School of Environment and Sustainable Development, Central University of Gujarat, Gandhinagar, Gujarat, 382030, India
Corresponding author Email: pankajb434@yahoo.com
Article Publishing History
Received: 19/07/2019
Accepted After Revision: 20/09/2019
Mycorrhiza is a mutualistic relationship between microorganism and plant roots. The best communal relationship is the vesicular-arbuscular, which creates fungus assemblies like arbuscules and vesicles in the cortex section of the plant roots. The present research work assesses the potential of Sorghum bicolor for development of mycorrhizal soil. The soil-based mycorrhizal inoculum was established by Sorghum as a host plant in the greenhouse for two and a half months using pot culture technique. The physicochemical properties of soil like pH, electrical conductivity, soil moisture, water holding capacity, organic carbon, organic matter, available phosphorus, potassium, nitrate, nitrite, exchangeable ammonia were analyzed during the development of mycorrhizal soil. Bacterial and fungus populations were evaluated at every 15 days in the rhizospheric soil samples. Along these lines, developing mycorrhizae prompt a progression of changes in supplement accessibility, microbial structure and enzymatic exercises in the soil that may decide the result of a phytoremediation effort. In the present research work, the established mycorrhizal inoculum may contribute to building up a compelling mycorrhizosphere that can give nature to the improved degradation of toxins present in the soil. The findings revealed that the inoculum might comprise a very high percentage of organic carbon and other nutrients. The microbial populations were also increased with the progression of the study period.
Heavy Metals, Mycorrhiza, Mycorrhizal Inoculum, Rhizospheric Bioremediation, Sorghum.
Kumar P, Fulekar M. H. Mycorrhizal Soil Development Using Sorghum bicolor for Rhizospheric Bioremediation of Heavy Metals. Biosc.Biotech.Res.Comm. 2019;12(3).
Kumar P, Fulekar M. H. Mycorrhizal Soil Development Using Sorghum bicolor for Rhizospheric Bioremediation of Heavy Metals. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2mbvDeF
Copyright © Kumar and Fulekar, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Plants in natural environments have fluctuating degrees of dependency on mycorrhizal associations, as inclined by the accessibility of nutrients in the soil in which they certainly occur (Hindumathi and Reddy 2011). Plants are reliant on the mycorrhizal colonization to show extreme progress at a specified level of soil productiveness is known as Mycorrhizal dependence (Gerdermann 1975). Development of phytoremediation procedures needs a comprehensive consideration and supervision of the composite communications in the mycorrhizosphere. The main role of mycorrhizosphere organisms may have been marginalized in intensive farming since bacterial populations in conventional farming systems have been improved due to tillage and great efforts of inorganic manures (Abbasi et al., 2015). To enrich nutrient limits and contaminant noxiousness benefit may be taken in increasing and handling rhizosphere (Dubey and Fulekar 2011). Vesicular-arbuscular mycorrhiza fungi are dynamic constituents of the environment for the conservation of soil assembly and restoration of tainted lands (Caravaca et al. 2005; Fulekar et al. 2017,Chen et al. 2018).
Vesicular-arbuscular mycorrhiza is well-known to enhance soil nutrients thus enlightening the development and healthiness of the plants. VAM has been designated for nutrient such as phosphorous which has limited movement in the soil. It is perceived that VAM populated plants accrue and engage more phosphorous compared to plants that are not colonized, grown in soil that have low phosphorous (Azcón et al. 2003). The Vesicular-arbuscular mycorrhiza colonized roots not only enhanced the uptake of phosphorous but these also help in the accumulation of macro and micronutrients like copper, calcium, magnesium, iron (Allen et al. 2003).Mycorrhizal interdependences not only profit to plant progress but also to plant safety, particularly against ecological strains. Insufficiency of important nutrients specifically phosphorus, mainly limits crop development and efficiency (Nagarathna et al. 2007). The co-operative relation offers numerous welfares, comprising higher uptake of poorly movable soil nutrients and reduced vulnerability of roots to soil-borne pathogens (Quilambo 2003). The plants, developed in rhizospheric soil are extra viable and more tolerable to ecological pressures than normal plants, possibly improving contaminant accessibility (Dubey and Fulekar 2011; Gaur and Adholeya 2004).
Numerous investigation studies showed that persistent organic pollutants like PAH, aromatic hydrocarbons and hexachloro cyclohexane may be converting into less poisonous form, in the mycorrhizosphere (Huang et al. 2006; Sainz et al. 2006; Volante et al. 2005). Subsequently, the expansion of the rhizosphere by immunization of mycorrhiza has shown leniency to poisonous mixtures and also transforms the quality and richness rhizo-microbial communities accountable for biodegradation of polluted soil. The profits of the symbiotic relationship between plants and AMF are accredited to the more quantity of soil explored by the fungal hyphae, which delivers maximum water and nutrients fascination by the plant, moreover the removal and immobilization of heavy metals by fungal tissues (Wang et al. 2017).
Although the potential of AM fungi for the protection of plant is generally acknowledged, it should be noted that in some cases, mycorrhizal crops have no benefits from AM, or may even exhibit reduced growth and fitness (Chen et al. 2018; Jacott et al. 2017).Sorghum bicolor has been cultured in sub-Saharan Africa and South Asia for over 5000 years and highly receptive to AM fungi, for the growth of plants, in low-fertility soil (Cobb et al. 2016). Some microbes have been used in relationship with plants to lighten the stress instigated by metal contamination ( Leal et al. 2016). Arbuscular Mycorrhizal Fungi form effective cooperation with many plants and helps in progress and conferring better lenience to ecological strains, including heavy metal contamination (Ogar et al. 2015; Rajkumar et al. 2012; Wang et al. 2017 Chen et al. 2018).
For rhizospheric bioremediation studies, development of a cost-effective technique for the bulk invention of mycorrhizal inoculum is of extreme significance. Since the above-mentioned facts, current research work was carried out to develop and evaluate the potential of soil-based mycorrhizal inoculum for effective rhizosphere bioremediation of hazardous compound and to study the influence of mycorrhiza on soil physicochemical parameters during the development of mycorrhizosphere.
Material and Methods
Host Plant and Testing
The experiment was conducted in the greenhouse of the School of Environment and Sustainable Development, Central University of Gujarat, Gandhinagar, Gujarat. Sorghum bicolor commonly called as Sorghum a fibrous root grass which is widely used for culturing the mycorrhiza was chosen as a host plant in the current study for the development of mycorrhizal soil at laboratory scale. The sorghum seeds were procured from Department of Seed Technology, Indian Grassland and Fodder Research Institute, Jhansi, Uttar Pradesh. Prior to their use in the experiment, the seeds were surface-sterilized for 5 mins with 0.1 percent HgCl2 and consequently washed numerous times with distilled water to equivocate fungus infection. The washed seeds were kept in the petri dishes holding wet filter paper and incubated for a period of three days in the dark. The germinated seeds were used for the development of mycorrhizal soil.
Collection of Soil for Experiment
The experimental soil used for the development of mycorrhizal soil was collected from the bank of Sabarmati River, Gandhinagar, Gujarat from a depth of 0-15 cm. The soil was then dehydrated and screened using 2 mm sieve. The soil was mixed strongly for 20 mins in deionized water and physicochemical parameters viz. pH, EC, moisture, organic carbon, water holding capacity, organic matter, available phosphorus, potassium, nitrate, nitrite, exchangeable ammonia were analyzed using standard procedures.
Experimental Design
A greenhouse experiment was carried out for the development of mycorrhizal soil using pot culture technique under the controlled environmental condition with the help of inoculum (VAM) in the form of a combination of soil, spores, root fragments, and inoculum was acquired from Department of Microbiology, Indian Agricultural Research Institute, New Delhi.The greenhouse experiment was performed in 3 kg capability pots perforated at the base. The mixture of collected soil, sand and starter mycorrhizal inoculum (3:1:1) used for the experiment to offer uniformity and permeability to the soil. After that twenty-five, external sanitized sorghum seeds were sown in each pot and their progression was observed for two and a half months. The Hogland solution was provided to the plants to keep free from phosphorous. Around 10 ml of Hogland solution was delivered per pot. The pots were kept in a greenhouse with natural sunlight at temperatures of 27-28oC during the day and 24-26oC during the night. The growth of mycorrhiza (mycorrhizal properties) was dignified for physicochemical changes happening in soil by collecting the rhizospheric soil and roots at the regular intervals of 15 days for the entire period of 75 days. The soil was dehydrated at 105ºC for water holding capacity determination. The spore count (per 100 gm. soil) and % colonization by mycorrhizal fungi was also carried out. Sorghum being a fibrous root grass found to grow well for the period of 21/2 months in greenhouse condition and delivered an enormous network of fine roots.
Soil Sampling and Analysis
The soil samples from the set up were collected at a regular interval of 15 days in order to determine soil physicochemical properties. The samples were collected in triplicate and preserved in the plastic bags, labelled and analyzed for following parameters: the estimation of pH was (1:2.5 w/v) by digitalized pH meter (Woermann 1973), Electrical conductivity was calculated (1:2.5 w/v) by conductivity meter. Organic carbon and Organic matter were also calculated (Osuji and Adesiyan 2005), Available Phosphorus was measured (Kovar et al. 2009). Soil moisture, water holding capacity, nitrate, and potassium were also analyzed as per the standard methods.
Description of Bacterial and Fungal Population
The classification for the microbial population started within 5-6 hours of soil collection using serial dilution technique or plate count method (Yee et al. 1998). 1gm of collected soil samples was mixed with 10 mL of sterile water. Once the dilution is completed, the sample was then supplied onto petri plates having an agar growth medium with added nutrients. Afterward, an aliquot of 0.1 mL of dilutions was spread onto agar plates from the suitable dilution tubes and incubated at 37oC. The microbial population was calculated after twenty-four hours. The fungus population was calculated after 48 to 72 hours (Kumar and Fulekar 2018).
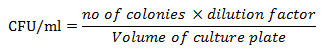
Root Colonization
1 gm. of root segments were cautiously rinsed with distilled water and divided into 1cm long sections. The fragments of root were cleaned with 10 percent potassium hydroxide (KOH) at 90oC for 15 to 30 min and rinsed with distilled water, before acidification through 2 percent Hydrochloric acid (HCl) for 10 min. after that the roots were stained with 0.01 percent acidic magenta dye (Kormanik, P. P., Bryan, W. C., and Schultz 1980).
Statistical Analysis
The physicochemical characterizations of every sample were calculated in triplicates and the obtained values were described as Mean±SD. Correlation factor was determined to ascertain the affiliation between physicochemical properties.
Results and Discussion
Characterization of Soil
The soil was obtained from Sabarmati river basin for experimental purpose and analyzed for physicochemical analysis. The collected soil was sandy loamy in nature. The pH was found with an average value of 7.83±0.08. Electrical conductivity was found with an average value of 0.36±0.03mS/cm. Soil moisture content with an average value of 12.09±1.08%. The mean water holding capacity of the soil was found 59.25±1.15%. Organic carbon with an average value of 0.36±0.02% and the mean value of organic matter was 0.61±0.03%. Available phosphorus was found with an average value of 0.97±0.04mg/kg Potassium was found with an average concentration of 19.20±0.28mg/kg. The calcium was very high in the collected soil with an average value of 11817±2.65ppm and magnesium was found with an average value of 1270.17±2.75ppm. Nitrate was found with a mean value of 12.62±0.37ppm. Table 1 is showing the physicochemical characterization of soil collected for experimental purpose.
Table 1: Physicochemical Properties of Collected Soil (Sandy loamy)
| Physicochemical Properties | Mean±SD |
|
pH |
7.83±0.08 |
|
EC (mS/cm) |
0.36±0.03 |
|
Soil moisture (%) |
12.09±1.08 |
|
Water Holding Capacity (%) |
59.25±1.15 |
|
Organic Carbon (%) |
0.36±0.02 |
|
Organic Matter (%) |
0.61±0.03 |
|
Available Phosphorus (mg/kg) |
0.97±0.04 |
|
Potassium (mg/kg) |
19.20±0.28 |
|
Sulphate (mg/kg) |
5.08±0.15 |
|
Calcium (ppm) |
11817.00±2.65 |
|
Magnesium (ppm) |
1270.17±2.75 |
|
Nitrates (ppm) |
12.62±0.37 |
|
VAM colonization (%) |
Nil |
|
Spore count (per 100gm soil) |
Nil |
Physicochemical Categorization of Mycorrhizal Inoculum
The mycorrhizal inoculum was acquired from the Department of Microbiology, Indian Agricultural Research Institute, New Delhi and characterized for different parameters (Table 2). The pH was found with an average value of 8.23±0.09. Electrical conductivity was found with an average value of 1.34±0.10mS/cm. Soil moisture content with an average value of 31.20±0.93%. Water holding capacity of the soil was found 41.33±1.26%. Organic carbon with an average value of 0.61±0.02%. Organic matter was found 1.05±0.03%. Available phosphorus was found with an average value of 1.24±0.04mg/kg. Potassium was found with an average concentration of 20.37±0.14mg/kg. Nitrate, Nitrite and Exchangeable ammonia were found with an average value of 7.41±0.14mg/kg and 1.91±0.03mg/kg and 35.65±0.30mg/kg respectively.
Table 2: Characterization of Mycorrhizal Inoculum
| Physicochemical parameters | Mean±SD |
| pH | 8.23±0.09 |
| EC (mS/cm) | 1.34±0.10 |
| Soil moisture (%) | 31.20±0.93 |
| Water Holding Capacity (%) | 41.33±1.26 |
| Organic Carbon (%) | 0.61±0.02 |
| Organic Matter (%) | 1.05±0.03 |
| Available Phosphorus (mg/kg) | 1.24±0.04 |
| Potassium (mg/kg) | 20.37±0.14 |
| Nitrate (mg/kg) | 7.41±0.14 |
| Nitrite (mg/kg) | 1.91±0.03 |
| Exchangeable Ammonia (mg/kg) | 35.65±0.30 |
|
VAM colonization (%) |
76 |
|
Spore count (per 100gm soil) |
450 |
Mycorrhizal soil – Development and Characterization
Mycorrhizal soil has been developed and classified for different physicochemical properties at regular interval of 15 days (Table 3). The pots kept in the greenhouse for the development of mycorrhizal soil (Figure 7).
Table 3: Variations in different properties of rhizospheric soil during mycorrhizal development
| Physicochemical
parameters |
15 Days
Mean±SD |
30 Days
Mean±SD |
45 Days
Mean±SD |
60 Days
Mean±SD |
75 Days
Mean±SD |
| pH | 8.21±0.02 | 8.40±0.02 | 8.26±0.06 | 8.22±0.07 | 8.10±0.07 |
| EC (mS/cm) | 1.89±0.02 | 1.80±0.01 | 2.53±0.04 | 3.01±0.09 | 2.40±0.22 |
| Soil moisture (%) | 31.67±0.45 | 41.50±0.60 | 43.81±0.25 | 45.92±0.33 | 46.88±1.49 |
| Water Holding Capacity (%) | 34.87±0.35 | 40.50±1.00 | 42.46±0.54 | 40.70±0.44 | 41.63±0.38 |
| Organic Carbon (%) | 0.39±0.02 | 0.35±0.02 | 0.55±0.02 | 0.46±0.02 | 0.58±0.07 |
| Organic Matter (%) | 0.67±0.03 | 0.59±0.04 | 0.94±0.04 | 0.78±0.03 | 0.87±0.02 |
| Available Phosphorus (mg/kg) | 0.95±0.02 | 0.88±0.09 | 0.95±0.02 | 0.97±0.05 | 1.38±0.47 |
| Potassium (mg/kg) | 8.79±0.04 | 7.48±0.07 | 10.83±0.14 | 9.98±0.14 | 11.11±0.76 |
| Nitrate (mg/kg) | 14.43±0.46 | 9.40±0.16 | 11.66±0.41 | 13.42±0.39 | 14.63±0.51 |
| Nitrite (mg/kg) | 8.58±0.30 | 6.04±0.06 | 7.84±0.25 | 9.36±0.45 | 10.65±0.39 |
| Exchangeable Ammonia (mg/kg) | 12.33±0.34 | 11.20±0.32 | 15.56±0.36 | 16.80±0.22 | 17.55±0.33 |
|
VAM colonization (%) |
14 | 29 | 34 | 59 | 76 |
|
Spore count (per 100gm soil) |
75 | 190 | 260 | 380 | 560 |
Correlation Matrix
It is determined from the Table 4 that significant positive association between the pairs of some physicochemical properties of rhizospheric soil followed as-soil moisture with water holding capacity (r = 0.93), organic carbon with organic matter (r = 0.95), with potassium (r = 0.97), organic matter with potassium (r = 0.96), potassium with exchangeable ammonia (r = 0.92), nitrate with nitrite (r = 0.91), and VAM colonization is highly positively correlated with spore count (r = 0.99).
Table 4: Pearson’s correlation coefficient (r) among physicochemical factors of rhizospheric soil during mycorrhizal development
| A | B | C | D | E | F | G | H | I | J | K | L | M | |||
| A | 1 | ||||||||||||||
| B | -0.41 | 1 | |||||||||||||
| C | -0.17 | 0.68 | 1 | ||||||||||||
| D | 0.05 | 0.52 | 0.93 | 1 | |||||||||||
| E | -0.70 | 0.58 | 0.61 | 0.59 | 1 | ||||||||||
| F | -0.57 | 0.66 | 0.56 | 0.59 | 0.95 | 1 | |||||||||
| G | -0.81 | 0.21 | 0.46 | 0.28 | 0.72 | 0.48 | 1 | ||||||||
| H | -0.77 | 0.70 | 0.56 | 0.49 | 0.97 | 0.96 | 0.65 | 1 | |||||||
| I | -0.94 | 0.32 | -0.11 | -0.36 | 0.45 | 0.35 | 0.61 | 0.57 | 1 | ||||||
| J | -0.97 | 0.55 | 0.31 | 0.04 | 0.68 | 0.55 | 0.81 | 0.76 | 0.91 | 1 | |||||
| K | -0.74 | 0.85 | 0.73 | 0.56 | 0.87 | 0.82 | 0.68 | 0.92 | 0.56 | 0.82 | 1 | ||||
| L | -0.59 | 0.64 | 0.85 | 0.62 | 0.67 | 0.51 | 0.79 | 0.66 | 0.37 | 0.72 | 0.86 | 1 | |||
| M | -0.60 | 0.60 | 0.86 | 0.67 | 0.74 | 0.58 | 0.83 | 0.71 | 0.35 | 0.71 | 0.86 | 0.99 | 1 | ||
A – pH, B – Electrical conductivity, C – Soil moisture , D – Water holding capacity, E – Organic carbon, F – Organic matter, G – Available phosphorus, H – Potassium, I – Nitrate, J – Nitrite, K – Exchangeable Ammonia, L – VAM colonization, M – Spore count .
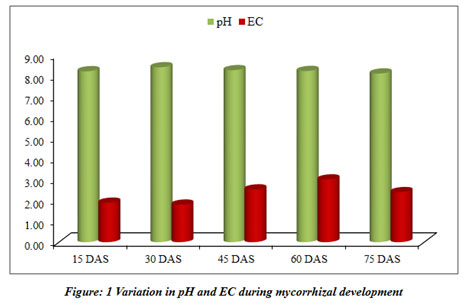 |
Figure 1 |
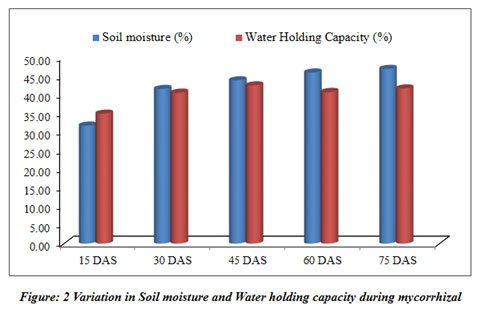 |
Figure 2 |
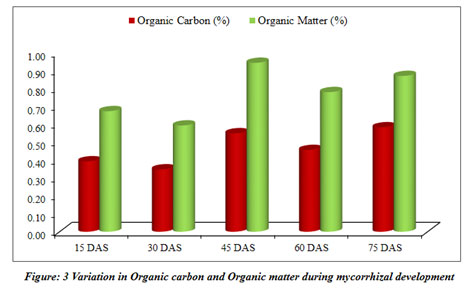 |
Figure 3 |
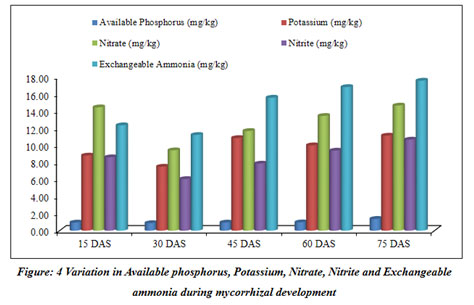 |
Figure 4 |
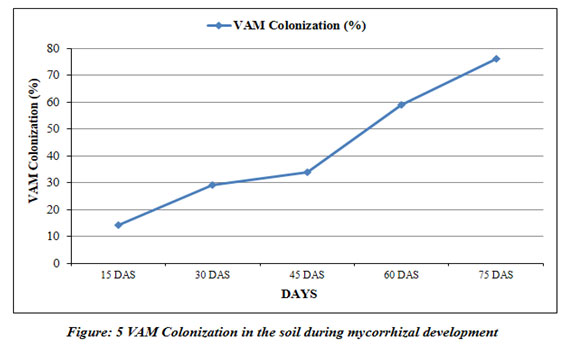 |
Figure 5 |
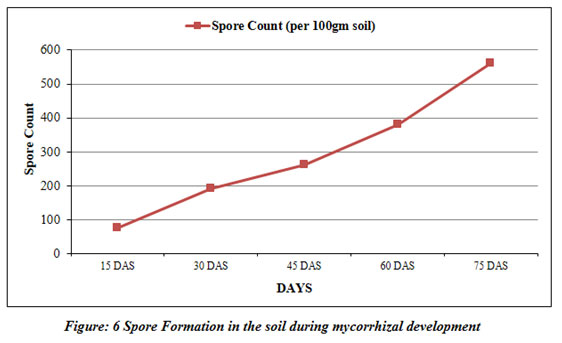 |
Figure 6 |
 |
Figure 7 |
Morphological Characteristics of Plant
The morphological properties of the Sorghum plant were also evaluated; root length and shoot length were measured at every 15 days during the development of mycorrhizal soil (Table 5).
Table 5: Characteristics (Root Length and Shoot Length) of Plant
|
Days |
Root Length (cm)
Mean±SD |
Shoot Length (cm)
Mean±SD |
| 15 DAS | 3.20 ± 0.24 | 8.92 ± 0.96 |
| 30 DAS | 4.80 ± 0.22 | 17.37 ± 0.66 |
| 45 DAS | 5.77 ± 0.31 | 25 ± 0.41 |
| 60 DAS | 9.20 ± 1.75 | 30.50 ± 1.08 |
| 75 DAS | 12.50 ± 0.82 | 37.97± 1.23 |
Estimation of Plant’s Biomass
The total biomass of the plant was evaluated by the addition of root dry weight and shoot dry weight (Table 6).
|
Days |
Root Dry Weight (gm.) |
Shoot Dry Weight (gm.) |
Total Plant Biomass (gm.) |
||
| 15 DAS | 0.025 ± 0.0029 | 0.884 ± 0.0006 | 0.909 ± 0.0008 | ||
| 30 DAS | 0.054 ± 0.0016 | 1.184 ± 0.0095 | 1.238 ± 0.0095 | ||
| 45 DAS | 0.097 ± 0.0009 | 1.943 ± 0.0113 | 2.040 ± 0.0113 | ||
| 60 DAS | 0.1117± 0.0090 | 2.56 ± 0.0370 | 2.657 ± 0.0923 | ||
| 75 DAS | 0.226 ± 0.0045 | 3.15 ± 0.0364 | 3.379 ± 0.0392 |
Microbial Status
Mycorrhiza is well-known to collaborate and regulate the bacterial populations and their richness in the soil (Pilon-Smits 2005). Thus the present research work includes the assessment of biotic factors along with physicochemical properties of the soil. The microbial population was evaluated at five intervals (Table 7).
Table 7: Microbial Counts during the Development of Mycorrhizal Soil
| Microorganism | 15 Days
|
30 Days
|
45 Days
|
60 Days
|
75 Days
|
|
Bacterial Count |
2.9X106 | 3.7X106 | 4.9X106 | 5.6X106 | 6.8X106 |
|
Fungal count |
1.6X104 | 1.9X104 | 2.1X104 | 2.6X104 | 2.8X104 |
Table 8: Microbial Species Obtained during Mycorrhizal Soil Development
| Bacterial Genera | Fungal Genera |
| Streptococcus spp. | Aspergillus niger |
| Bacillus spp. | Rhizopus spp. |
| Pseudomonas spp. | Aspergillus flavus |
| Alcaligenes spp. | Mucor spp. |
The current study mainly focuses on mycorrhizal soil development using Sorghum bicolor under the controlled condition of the greenhouse. The physical and chemical properties of soil were also analyzed throughout the progression of mycorrhiza at every 15 days and the results revealed that developed mycorrhizal soil is having enhanced pH, organic carbon and other properties than in the soil collected for the experiment and found to be increasing (Table 3). The pH of the soil remained in the range of 8.2 to 8.1 during the experiment of total two and a half months, while the EC was increasing throughout the experiment (Table 3). The colonization of mycorrhiza is encouraged in well-aired soil so the moisture of the soil was increasing from 31.67% to 46.88%. The soil collected for the experiment was detected to be low in organic carbon 0.36%, which increased during the experiment and ranged from 0.39% to 0.58%. Phosphorus was also observed increasing from 0.95% to 1.38%. It is a critical soil nutrient that AM fungi acquire and transfer to host plants (Cobb et al. 2018). Nitrate and nitrite content did not vary significantly. Increase in carbon during the study as may be contributing to the enhanced progress and fitness of the plants. Vesicular-arbuscular mycorrhiza is sensitive to the moisture of the soil and optimum moisture is essential for vesicular-arbuscular mycorrhiza sporulation (Redhead 1975). The response of vesicular-arbuscular mycorrhiza fungi to soil pH might reliant on the strains and species unifying the native vesicular-arbuscular mycorrhiza flora (Robson 1989). The early research studies reported that Glomus species needs alkaline to neutral soil for their predominance (Mosse et al. 1981). The role of nitrogen is very important in inducing the formation of mycorrhiza and functions through stimulating or suppressing the colonization of root and production of spores by arbuscular mycorrhiza fungi (Sylvia and Neal 1990).
Mycorrhizal classification carried out by assessing the colonization of vesicular-arbuscular mycorrhiza (Fig 5) and counting of spore formation at every 15 days (Fig 6). The colonization of host plant’s roots was flourished by the 15th day. The establishment of the roots by vesicular-arbuscular mycorrhiza heightened from 14% to 76% from 15th day to 75th day. The colonization was clearly found to be more in the last week and amplified by the end of the experiment. Mickan et al., (2016) reported similar results of amplified colonization for subterranean clover (Trifolium subterraneum) grown in water-limited agricultural soils. The most significant period of the existence of vesicular-arbuscular mycorrhiza is spores formation. Its morphological attributes help distinguishing proof and order of VAM into discrete genera (Quilambo 2003).
The findings of the current study have clearly showed that the spore formation was expanded with time (Fig 6). It is clear from our findings that the colonization of root indicated a positive relationship with the number of spores formed in mycorrhizal soil and expected that proliferation in root settlement caused a rise in spore formation throughout the mycorrhiza development. Successive spore formation and colonization of roots may be dependent on the edaphic factors, host plant and microbial population (Fulekar et al. 2017; Khalil et al. 1992).
Conversely, certain investigators described that no substantial association between the density of spores and colonization of VAM (Li et al. 2007). Usually, the spore accessibility affects the colonization of AMF (Muthukumar et al. 2003). The relationships between the mycorrhiza and a bacterial associate may affect other members of the mycorrhizosphere community.
The presence of a significant positive correlation between spore density and root colonization can be ascribed that greater spore density is associated with the rate and magnitude of mycorrhizal development (Table 4). The current research work is in agreement with the earlier findings of Wu et al (2006) in Citrus. The insignificant correlation was noted between spore density and other soil properties like pH, electrical conductivity, organic carbon indicating that these factors had no effect or may poorly reflect spore population density (Hindumathi and Reddy 2011). Various studies have assessed the effect of organic matter on arbuscular mycorrhizae (Gaur and Adholeya 2002; Gryndler et al. 2002) with different results indicating their variable responses on plants and fungi. However, root colonization was significantly negatively correlated with soil pH. The present study suggests that the colonization of roots is prejudiced by soil edaphic features like nutrient status, pH, electrical conductivity, organic carbon and host cultivar vulnerability. Mycorrhizal dependency is a constitutive property of plant species. Rapid colonization of roots during the early stages of the host plant is an essential requirement for good host plant response to mycorrhiza (Hindumathi and Reddy 2011).
Viable plate counts
Microbial population and biochemical processes linked with roots may be affected by arbuscular mycorrhiza fungi colonized roots. Microbial population in the soil can be modified by interacting mycorrhizae, so the bacterial and fungi population was evaluated throughout the mycorrhizal soil inoculum growth. Bacterial CFU enlarged along the period and found 2.9X106 at 15th day, 3.7X106 at 30th day, 4.9X106 at 45th day, 5.6X106 at 60th day and 6.8X106 at the 75th day (Table 7). The bacterial species found during the development of mycorrhizal soil are Streptococcus spp., Bacillus spp., Pseudomonas spp., Alcaligenes spp. (Table 8).The fungal population was observed with the marginal proliferation 1.6X104 at 15th day, 1.9X104 at 30th day, 2.1X104 at 45th day, 2.6X104 at 60th day and 2.8X104 at the 75th day (Table 7). The recognized fungal species were Aspergillus niger, Rhizopus spp., Aspergillus flavus Mucor spp. (Table 8).
Role of AMF for Rhizosphere Bioremediation of Heavy Metal Contaminated Soils
During the last decades, the potential of plants has been explored to reduce the heavy metal pollution in soils and AM fungi might possibly play a vital role in such approaches (Chen et al. 2018). Several laboratory studies have been carried out to explore the potential of AM in bioremediation of the soil, however, only few field studies have addressed the applicability of this approach to large scale conditions (Chibuike 2013).
Microorganisms present in the soil play a very important role in the mobilization of metal ions, thus altering their accessibility to plants. Vesicular-arbuscular Mycorrhizal Fungi are usually present in soil and form a significant functional factor of the soil-plant system including anxious soils. Arbuscular Mycorrhizal Fungi might be exaggerated by metal toxicity, but it is revealed that plants developing in soils polluted with metals are populated by Arbuscular Mycorrhizal Fungi ( Mathur et al. 2007). Many reports concerning this have quantified spores and estimated root colonization in situ. Others have gone further and described metal tolerant AMF in heavy metal polluted soils (Weissenhorn and Leyval 1995). The mycorrhizal fungi can also affect plant tolerance to heavy metals by altering the antioxidant enzyme activities. Azcón et al. (2010) studied the effects of autochthonous microbes on the antioxidant activities of plants developing in a heavy metal contaminated soil. They found that AMF inoculation expressively improved catalase, ascorbate peroxidase, or glutathione reductase activities and helped plants to limit oxidative damage to biomolecules in response to metal stress (Rajkumar et al. 2012). These fungi have the capability of metal chelation, which is provided by organic constituents occur at the edge of the plasma membrane and the separation of poisonous components in vesicles and spores, functioning as a tool that delivers plant lenience to heavy metal (Cornejo et al. 2013).
The rhizoremediation process exploits on the distinctive potentials of flora and the possibilities for transformation of contaminants in the rhizosphere. Hence, scientists are aiming at the rhizospheric zone as of upgraded degradation and plant-microbe communications (Korade and Fulekar 2009; Olson et al. 2003). The utilization of Vesicular-arbuscular mycorrhizal fungi as a clean and cost-effective management strategy may help in the better growth of plants and also in the removal of heavy metal polluted soil.
Conclusion
The developed mycorrhizal soil in the current study was further efficiently utilized in greenhouse experiment conducted using other grass species for the rhizosphere bioremediation of heavy metals. Therefore inoculum was found to have an advantageous relationship of microscopic organisms with root zone in the rhizosphere; consequently subsidizing essentially to the foundation of compelling rhizosphere that can deliver the surroundings for the existence and development of microbes just as an increment in natural carbon which additional supports to improve deprivation of pollutants in the soil. Arbuscular mycorrhizal fungi endorse many features of plant life, in specific better nutrition, enhanced growth, stress tolerance, and disease resistance. The results also concluded that mycorrhizosphere relations can enhance the growth and health of plants and soil quality. By limited efforts, a fruitful rhizoremediation system could be more useful to many other clean-up technologies.
Acknowledgments
The research work was supported by the University Grants Commission, New Delhi, India, under the scheme of Rajiv Gandhi National Fellowship (F1-17.1/2014-15/RGNF-201415-SC-UTT-69661). The author is also thankful to the Central Instrumentation Facilities provided by Central University of Gujarat, Gandhinagar, India.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
Abbasi, H., Ambreen, A. and Rushda, S. (2015). Vesicular Arbuscular Mycorrhizal (VAM) Fungi: A Tool for Sustainable Agriculture. American Journal of Plant Nutrition and Fertilization Technology 5 (2): 40-49.
Allen, M. F., Swenson, W., Querejeta, J. I., Egerton-Warburton, L. M., and Treseder, K. K. (2003). Ecology of mycorrhizae: A Conceptual Framework for Complex Interactions Among Plants and Fungi. Annual Review of Phytopathology, 41(1), 271-303.
Azcón, R, Ambrosano, E., and Charest, C. (2003). Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Science, 165(5), 1137-1145.
Azcón, Rosario, del Carmen Perálvarez, M., Roldán, A., and Barea, J.-M. (2010). Arbuscular Mycorrhizal Fungi, Bacillus cereus, and Candida parapsilosis from a Multicontaminated Soil Alleviate Metal Toxicity in Plants. Microbial Ecology, 59(4), 668-677.
Caravaca, F., Alguacil, M. M., Barea, J. M., and Roldán, A. (2005). Survival of inocula and native AM fungi species associated with shrubs in a degraded Mediterranean ecosystem. Soil Biology and Biochemistry, 37(2), 227-233.
Chen, M., Arato, M., Borghi, L., Nouri, E., and Reinhardt, D. (2018). Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Frontiers in Plant Science, 9(September), 1-14.
Chibuike, G. U. (2013). Use of mycorrhiza in soil remediation: A review. Scientific Research and Essays, 8(35), 679-1687.
Cobb, A. B., Wilson, G. W. T., and Goad, C. L. (2018 ) Linking sorghum nutrition and production with arbuscular mycorrhizal fungi and alternative soil amendments. Journal of Plant Nutrition and Soil Science, 181(2), 211-219.
Cobb, A. B., Wilson, G. W. T., Goad, C. L., Bean, S. R., Kaufman, R. C., Herald, T. J., and Wilson, J. D. (2016). The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: Enhancing sustainability through plant-microbial partnership. Agriculture, Ecosystems and Environment, 233, 432-440.
Cornejo, P., Pérez-Tienda, J., Meier, S., Valderas, A., Borie, F., Azcon-Aguilar, C., and Ferrol, N. (2013). Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil Biology and Biochemistry, 57, 925-928.
Dubey, K., and Fulekar, M. (2011). Mycorrhizosphere development and management: The role of nutrients, micro-organisms and bio-chemical activities. Agriculture and Biology Journal of North America, 2(2), 315-324.
Fulekar, J., Pathak, B., and Fulekar, M. H. (2017). Development of Mycorrhizosphere Using Sorghum bicolor for Rhizosphere Biormediation. International Journal of Current Research and Academic Review, 5(6), 42-48.
Gaur, A., and Adholeya, A. (2002). Arbuscular-mycorrhizal inoculation of five tropical fodder crops and inoculum production in marginal soil amended with organic matter. Biology and Fertility of Soils, 35(3), 214-218.
Gaur, A., and Adholeya, A. (2004). Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Current Science, 86.
Gerdermann, J. W. (1975). Vesicular-arbuscular mycorrhizae. In J. G. Torrey and D. T. Clarkson Eds (Ed.), The Development and Function of Roots. (pp. 491–575). Academic Press, New York.
Gryndler, M., Vosátka, M., Hršelová, H., Chvátalová, I., & Jansa, J. (2002). Interaction between arbuscular mycorrhizal fungi and cellulose in growth substrate. Applied Soil Ecology, 19(3), 279–288.
Hindumathi A. and Reddy B. N. (2011). Dependency of Sorghum on Arbuscular Mycorrhizal Colonization for Growth and Development. J Mycol Plant Pathol, 41(4).
Huang, H., Zhang, S., Chen, B.-D., Wu, N., Shan, X.-Q., and Christy, P. (2006). Uptake of Atrazine and Cadmium from Soil by Maize (Zea mays L.) in Association with the Arbuscular Mycorrhizal Fungus Glomus etunicatum. Journal of Agricultural and Food Chemistry, 54(25), 9377–9382.
Jacott, C., Murray, J., and Ridout, C. (2017). Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding. Agronomy, 7, 75.
Khalil, S., Loynachan, T. E., and McNabb, H. S. (1992). Colonization of Soybean by Mycorrhizal Fungi and Spore Populations in Iowa Soils. Agronomy Journal, 84(5), 832–836.
Korade, D. L., and Fulekar, M. H. (2009). Development and evaluation of mycorrhiza for rhizosphere bioremediation. Journal of Applied Biosciences, (September), 922–929.
Kormanik, P. P., Bryan, W. C., and Schultz, R. C. (1980). Procedures and equipment for staining large numbers of plant-roots samples for endomycorrhizal assay. Can. J. Microbiol, 26, 536–538.
Kovar, J. L., Pierzynski, G. M., and Hodges, S. C. (2009). Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters Second Edition.
Kumar, P., and Fulekar, M. H. (2018). Rhizosphere Bioremediation of Heavy Metals (Copper and Lead) by Cenchrus ciliaris. Research Journal of Environmental Sciences, 12(4), 166-176.
Li, N. F., Zhang, Y., and Zhao, Z. W. (2007). Arbuscular mycorrhizal colonization and spore density across different land-use types in a hot and arid ecosystem, southwest China. Journal of Plant Nutrition and Soil Science, 170(3), 419-425.
Lopes Leal, P., Varón-López, M., Gonçalves de Oliveira Prado, I., Valentim dos Santos, J., Fonsêca Sousa Soares, C. R., Siqueira, J. O., and de Souza Moreira, F. M. (2016). Enrichment of arbuscular mycorrhizal fungi in a contaminated soil after rehabilitation. Brazilian Journal of Microbiology, 47(4), 853-862.
Mickan, B. S., Abbott, L. K., Stefanova, K., and Solaiman, Z. M. (2016). Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza, 26(6), 565-574.
Mosse, B., Stribley, D. P., and LeTacon, F. (1981). Ecology of Mycorrhizae and Mycorrhizal Fungi BT – Advances in Microbial Ecology. In M. Alexander (Ed.), (pp. 137–210). Boston, MA: Springer US.
Muthukumar, T., Sha, L., Yang, X., Cao, M., Tang, J., and Zheng, Z. (2003). Mycorrhiza of plants in different vegetation types in tropical ecosystems of Xishuangbanna, southwest China. Mycorrhiza, 13(6), 289-297.
Nagarathna, T. K., Prasad, T. G., Bagyaraj, D. J., and Shadakshari, Y. G. (2007). Effect of arbuscular mycorrhiza and phosphorus levels on growth and water use efficiency in Sunflower at different soil moisture status. Journal of Agricultural Technology, 3(2), 221-229.
Mathur, N., Singh, J., Bohra, S., and Quaizi, A. V. (2007). Arbuscular Mycorrhizal Fungi: A Potential Tool for Phytoremediation. Journal of Plant Sciences, 2(2), 127-140.
Ogar, A., Sobczyk, Ł., and Turnau, K. (2015). Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environmental Science and Pollution Research, 22(23), 19142-19156.
Olson, P. E., Reardon, K. F., and Pilon-Smits, E. A. H. (2003, September). Ecology of Rhizosphere Bioremediation. Phytoremediation.
Osuji, L. C., and Adesiyan, S. O. (2005). The Isiokpo Oil-Pipeline Leakage: Total Organic Carbon/Organic Matter Contents of Affected Soils. Chemistry & Biodiversity, 2(8), 1079-1085.
Pilon-Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology, 56(1), 15–39.
Quilambo, O. A. (2003). The vesicular-arbuscular mycorrhizal symbiosis. African Journal of Biotechnology, 2(12), 539-546.
Rajkumar, M., Sandhya, S., Prasad, M. N. V., and Freitas, H. (2012). Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnology Advances, 30(6), 1562-1574.
Redhead, J. (1975). Endotrophic mycorrhizal in Nigeria: some aspects of the ecology of the endotrophic mycorrhizal association of Khaya gandiflora C.D.C. In F.E.Sanders; B.Mosse and P.B Tinker (Ed.), Ectomycorrhizas (pp. 447–460). New York Academic Press.
Robson AD, A. L. (1989). The effect of soil acidity on microbial activity in soils. In A.D. Robson (Ed.), Soil acidity and plant growth (pp. 139–165). Sydney Academic Press.
Sainz, M. J., González-Penalta, B., and Vilariño, A. (2006). Effects of hexachlorocyclohexane on rhizosphere fungal propagules and root colonization by arbuscular mycorrhizal fungi in Plantago lanceolata. European Journal of Soil Science, 57(1), 83–90.
Sylvia, D. M., and Neal, L. H. (1990). Nitrogen affects the phosphorus response of VA mycorrhiza. New Phytologist, 115(2), 303-310.
Volante, A., Lingua, G., Cesaro, P., Cresta, A., Puppo, M., Ariati, L., and Berta, G. (2005). Influence of three species of arbuscular mycorrhizal fungi on the persistence of aromatic hydrocarbons in contaminated substrates. Mycorrhiza, 16(1), 43-50.
Wang, L., Ji, B., Hu, Y., Liu, R., and Sun, W. (2017). A review on in situ phytoremediation of mine tailings. Chemosphere, 184, 594–600.
Weissenhorn, I., and Leyval, C. (1995). Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomus mosseae and cadmium uptake in sand culture. Plant and Soil, 175, 233-238.
Woermann, D. (1973). R. G. Bates: Determination of pH, Theory and Practice. 2nd Edition, John Wiley & Sons, New York, London, Sydney, Toronto 1973. 479 Seiten. Preis: £ 10.00. Berichte der Bunsengesellschaft für physikalische Chemie, 77(9), 737-737.
Yee, D. C., Maynard, J. A., and Wood, T. K. (1998). Rhizoremediation of trichloroethylene by a recombinant, root-colonizing Pseudomonas fluorescens strain expressing toluene Ortho-monooxygenase constitutively. Applied and Environmental Microbiology, 64(1), 112–118.


