests that verapamil attenuated anxiety/manic-like behaviour induced via deprivation of REM phase sleep, through a mechanism believed to be associated with reduced oxidative stress, and neuronal damage. Verapamil also significantly restored antioxidant levels and protected against loss of caudate neurons. In conclusion, verapamil ameliorates manic-like symptoms and anxiety in mice derived of sleep, while protecting brain neurons against oxidative stress damage induced by sleep deprivation.
ACKNOWLEDGEMENTS:
This work was technically supported by the laboratory technicians of Pharmacology laboratory, DELSU.
Conflict of interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Abrial E, Etievant A, Betry C, et al. (2013). Protein kinase C regulates mood related behaviours and adult hippocampal cell proliferation in rats. Progress in Neuropsychopharmacology and Biological Psychiatry, 43:40–48.
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, (4).
Armani F, Andersen ML, Andreatini R, et al. (2012). Successful combined therapy with tamoxifen and lithium in a paradoxical sleep deprivation-induced mania model. CNS Neuroscience and Therapeutics, 18:119–125.
Baker JT, Dillon DG, Patrick LM, et al. (2019). Functional connectomics of affective and psychotic pathology. Proceedings of the National Academy of Sciences of the United States of America, 116(18), pp.9050-9059.
Ben-Azu B, Emokpae O, Ajayi AM, et al. (2020). Repeated psychosocial stress causes glutamic acid decarboxylase isoform67, oxidative-Nox-2 changes and neuroinflammation in mice: Prevention by treatment with a neuroactive flavonoid, morin. Brain Research 1744, 146917.
Benedetti F, Fresi F, Maccioni P., et al. (2008). Behavioural sensitization to repeated sleep deprivation in a mice model of mania. Behavioural Brain Research, 187:221–227.
Colavito V, Fabene PF, Grassi-Zucconi G, et al. (2013). Experimental sleep deprivation as a tool to test memory deficits in rodents. Frontiers in System Neuroscience, 7.
Dailey, M.W. and Saadabadi, A., (2018). Mania, In: StatPearls (internet), Treasure Island (FL). Available from: https://www.ncbi.nlm.nih.gov/books/NBK493168/
Das R and Plow EF. (2010). A new function for old drugs. Cell Cycle 9(4): 638-639.
De Miranda AS., Vieira ÉL, dos Reis-Bastos J, et al. (2020). Role of gut microbiota in the GBR12909 model of mania-like behavior in mice. Journal of Neuroimmunology, 577292. doi:10.1016/j.jneuroim.2020.577292 .
Dubovsky SL. (1994) Why don’t we hear more about the calcium antagonists? An industry-academia interaction. Biol Psychiatry, 251;149-150.
Eduard V and Sanchez-Moreno J. (2008). Acute and long-term treatment of mania. Dialogues Clin Neurosci. ;10(2):165-179.
Faraut B, Leger D, Medkour T, et al. (2015). Napping reverses increased pain sensitivity due to sleep restriction. PLoS ONE 10(2):e0117425.
Fratta W, Collu M, Martellotta MC, et al. (1987). Stress-induced insomnia: opioid-dopamine interactions. European Journal of Pharmacology, 142U: 437–44Y.
Garg R. and Kumar A. (2008). Possible role of citalopram and desipramine against sleep deprivation-induced anxiety like-behaviour alterations and oxidative damage in mice. Indian Journal of Experimental Biology 46:770e6.
Gessa GL, Pani L, Fadda P. et al. (1995). Sleep deprivation in the rat: an animal model of mania. European Journal of Neuropsychopharmacology, 5: 89–93.
Goldstein JA. (1985). Calcium channel blockers in the treatment of panic disorder. J Clin Psychiatry, 46546.
Gonzalez-Castañeda RE, Galvez-Contreras AY, Martínez-Quezada CJ, et al. (2016). Sex-related effects of sleep deprivation on depressive-and anxiety-like behaviours in mice, Experimental Animal, Tokyo, 65: 97–107.
Hicks RA, Moore JD, Hayes C, et al. (1979). REM sleep deprivation increases aggressiveness in male rats. Physiology and Behaviour. 22: 1097–1100.
Hirshkowitz M, Whiton K, Albert SM, et al. (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep health, 1(1), 40-43
Koyuncu A, İnce E, Ertekin E, et al. (2019). Comorbidity in social anxiety disorder: diagnostic and therapeutic challenges. Drugs in context, 8.
Kuloğlu M, Ustundag B, Atmaca M, et al. (2002). Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochemistry and Function, 20:171-175.
Kumar A. and Garg R. (2008). A role of nitric oxide mechanism involved in the protective effects of venlafaxine in sleep deprivation. Behavioural Brain Research, 194:169-173.
Lautenbacher S, Kundermann B, and Krieg JC. (2006). Sleep deprivation and pain perception. Sleep Med Rev. 10(5):357-369.
Lentz MJ, Landis CA, Rothermel J, et al. (1999). Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle-aged women. J Rheumatol. 26(7):1586-1592.
Malkoff-Schwartz S, Frank E, Anderson B, et al. (1998). Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Archives of General Psychiatry, 55: 702–707.
Mansell W. and Pedley R. (2008). The ascent into mania: a review of psychological processes associated with the development of manic symptoms. Clinical Psychological Review, 28: 494–452Y.
Martinez-Aran A, Vieta E, Reinares M, et al. (2004). Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 161(2):262-270.
Martins PJF, Nobrega JN, Tufik S. et al. (2008). Sleep deprivation-induced gnawing—relationship to changes in feeding behaviour in rats, Physiology and Behaviour, 93:229–234.
Mathangi DC, Shyamala R. and Subhashini AS. (2012). Effect of REM sleep deprivation on the antioxidant status in the brain of Wistar rats. Annals in Neuroscience, 19: 161.
McCarty R, Josephs T, Kovtun O, et al. (2021). Enlightened: addressing circadian and seasonal changes in photoperiod in animal models of bipolar disorder. Translational Psychiatry, 11:373.
McClung CA. (2007). Circadian genes, rhythms and the biology of mood disorders. Pharmacology and Therapeutics, 114: 222–232.
McClung CA. (2011). Circadian rhythms and mood regulation: insights from pre-clinical models. European Neuropsychopharmacology 21 (Suppl. 4R): S683–S693.
Ozcan ME, Gulec M, Ozerol E, et al. (2004). Antioxidant enzyme activities and oxidative stress in affective disorders. International Clinical Psychopharmacology, 19:89-95.
Paul M. and Allan Y. (2012). Bipolar disorders. Wright P, Stern J, Phelan M. (Eds.) Core Psychiatry 21; 313-334.
Ramanathan L, Gulyani S, Nienhuis R. et al. (2002). Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem, Neuroreport, 13(11): 1387–1390.
Ranjekar PK, Hinge A, Hegde MV, et al. (2003). Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Research, 121:109-122.
Roehrs T, Hyde M, Blaisdell B, et al. (2006). Sleep loss and REM sleep loss are hyperalgesic. Sleep. 29(2):145-151.
Salvadore G, Quiroz JA, Machado-Vieira R, et al. (2010). The Neurobiology of the Switch Process in Bipolar Disorder: A Review. The Journal of Clinical Psychiatry. 71 (11): 1488–1501.
Schrimpf M, Liegl G, Boeckle M, et al. (2015). The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med. 16(11):1313-1320
Schuh-Hofer S, Wodarski R, Pfau DB, et al. (2013). One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain, 154;9: 1613-1621.
Singh R, Kiloung J, Singh S. et al. (2008). Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats, Biogerontology, 9(3): 153–162.
Smith MT, Perlis ML, Smith MS, et al. (2000). Sleep quality and presleep arousal in chronic pain. J Behav Med. 23(1):1-13
Stroemel-Scheder C, Kundermann B, and Lautenbacher S. (2020). The effects of recovery sleep on pain perception: A systematic review. Neurosci Biobehav Rev 113:408-425.
Tabet N, Mantle D. and Orrell M. (2000). Free radicals as mediators of toxicity in Alzheimer’s disease: a review and hypothesis, Adverse Drug Reaction and Toxicology Review. 19: 127–152.
Tamaddonfard E, Erfanparast A, Taati M, et al. (2014). Role of opioid system in verapamil-induced antinociception in a rat model of orofacial pain. Veterinary Research Forum 5 (1) 49 – 54
Umukoro S, Bakre TO, and Onwuchekwa C. (2010). Anti-psychotic and sedative effect of calcium channel blockers in mice. Afr J Med Med Sci. 39 Suppl:61-66.
Urien S, Pinquier JL, Paquette B, et al. (1987). Effect of the binding of isradipine and darodipine to different plasma proteins on their transfer through the rat blood-brain barrier. Drug binding to lipoproteins does not limit the transfer of drug. J Pharmacol Exp Ther 242:349-353.
Wehr TA. (1992). Improvement of depression and triggering of mania by sleep deprivation. Journal of American Medical Association, 267 (4R): 548–551.


