Post Graduate Department of Zoology, Natural and Applied Sciences (Zoology) Research Centre,
Raja Narendra Lal Khan Women’s College, Paschim Medinipur, West Bengal, India.
Corresponding author email: chandaangsuman182@gmail.com
Article Publishing History
Received: 11/09/2021
Accepted After Revision:
Present study is a first-time report of flathead Sillago, Sillaginopsis panijus (Hamilton, 1822), from Rupnarayan River of West Bengal. Seasonal sampling performed from January 2019- February 2020 by collection of water sample and fish sample in the morning time 5.00 A.M. – 8.00 A.M. A total of 116 specimens of Sillaginopsis panijus (Hamilton, 1822) were collected from four different sampling stations of Rupnarayan river (22.23°N 88.03°E to 22.40°N 87.36°E), West Bengal, India. Present work is a morphometric and meristic data analysis has been provided in detail. Total 23 morphometric characters and 13 meristic characters were analyzed. Morphological characteristics of the species were present to confirm the occurrence and distribution of Sillaginopsis panijus (Hamilton, 1822) along the riverine water of Rupnarayan.
The physico-chemical parameters of water have been measured such as temperature of water, dissolved oxygen, pH and salinity. The statistical analysis of multivariate test with post-Hoc analysis and correlation were established with the abundance of S. panijus (Hamilton, 1822) in relation to water parameters. The result shows the dissolved oxygen, temperature, pH and salinity played a most important role in the distribution of S. panijus (Hamilton, 1822). The result shows a statistically significant difference in distribution of fish species, F (12, 8) =18.86, p<0.0005; Wilk’s Λ=0.001, partial η2=0.966.
Present study certainly provides the baseline information of Sillaginopsis panijus (Hamilton, 1822) from the Rupnarayan river of West Bengal, India. This record of Sillaginopsis panijus (Hamilton, 1822) may assist the fishery scientist, researchers, policy planners and conservationists to develop sustainable fishery management. Therefore, this study was considered as a first step on morphometric characters for its development and documenting the extension of the distribution and ecological changes in its natural habitat which helps to conserve this species abundance in this area and prevent overexploitation.
Biometry, First Report, Physico-Chemical Parameters, Rupnarayan River, Sillaginopsis Panijus.
Mukherjee M. M, Chanda A. Morphometric and Meristic Analysis of Sillaginopsis panijus Along with Seasonal Variation Recorded from Rupnarayan River, West Bengal, India. Biosc.Biotech.Res.Comm. 2021;14(4).
Mukherjee M. M, Chanda A. Morphometric and Meristic Analysis of Sillaginopsis panijus Along with Seasonal
Variation Recorded from Rupnarayan River, West Bengal, India. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3c54iSl“>https://bit.ly/3c54iSl</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
The Sillaginopsis panijus which is a flathead sillago of the family Sillaginidae and order Perciformes is a migratory amphidromous fish found in the areas of Gangetic delta (Type locality), Pondicherry (Coromondoal coast), Bangladesh (Siddik et al. 2015), Burma-Malaysia and rarely to the Indonesia (Hamilton 1822; Hamilton- Buchanon 1822; Rayappa et al. 1962; Roper et al. 1984; Talwar and Jhingran 1991; Rahman 2005; Azim et al. 2012). This family is found widespread in the Indian ocean and western Pacific Ocean also. The sillaginids are easily identified with their uniformity of body shapes. The morphological identifications are considered the most common cost-effective tool in the characterisation of fish species (Cadrin and Silva 2005; Chaklader et al. 2015; Sidik et al. 2021).
S. panijus is an estuarine and inshore marine fish but adapted in the muddy substrate in shallow water and it migrates to the upper reaches of the tidal river for extending their habitat for breeding and in search of food (Hamilton 1822; Talwar and Jhingran 1991; Alam et al. 2007). The spawning of S. panijus (Hamilton, 1822) occurs twice in a year (probably August-September and November- February) and the juveniles migrate toward the upper region of the tidal river during the month of December and March-April (Talwar and Jhingran 1991; Liu et al. 2021).
It comes with a predatory habit and consumes small fish, planktonic crustaceans and algae. The morphometric and meristic study such as measuring of length, counting of fins and fin-rays, counting of scales and other parameters are important tools used for the proper identification of the species (Cavalcanti et al. 1999). Fish populations are highly dependent upon the physico-chemical parameters of the riverine water body, which supports the abundance of fish population and to perform their biological functions (Ali 1999).
Among all the physico-chemical factors salinity, pH, temperature and dissolved oxygen (DO) are the determinants and by their regular or irregular fluctuations a fish population is determined (Thirumala. et al. 2011). Literature survey reveals that a very little work has been done on the fish faunal diversity till date in the river Rupnaryan except the work done by Mishra et al. in (2003) and Ghorai et al. in (2015) and they have listed seventeen and thirty-eight number of species respectively. The present investigation reveals strong evidence that the existence of S. panijus, will certainly enrich the biodiversity data of the river Rupnarayan (Hamilton 1822; Chakraborty et al. 2021).
MATERIAL AND METHODS
During present study a total of 116 specimens were collected seasonally by the help of the local fishermen, which captured in the early morning using trawl nets and gill nets from four different study sites of the Rupnarayan River namely Bandarghat (SI), Baksi (S2), Kolaghat (S3), and Gadiara (S4). After collection, photographs were taken for the fresh specimens and were preserved in a wide mouth jar having 4% formalin solution and brought to the laboratory of Raja N. L. Khan Women’s College (Autonomous) for further studies. A total of 27 individuals of various size ranges of S. panijus were studied morpho metrically and meristically. Twenty-three morphometric characters were measured by using a digital slide calipers scale with 0.1 cm accuracy (Hamilton 1822; Bagra and Das 2016).
Morphometric characteristics were studied by the help of existing literature like Talwer and Jhingran (1991); Jayaram (1999), Turan (1999), and the standard method followed after Hubbs and Lagler (1958) and Hubbs and Lagler (2016). Statistical analysis has done by using correlation matrix, multivariate tests, the test of between-subjects effects, multiple comparisons with Tukey HSD test method were performed to established the significant variation among the water parameters (pH, DO, Temparature, Salinity) with occurrence of fish species population seasonally in different four sampling stations (Gonzalez 2013; Nanda et al. 2021).
RESULTS AND DISCUSSION
The fresh fish have a shiny reddish silver colour in the anterior part and whitish silver colour in the abdomen (Fig.1). Fins are pale brownish with black dusting spots. The body shape is elongated and sub cylindrical with a greatly depressed head with scale. Mouth is small and terminal with villiform feeble teeth. Eyes are laterally present, slightly upwards with fleshy orbits. Body covered with small ctenoid scales, lateral line with 91 to 93 scales. Presence of two well separated dorsal fins with ten fin rays in first dorsal fin in where second dorsal spine of first dorsal fin is very elongated and extended to the caudal fin and second dorsal fin with 26-27 fin rays. The paired pectoral fin with 19 to 22 fin rays and the anal fin with two spine and 25 to 27 soft finrays. The caudal fin with 18 to 20 fin rays. Opercle with a well-developed spine.
Fin formula:
D1 X, D2 I+26-27, P 19-22, P I+V, A II+26-27, C18-20 (Present Study)
D1 X, I+24-28, P 17-22, P I+5, A II+25-27 (Pradhan et al. 2020)
D IX, I+27-28, P 23-24, P I+5, A II+24-28 (Islam et al. 2012)
D IX, I+26-27, P 23-24, P I+5, A II+25-26 (Rahman 1989,2005)
D X, I+26-27, P 24, V I+5, A II+24-26 (Talwar and Jhingran,1991)
Synonyms: Cheilodipterus panijus (Hamilton-Buchanon 1822) (Fishes of Ganges:221,381), Sillago panijus (Hamilton 1822) (i-vii + 1-405, Pls. 1-39), Sillago domina (Cuvier 1829) (Histoire naturelle des poissons. v. 3: i-xxviii + 2 pp. + 1-500, Pls. 41-71.), Sillaginopsis domina (Cuvier 1829) (Histoire naturelle des poissons. v. 3: i-xxviii + 2 pp. + 1-500, Pls. 41-71).
Type locality: Ganges estuaries (Hamilton-Buchanon 1822) (Fishes of Ganges:221,381)
Conservation status: According to IUCN Red List 2017 – 2020 Report, not evaluated in Bay of Bengal, India (Image and Bat 2020). Not evaluated globally (Pramanik et al. 2017).
Figure 1: Lateral View of Sillaginopsis panijus (Hamilton,1822) Showing Different Body Parts Measurements (Morphometry). aq-Total Length, ap- Fork Length, ar-Standard Length, ae- Head Length, ab- Snout Length, cd- Eye Diameter, ac- Pre-Orbital Length, ad- Post-Orbital Length, af- Pre-Pectoral Length, fv- Pectoral Fin Length, ag- 1st Pre-Dorsal Length, ai- 2nd Pre-Dorsal Length, gj- Longest Fin Ray, ik- 2nd Dorsal Fin Length, ay-Pre-Pelvic Length, yx- Pelvic Fin Length, au- Pre-Anal Fin Length, ut- Anal Fin Length, nq- Caudal Fin Length, hw- Body Depth, ms- Caudal Peduncle, az- Jaw Length.

The descriptive data of 23 morphometric characters of 27 identified samples of S. panijus comprised the range of minimum and maximum value, mean value, standard deviation and standard error of each of the characteristics presented in Table 2 and thirteen meristic counts of identified specimens are enlisted in table 1. The morpho-meristic characters differ in the same species due to environmental conditions of different geographical areas (Hamilton 1822; Franičević et al. 2005).
The collected specimen is agreed with some diagnosis done by Talwar and Jhingran (1991) except pectoral fin with nineteen to twenty-two fin rays, anal fin with twenty-six to twenty-seven fin rays and caudal fin with eighteen to twenty finrays. Such differences in count of second dorsal fin rays, pectoral fin rays and caudal fin rays are observed in the previous studies (Pradhan et al. 2020).
Some earlier authors also established the meristic counts like Talwar and Kacker (1967); Robins (1986); Rahman (1989); McKay (1992); Rahman (2005), Kaga and Ho (2012); Islam et al. (2012). Species distribution is influenced by a large number of physico-chemical factors such as surface water temperature, pH, salinity, dissolved oxygen was recorded during the study period. The multivariate tests (Table 3) represent the statistically significant difference in distribution of fish species, F (12, 8) =18.86, p<0.0005; Wilk’s Λ=0.001, partial η2=0.966.
The correlation matrix (Table 4) showed significance at the level 0.05 in the single star marking values and correlation is significant at the 0.01 level marked with double star marking values. The positive correlation value shows salinity-spot (0.665*), pH- season (0.603*) at 0.05 level and species no.- spot (0.861**)at0.01significancelevel (Mallya 2007; Ross and Behringer 2019; Velasco et al.2019).
The negative correlation value shows salinity-season (0-.637*) at 0.05 level and season-temp (-0.927**), pH-temp (-0.751**) at 0.01 level. The test of between-subject effects (Table 5) shows the significant value of the pH= F (2, 9) = 10.60; p < 0.004; partial η2 = 0.702; Temperature= F (2, 9) = 262.19; p < 0.001; partial η2 = 0.983 and the not significant values are DO = F (2, 9) = 0.09; p > 0.915; partial η2 = 0.019; Species No. = F (2, 9) = 0.38; p > 0.697; partial η2 = 0.077; Spots= F (2, 9) = 0.001; p > 1; partial η2 = 0.001; Salinity= F (2, 9) = 3.29; p > 0.085; partial η2 = 0.422. Post Hoc Tukey HSD test for multiple comparisons (Table 6) the significant values are in pH of the pre -monsoon -post monsoon (0.022), monsoon – post monsoon (0.004); temperature of pre-monsoon- monsoon (0.012), pre-monsoon- post monsoon (0.001) at the level p<0.05. The values of hydrogen ion concentration of water varied from (6.55-7.93) (Velasco et al. 2019).
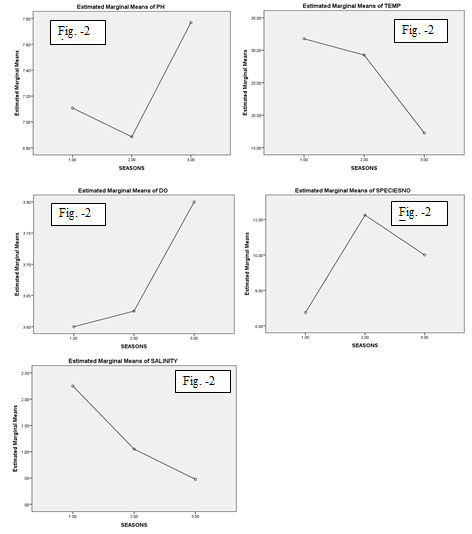
The marginal means of pH value (Fig.2 A) in the pre-monsoon season ranged between7.00-7.20 and in the monsoon was low (below 7.00) and in post- monsoon was high. It was minimum in monsoon at S1 and maximum in post monsoon time in S3. River water with a pH 5.5 and below is particularly at risk (Sulochanan and Muniyandi 2005). The graphical representation of temperature value (Fig.2 B) showed a lower temperature in post monsoon season than the monsoon season and the surface water temperature high in pre-monsoon season.
The minimum temperature (16°c) was recorded in post monsoon period (December, 2019) in station S3 and the maximum temperature (33°c) in summer season (May, 2019) in S4. The surface water temperature depends on the intensity of solar radiation, evaporation, tidal flow etc. similar findings reported in the previous studies (Thangaraj 1984; Raju et al. 2016; Cheung et al. 2018; Roy and Shamim 2020a; Rahman 2021).
The estimated marginal means of dissolved oxygen (DO) represented in (Fig.2 C) low level in pre-monsoon and in post-monsoon it was higher than the monsoon period. Dissolve oxygen (DO) is one of the most important parameters which reflects the physical and biological processes of water (Kibria 2017). The average concentration of DO in the water body varied 3.0- 5.6 during the study time. The minimum DO was recorded in the post monsoon season in S1 and maximum in S3 station. The individuals of S. panijus were established in a larger number in monsoon season than post-monsoon and a very few in pre-monsoon season (Fig.2 D).
Salinity was observed throughout the study period, minimum salinity (0.1ppm) recorded in post-monsoon season in S1 & S2 and the maximum salinity (4.0ppm) in pre-monsoon season in S4. The salinity ranged lower in the post-monsoon season than the monsoon and salinity level was high in the pre-monsoon season (Fig.2 E). The present result agrees with the result of Mahapatro et al.
(2017), that fish always seek better environmental conditions and they extended their habitat and geographical location due to environmental changes which depends on variable environmental parameters, as a result the species distributed in new areas far from their natural habitat (Hamilton 1822; Hanif et al. 2017; Cheung et al. 2018). The different parameters in an optimum level control the water quality which helps the proper growth of aquatic life (Roy et al. 2021). Several studies on different rivers in India were conducted and portrayed the deterioration of the water body and depletion of valuable aquatic life in its natural habitat (Roy and Shamim 2020a; Rahman 2021).
Table 1. Meristic count of examined Sillaginopsis panijus
(Hamilton, 1822) from Rupnarayan River
| Sl. No. | Meristic characters | Number |
| 1 | First dorsal fin rays | 10 |
| 2 | Second dorsal fin rays | 26-28 |
| 3 | Pectoral fin rays | 19-22 |
| 4 | Pelvic fin rays | 6 |
| 5 | Anal fin rays | 26-27 |
| 6 | Caudal fin rays | 18-20 |
| 7 | Scales on lateral line | 91-93 |
| 8 | Scales above lateral line | 6 |
| 9 | Scales below lateral line | 14-15 |
| 10 | First gill raker (Upper) | 3-4 |
| 11 | First gill raker (Lower) | 7-8 |
| 12 | Pre dorsal scale | 37-39 |
| 13 | Circumpeduncular scale | 9-10 |
Table 2. Morphometric measurement of examined Sillaginopsis panijus
(Hamilton, 1822) from Rupnarayan River
| Sl. No. | Morphometric characters | Maximum (cm) |
Minimum (cm) |
Mean | SD | SE |
| 1 | Total length | 25.5 | 8.6 | 18.1 | 6.8964 | 3.0842 |
| 2 | Fork length | 24.29 | 8 | 17.3 | 6.6421 | 2.9705 |
| 3 | Standard length | 22.5 | 7.8 | 15.8 | 6.2043 | 2.7747 |
| 4 | Head length | 6.38 | 1.66 | 4.35 | 1.9221 | 0.8596 |
| 5 | Head depth | 2.01 | 0.7 | 1.5 | 0.544 | 0.2433 |
| 6 | Eye diameter | 0.87 | 0.3 | 0.65 | 0.2316 | 0.1036 |
| 7 | Snout length | 2.55 | 0.6 | 1.74 | 0.8 | 0.3578 |
| 8 | First pre dorsal length | 7.34 | 2.57 | 5.06 | 1.9635 | 0.8781 |
| 9 | Second pre dorsal length | 9.92 | 3.49 | 6.98 | 2.7281 | 1.2201 |
| 10 | Pre pectoral length | 6.67 | 2.3 | 4.58 | 1.7551 | 0.7849 |
| 11 | Pre pelvic length | 6.93 | 2.4 | 4.83 | 1.8964 | 0.8481 |
| 12 | Pre anal length | 10.9 | 4 | 7.76 | 2.8248 | 1.2633 |
| 13 | Length of longest fin-ray | 13.65 | 3 | 8.58 | 4.3226 | 1.9331 |
| 14 | Pectoral fin length | 3.57 | 1.42 | 2.73 | 0.9924 | 0.4438 |
| 15 | Pelvic fin length | 2.64 | 0.9 | 1.94 | 0.7387 | 0.3304 |
| 16 | Anal fin length | 7.64 | 2.73 | 5.63 | 2.1222 | 0.9491 |
| 17 | Caudal fin length | 3.31 | 1.27 | 2.41 | 0.8194 | 0.3665 |
| 18 | Body depth | 3.27 | 1.1 | 2.13 | 0.824 | 0.3685 |
| 19 | Pre orbital length | 2.61 | 0.94 | 1.82 | 0.7283 | 0.3257 |
| 20 | Post orbital length | 3.41 | 1.22 | 2.21 | 0.8148 | 0.3644 |
| 21 | Lower jaw length | 1.04 | 0.33 | 0.72 | 0.3015 | 0.1348 |
| 22 | Upper jaw length | 1.44 | 0.49 | 0.97 | 0.3871 | 0.1731 |
| 23 | Length of Caudal peduncle | 1.61 | 0.47 | 1.16 | 0.4248 | 0.19 |
Table 3. The Multivariate Tests analysis of S. panijus (Hamilton,1822)
from Rupnarayan river, West Bengal.
| Effect | Value | F | Hypothesis df |
Error df |
Sig. | Partial Eta Squared |
|
| Intercept | Pillai’s Trace | 1 | 3972.3 | 6 | 4 | 0 | 1 |
| Wilks’ Lambda | 0 | 3972.3 | 6 | 4 | 0 | 1 | |
| Hotelling’s Trace | 5958.48 | 3972.3 | 6 | 4 | 0 | 1 | |
| Roy’s Largest Root | 5958.48 | 3972.3 | 6 | 4 | 0 | 1 | |
| Seasons | Pillai’s Trace | 1.694 | 4.612 | 12 | 10 | 0.011 | 0.847 |
| Wilks’ Lambda | 0.001 | 18.855 | 12 | 8 | 5E-04 | 0.966 | |
| Hotelling’s Trace | 260.418 | 65.104 | 12 | 6 | 5E-04 | 0.992 | |
| Roy’s Largest Root | 258.109 | 215.09 | 6 | 5 | 5E-04 | 0.996 |
Table 4. Correlation Matrix established with water parameter and number of species
of S. panijus from Rupnarayan river, West Bengal (Hamilton 1822).
| SPOTS | SEASO-NS | pH | TEMP | D.O. | SALIN-ITY | SPECIES NO | ||
| SPOTS | Pearson Correlation | 1 | 0 | 0.242 | 0.018 | 0.17 | 0.665* | 0.861** |
| Sig. (2-tailed) | 1 | 0.449 | 0.957 | 0.6 | 0.018 | 0 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| SEASONS | Pearson Correlation | 0 | 1 | 0.603* | -0.927** | 0.13 | -0.637* | 0.163 |
| Sig. (2-tailed) | 1 | 0.038 | 0 | 0.69 | 0.026 | 0.613 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| pH | Pearson Correlation | 0.242 | 0.603* | 1 | -0.751* | 0.32 | -0.216 | 0.068 |
| Sig. (2-tailed) | 0.449 | 0.038 | 0.005 | 0.31 | 0.501 | 0.833 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| TEMP. | Pearson Correlation | 0.018 | -.927** | -0.751* | 1 | -0.02 | 0.572 | 0-.067 |
| Sig. (2-tailed) | 0.957 | 0 | 0.005 | 0.52 | 0.052 | 0.836 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| D.O. | Pearson Correlation | 0.169 | 0.128 | 0.319 | -0.204 | 1 | -0.075 | -0.109 |
| Sig. (2-tailed) | 0.599 | 0.692 | 0.313 | 0.524 | 0.817 | 0.736 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| SALINITY | Pearson Correlation | 0.665* | -0.637* | -0.216 | 0.572 | -0.07 | 1 | 0.463 |
| Sig. (2-tailed) | 0.018 | 0.026 | 0.501 | 0.052 | 0.82 | 0.13 | ||
*. Correlation is significant at the 0.05 level (2-tailed).
**. Correlation is significant at the 0.01 level (2-tailed).
Table 5. Tests of Between-Subjects Effects analyses with water parameter and seasonal occurrences
of S. panijus from Rupnarayan river, West Bengal (Hamilton 1822).
| Source | Dependent Variable | Type III Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squared |
| Corrected Model | PH | 1.696a | 2 | 0.848 | 10.606 | 0.004 | 0.702 |
| TEMP | 480.667b | 2 | 240.333 | 262.182 | 0 | 0.983 | |
| DO | 0.095c | 2 | 0.047 | 0.089 | 0.915 | 0.019 | |
| SPECIES NO | 61.167d | 2 | 30.583 | 0.375 | 0.697 | 0.077 | |
| SPOTS | 0.000e | 2 | 0 | 0 | 1 | 0 | |
| SALINITY | 6.562f | 2 | 3.281 | 3.285 | 0.085 | 0.422 | |
| Intercept | PH | 631.475 | 1 | 631.475 | 7900.3 | 0 | 0.999 |
| TEMP | 8164.08 | 1 | 8164.08 | 8906.27 | 0 | 0.999 | |
| DO | 162.068 | 1 | 162.068 | 304.67 | 0 | 0.971 | |
| SPECIES NO | 1121.33 | 1 | 1121.33 | 13.759 | 0.005 | 0.605 | |
| SPOTS | 75 | 1 | 75 | 45 | 0 | 0.833 | |
| SALINITY | 19.001 | 1 | 19.001 | 19.027 | 0.002 | 0.679 | |
| Seasons | PH | 1.696 | 2 | 0.848 | 10.606 | 0.004 | 0.702 |
| TEMP | 480.667 | 2 | 240.333 | 262.182 | 0.001 | 0.983 | |
| DO | 0.095 | 2 | 0.047 | 0.089 | 0.915 | 0.019 | |
| SPECIES NO | 61.167 | 2 | 30.583 | 0.375 | 0.697 | 0.077 | |
| SPOTS | 0 | 2 | 0 | 0.001 | 1 | 0.001 | |
| SALINITY | 6.562 | 2 | 3.281 | 3.285 | 0.085 | 0.422 | |
| Error | PH | 0.719 | 9 | 0.08 | |||
| TEMP | 8.25 | 9 | 0.917 | ||||
| DO | 4.787 | 9 | 0.532 | ||||
| SPECIES NO | 733.5 | 9 | 81.5 | ||||
| SPOTS | 15 | 9 | 1.667 | ||||
| SALINITY | 8.988 | 9 | 0.999 | ||||
| Total | PH | 633.89 | 12 | ||||
| TEMP | 8653 | 12 | |||||
| DO | 166.95 | 12 | |||||
| SPECIES NO | 1916 | 12 | |||||
| SPOTS | 90 | 12 | |||||
| SALINITY | 34.55 | 12 | |||||
| Corrected Total | PH | 2.415 | 11 | ||||
| TEMP | 488.917 | 11 | |||||
| DO | 4.882 | 11 | |||||
| SPECIES NO | 794.667 | 11 | |||||
| SPOTS | 15 | 11 | |||||
| SALINITY | 15.549 | 11 | |||||
| a. R Squared = .702 (Adjusted R Squared = .636); b. R Squared = .983 (Adjusted R Squared = .979); c. R Squared = .019 (Adjusted R Squared = -.198); d. R Squared = .077 (Adjusted R Squared = -.128); e. R Squared = .000 (Adjusted R Squared = -.222); f. R Squared = .422 (Adjusted R Squared = .294) | |||||||
Table 6. Multiple Comparisons
| Tukey HSD | |||||||
| Dependent Variable |
(I) SEASONS |
(J) SEASONS |
Mean Difference (I-J) |
Std. Error | Sig. | 95% Confidence Interval | |
| Lower Bound |
Upper Bound |
||||||
| pH | 1 | 2 | 0.2225 | 0.19991 | 0.53 | -0.3357 | 0.7807 |
| 3 | -0.6625* | 0.19991 | 0.022 | -1.2207 | -0.1043 | ||
| 2 | 1 | -0.2225 | 0.19991 | 0.53 | -0.7807 | 0.3357 | |
| 3 | -0.8850* | 0.19991 | 0.004 | -1.4432 | -0.3268 | ||
| 3 | 1 | 0.6625* | 0.19991 | 0.022 | 0.1043 | 1.2207 | |
| 2 | 0.8850* | 0.19991 | 0.004 | 0.3268 | 1.4432 | ||
| TEMP | 1 | 2 | 2.5000* | 0.677 | 0.012 | 0.6098 | 4.3902 |
| 3 | 14.5000* | 0.677 | 0.001 | 12.6098 | 16.3902 | ||
| 2 | 1 | -2.5000* | 0.677 | 0.012 | -4.3902 | -0.6098 | |
| 3 | 12.0000* | 0.677 | 0.001 | 10.1098 | 13.8902 | ||
| 3 | 1 | -14.5000* | 0.677 | 0.001 | -16.3902 | -12.6098 | |
| 2 | -12.0000* | 0.677 | 0.001 | -13.8902 | -10.1098 | ||
| DO | 1 | 2 | -0.025 | 0.51572 | 0.999 | -1.4649 | 1.4149 |
| 3 | -0.2 | 0.51572 | 0.921 | -1.6399 | 1.2399 | ||
| 2 | 1 | 0.025 | 0.51572 | 0.999 | -1.4149 | 1.4649 | |
| 3 | -0.175 | 0.51572 | 0.939 | -1.6149 | 1.2649 | ||
| 3 | 1 | 0.2 | 0.51572 | 0.921 | -1.2399 | 1.6399 | |
| 2 | 0.175 | 0.51572 | 0.939 | -1.2649 | 1.6149 | ||
| SPECIES NO | 1 | 2 | -5.5 | 6.38357 | 0.676 | -23.323 | 12.323 |
| 3 | -3.25 | 6.38357 | 0.869 | -21.073 | 14.573 | ||
| 2 | 1 | 5.5 | 6.38357 | 0.676 | -12.323 | 23.323 | |
| 3 | 2.25 | 6.38357 | 0.934 | -15.573 | 20.073 | ||
| 3 | 1 | 3.25 | 6.38357 | 0.869 | -14.573 | 21.073 | |
| 2 | -2.25 | 6.38357 | 0.934 | -20.073 | 15.573 | ||
| SPOTS | 1 | 2 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 |
| 3 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 | ||
| 2 | 1 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 | |
| 3 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 | ||
| 3 | 1 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 | |
| 2 | 0 | 0.91287 | 1 | -2.5487 | 2.5487 | ||
| SALINITY | 1 | 2 | 1.2 | 0.70662 | 0.258 | -0.7729 | 3.1729 |
| 3 | 1.775 | 0.70662 | 0.077 | -0.1979 | 3.7479 | ||
| 2 | 1 | -1.2 | 0.70662 | 0.258 | -3.1729 | 0.7729 | |
| 3 | 0.575 | 0.70662 | 0.704 | -1.3979 | 2.5479 | ||
| 3 | 1 | -1.775 | 0.70662 | 0.077 | -3.7479 | 0.1979 | |
| 2 | -0.575 | 0.70662 | 0.704 | -2.5479 | 1.3979 | ||
| Based on observed means. The error term is Mean Square (Error) = .999. | |||||||
| * The mean difference is significant at the .05 level. | |||||||
Figure 2: (A-E): Shows the graphical representation of the relation with pH, temperature, DO, specimen no and salinity in respect to the season of Sillaginopsis panijus (Hamilton, 1822) from Rupnarayan river, West Bengal.
CONCLUSION
The findings of the present study ensure the presence of Sillaginopsis panijus as a first-time record ever in the upstream and downstream of the Rupnarayan river of West Bengal, India. Present morphometric study describes thorough and vivid comparison among individuals in a species qualitatively. It provides the basic information for fishery management and research. The S. panijus population size is found larger in monsoon than post-monsoon and smaller in pre-monsoon season. This seasonal and morphometric study could be used as primary information in the near future in fish research and management.
ACKNOWLEDGEMENTS
This study was not financially supported by any other funding agency. Authors are also thankful to Principal, Raja Narendra Lal Khan Women’s College (Autonomous) and Head of the Department of Zoology, for providing laboratory facilities and support during the study periods. Authors are grateful to anonymous reviewers and editors for their valuable suggestions and feedback.
Conflict of Interests: Authors declare no conflict of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Intuitional Review Board (IRB) of Raja Narendra Lal Khan Women’s College Medinipur, West Bengal, India.
REFERENCES
Alam, A., Mustafa, M. G. and Azad, M. A. K. (2007). Water and sediment quality and plankton diversity of Posna beel, Tangail, Bangladesh J. Fish, 30, pp. 177–188.
Ali, S.S. (1999). Freshwater Fishery Biology. Naseem Book Depot, Hyderabad, Pakistan, (1), pp.108–14.
Azim, M. A., Islam, M. R. Hossain, M. B. et al. (2012). Seasonal variations in the proximate composition of Gangetic Sillago, Sillaginopsis panijus (Perciformes: Sillaginidae), Middle East Journal Of Scientific Research, 11(5), pp. 559–562.
Bagra, K. and Das, D. N. (2016). Fish Diversity of River Siyom of Arunachal Pradesh India: A Case Study Fish Diversity of River Siyom of Arunachal Pradesh India: A Case Study, (February 2011). doi: 10.3126/on.v8i1.4324.
Cadrin, S. X. and Silva, V. M. (2005). Morphometric variation of yellowtail flounder, ICES Journal of Marine Science, 62(4), pp. 683–694. doi: 10.1016/j.icesjms.2005.02.006.
Cavalcanti, M. J., Monteiro, L. R. and Lopes, P. R. D. (1999). Landmark-based morphometric analysis in selected species of serranid fishes (Perciformes: Teleostei), Zoological Studies, 38(3), pp. 287–294.
Chaklader, R., Bakar Siddik, M. A. and Nahar, A. (2015). Taxonomic diversity of paradise threadfin Polynemus paradiseus (Linnaeus, 1758) inhabiting southern coastal rivers in Bangladesh, Sains Malaysiana, 44(9), pp. 1241–1248. doi: 10.17576/jsm-2015-4409-04.
Chakraborty, T., Chatterjee, A. and Saha, N. C. (2021). Seasonal Fluctuations in Physicochemical Parameters in Relation to Fish Diversity in Muragacha Beel, West Bengal India. Bioscience Biotechnology Research Communications. 14. 768-774. 10.21786/bbrc/14.2.50.
Cheung, W., Bruggeman, J. and Butenschon, M. (2018). Chapter 4: Projected changes in global and national potential marine fisheries catch under climate change scenarios in the twenty-first century. Impacts of climate change on fisheries and aquaculture, p.63.
Cuvier, G.L.C.F.D. and Valenciennes, A., (1829). Histoire naturelle des poissons. Tome troisième. Suite du Livre troisième. Des percoïdes à dorsale unique à sept rayons branchiaux et à dents en velours ou en cardes. Histoire naturelle des poissons, 3, p.500.
Franičević, M., Sinovčić, G., Čikeš Keč, V et al. (2005). Biometry analysis of the Atlantic bonito, Sarda sarda (Bloch, 1793), in the Adriatic Sea, Acta Adriatica, 46(2), pp. 213–222.
Ghorai, M. et al. (2015) The Impact of Coal Fly Ash Power Station on Distribution and Biodiversity of Freshwater Fishes in Rupnarayan River, West Bengal, India, International Journal of Current Research, 7(12), pp. 23954–23961.
Gonzalez, R. (2013) Advanced topics in ANOVA, 9, pp. 1–61.
Hamilton – Buchanon (1822). An account of the fishes found in the river Ganges and its branches. Edinburgh & London.:221, 381
Hamilton, F., (1822). An account of the fishes found in the river Ganges and its branches. Archibald Constable, 1.
Hanif, M. A., Siddik, M. A. B., Nahar, A. et al. (2017). A new distribution of the buffon’s river garfish, Zenarchopterus buffonis (Valenciennes, 1847) in the southern coastal rivers of Bangladesh, Journal of Applied Ichthyology, 33(6), pp. 1211–1214. doi: 10.1111/jai.13462.
http://faunaofindia.nic.in/php/hpg/hpg_books_toc.php?book_id=003&type=hpg&book_title=Co mmercial+Sea+Fishes+of+India.
Hubbs, C. and Lagler, K. (2016). Fishes of the Great Lakes Region, Revised Edition, Fishes of the Great Lakes Region, Revised Edition. doi: 10.3998/mpub.17658.
Hubbs, C. L. and Lagler, K. F. (1958). Fishes of the Great Lakes region. IBH Publishing Co. Pvt. Ltd. New Delhi-Calcutta, India, p. 816-817.
Image, E. N. and Bat, R. F. (2020). IUCN Red List 2017 – 2020 Report.
Islam, M. R., Sultana, N, Hossain, M. B et al. (2012). Estimation of fecundity and gonadosomatic index (GSI) of Gangetic whiting, Sillaginopsis panijus (Hamilton, 1822) from the Meghna river estuary, Banglades, World Applied Sciences Journal, 17(10), pp. 1253–1260.
Jayaram K. C. (1999). The Freshwater Fishes of the Indian Region. Delhi, Narendra Publishing House, New Delhi, India.
Kaga, T. and Ho, H. (2012). Redescription of Sillago (Parasillago) indica McKay, Dutt & Sujatha, 1985 (Perciformes: Sillaginidae), with a reassignment to the subgenus Sillago, Zootaxa, 3513, pp. 61–67.
Kibria, G. (2017). Dissolved oxygen: The facts Environmental update-Dissolved oxygen : The facts, pp. 3–5. doi: 10.13140/RG.2.2.24591.28320.
Liu, Y., Tong, S. and Xiong, Y. (2021). Research Status and Prospect of Fish Habitat, IOP Conference Series: Earth and Environmental Science, 643(1). doi:10.1088/1755-1315/643/1/012112.
Mahapatro, D., Mishra, R. K. and Panda, S. (2017). Range extension of a vulnerable Sea horse Hippocampus fuscus (Actinopterygii: Syngnathidae) on the north-eastern Bay of Bengal coast, Marine Biodiversity Records, 10(1), pp. 4–9. doi: 10.1186/s41200-017-0108-z.
Mallya, Y. J. (2007). The Effect of Dissolved Oxygen on Fish Growth in Aquaculture, p. 30.
McKay, R. J., (1992). FAO Species Catalogue. Sillaginid fishes of the world (family Sillaginidae). An annotated and illustrated catalogue of the sillago, smelt or Indo-Pacific whiting species known to date. Rome: FAO. FAO Fish. Synop. 125(14):pp.87.
Mishra, S. S., Pradhan, P, Kar, S. et al. (2003). Ichthyofaunal diversity of Midnapore, Bankura and Hooghly Districts, South West Bengal, Records of the Zoological Survey of India, Miscellaneous Publication, Occasional Paper, 220, pp. 01.01.65.
Nanda, A. et al. (2021). Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control type I error, International Journal of Statistics and Applied Mathematics, 6(1), pp. 59–65. doi: 10.22271/maths.2021.v6.i1a.636.
Pradhan, S. K. et al. (2020). Biometry, length-weight and length-length relationships of flathead sillago Sillaginopsis panijus (Hamilton, 1822) (perciformes: Sillaginidae) from the north-western Bay of Bengal, Indian Journal of Fisheries, 67(3), pp. 144–151. doi: 10.21077/ijf.2020.67.3.95369-16.
Pramanik, M. M. H., Hasan, M. M., Bisshas, S. M. et al. (2017). Fish biodiversity and their present conservation status in the Meghna River of Bangladesh, International Journal of Fisheries and Aquatic Studies, 5(1), pp. 446–455.
Rahman, A. K. A. (1989). Freshwater fishes of Bangladesh. Zoological Society of Bangladesh, Department of Zoology, Dhaka University, Bangladesh, (1), pp. 318-319.
Rahman, A. K. A. (2005). Freshwater fishes of Bangladesh. Zoological Society of Bangladesh, Department of Zoology, Dhaka University, Bangladesh, (2), pp. 344-345.
Rahman, A., Jahanara, I. and Jolly, Y. N. (2021). Assessment of physicochemical properties of water and their seasonal variation in an urban river in Bangladesh, Water Science and Engineering, 14(2), pp. 139–148. doi: 10.1016/j.wse.2021.06.006.
Raju, C., Sridharan, G., Mariappan, P. et al. (2016). Studies on the Physico – Chemical Parameters of Vellaiyar Estuary of Vailankanni, Nagapattinam , East Coast of India, (1), pp. 33–39.
Rayappa, V., Pantulu and Sing, V. D. (1962). On the use of pectoral spines for the determination of age and growth of Pangasius pangasius (hamilton buch), ICES Journal of Marine Science, 27(2), pp. 192–216. doi: 10.1093/icesjms/27.2.192.
Robins, R. (1986). Queensland Museum, Australian Archaeology, 23(1), pp. 115-116. doi: 10.1080/03122417.1986.12093091.
Roper, C.F., Sweeney, M.J. and Nauen, C., (1984). Cephalopods of the world. An annotated and illustrated catalogue of species of interest to fisheries.
Ross, E. and Behringer, D. (2019). Changes in temperature, pH, and salinity affect the sheltering responses of Caribbean spiny lobsters to chemosensory cues, Scientific Reports, 9(1), pp. 1–11. doi: 10.1038/s41598-019-40832-y.
Roy, M., and Shamim, F. (2020a). Assessment of antropogenically induced pollution in the surface water of River Ganga: A study in the Dhakhineswar Ghat, W.B, India. Journal of Water Pollution & Purification Research, 7(1), 15–19.
Roy, M., Shamim, F. and Chatterjee, S. (2021). Evaluation of Physicochemical and Biological Parameters on the Water Quality of Shilabati River, West Bengal, India, Water Science, 35(1), pp. 71–81. doi: 10.1080/23570008.2021.1928902.
Siddik, M. A. B., Hanif, M. A., Chaklader, M. R. et al. (2015). Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh, Egyptian Journal of Aquatic Research, 41(4), pp. 307–313. doi: 10.1016/j.ejar.2015.11.001.
Sidiq, M., Ahmed, I. and Bakhtiyar, Y. (2021). Length-weight relationship, morphometric characters, and meristic counts of the coldwater fish Crossocheilus diplochilus (Heckel) from Dal Lake, Fisheries & Aquatic Life, 29(1), pp. 29–34. doi: 10.2478/aopf-2021-0003.
Sulochanan, B. and Muniyandi, K. (2005). Hydrographic parameters off Gulf of Mannar and Palk Bay during an year of abnormal rainfall, 47(2), pp. 198–200.
Talwar, P. K. and Jhingran, A. G. (1991). Inland fishes of India and adjacent countries, Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi-Culcutta, India, 2, pp. 816-817.
Talwar, P. K. and Kacker, R. K. (1967). Commercial Sea Fishes Of India, ZOOLOGICAL SURVEY OF INDIA HANDBOOK, 1(465), pp. 106–111. Available at:
Thangaraj, G. S. (1984). Ecology of the marine zine of Vellar estuary, Ph.D. Thesis, Annamalai University.
Thirumala., S., B.R, K. and Kantaraj.G.S (2011). Fish diversity in relation to physico-chemical characteristics of Bhadra reservoir of Karnataka, India, Pelagia Research Library, 2(5), pp.34–47.
Turan, C. (1999). A note on the examination of morphometric differentiation among fish populations: The Truss System, Turkish Journal of Zoology, 23(3), pp. 259–263.
Velasco, J., Gutiérrez-Cánovas, C., Botella-Cruz, M. et al. (2019). Effects of salinity changes on aquatic organisms in a multiple stressor context, Philosophical Transactions of the Royal Society B: Biological Sciences, 374(1764). doi: 10.1098/rstb.2018.0011.


