Department of Pharmaceutical Analysis, RBVRR Women’s College of Pharmacy, Barkatpura, Hyderabad, Telangana, India.
Corresponding author email: bhavya.khagga@gmail.com
Article Publishing History
Received: 18/10/2020
Accepted After Revision: 12/12/2020
A new, simple, precise, accurate, reproducible and economic stability indicating UV spectroscopic method was developed and validated for simultaneous estimation of Dapagliflozin and Metformin in pure and combined pharmaceutical dosage form. The UV spectrophotometric estimation of Dapagliflozin and Metformin was determined using the Q absorption ratio method at 222 nm and 232 nm respectively using water as diluent. The linearity ranges for Dapagliflozin and Metformin was 2 – 32 μg/ml and 1–20μg/ml respectively with their correlation coefficient values (R2) 0.999. The percentage recovery at various concentration levels varied from 96.82 – 99.8 % for Dapagliflozin and 98.15 to 99.35 % for Metformin confirming that the method is accurate. LOD and LOQ for Dapagliflozin was found to be 0.0241μg/ml and 0.0293 μg/ml and for Metformin 0.0732 μg/ml and 0.0890 μg/ml. In the precision study, the% RSD value was found to be 0.1845 % and 0.2052 % for Dapagliflozin and Metformin respectively. Degradation studies were performed, both the drugs were found to be degraded in acid by using Hydrochloric acid, in base using sodium hydroxide solution, in peroxide using hydrogen peroxide solution, temperature and in light by exposing it to UV light in UV chamber. The results for estimation of Dapagliflozin and Metformin Hydrochloride and validation parameters like accuracy, precision, ruggedness, linearity were studied for the method and were found to be within the limits. The developed method was free from the interferences due to excipients present in the formulation and it can be used for routine quality control analysis.
Dapagliflozin, Degradation Studies, Metformin, Q absorption ratio Method, Stability.
Bhavyasri K, Surekha T, Sumakanth M. Method Development, Validation and Stress Studies of Dapagliflozin and Metformin Hydrochloride Using Ultraviolet-Visible Spectroscopy in Bulk and Combined Pharmaceutical Formulations. Biosc.Biotech.Res.Comm. 2020;13(4).
Bhavyasri K, Surekha T, Sumakanth M. Method Development, Validation and Stress Studies of Dapagliflozin and Metformin Hydrochloride Using Ultraviolet-Visible Spectroscopy in Bulk and Combined Pharmaceutical Formulations. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3mQGtAU
Copyright © Bhavyasri et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
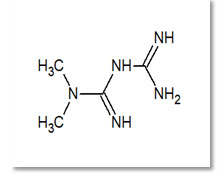
Metformin (MET) hydrochloride (Fig. 1) which chemically known as (3-(diamino methylidene)-1, 1-dimethylguanidine; hydrochloride. It has molecular formula of C4H11N5 and molecular weight is 165.62 g/mol. Metformin is an oral anti-hyperglycaemic agent (Type 2 diabetes) belongs to class of biguanides and useful for treating non-insulin-dependent diabetes mellitus. It decreases blood sugar levels by decreasing hepatic glucose production, decreasing intestinal absorption of glucose, and improving insulin sensitivity by increasing peripheral glucose uptake and utilization. These effects are mediated by the initial activation by AMP-activated protein kinase, a liver enzyme that plays an important role in insulin signalling, whole body energy balance, and the metabolism of glucose and fats (Mishra 2011; Urooj 2017).
Figure 1: Structure of Metformin.
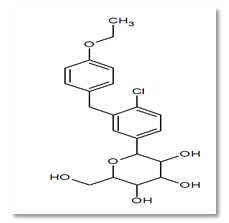
Dapagliflozin (DAPA) (Fig. 2) is an antidiabetic drug. Its chemical name is (2S, 3R, 4R, 5S, 6R)-2-[4-chloro-3-(4-ethoxybenzy) phenyl]-6- (hydroxymethyl) tetrahydro-2H-pyran-3, 4, 5-triol. It acts as SGLT-2 inhibitor. Inhibition of this enzyme system reduces the rate of digestion of carbohydrates (Nalwade, 2012).
Figure 2: Structure of Dapagliflozin
A literature survey has revealed that only few articles on UV spectroscopic method for the simultaneous estimation of Dapagliflozin and Metformin (Mishra 2011; Jain 2015). The aim of our work was development of new, stability indicating UV method for determination ofDapagliflozin and Metformin HCL which possess the following advantages when compared to the already existing UV methods which is simple, cost-effective, and economic (Jain 2015). The main target for our new developed method is estimation of Dapagliflozin and Metformin HCL in the Pharmaceutical dosage forms.
MATERIAL AND METHODS
Spectrophotometric measurements were made in (ELICO) Double beam SL 210 UV-Visible spectrometer with 0.5 cm quartz cells. Drug Dapagliflozin and Metformin HCl were supplied as a gift sample from laboratory.Solubility of drugs 10mg of Dapagliflozin and Metformin HCL of each was weighed and checked in water, methanol and acetonitrile. Both the drugs were found to be soluble in water.
For the selection of wavelength scan the standard solutions in UV spectrophotometer between 200 nm to 400 nm on spectrum mode, using water as a blank. The two drugs show λ max at 222 nm and 232 nm for Dapagliflozin and Metformin HCl respectively. 10 ppm solutions of both the drugs are scanned in the overlay mode to determine isosbestic point.
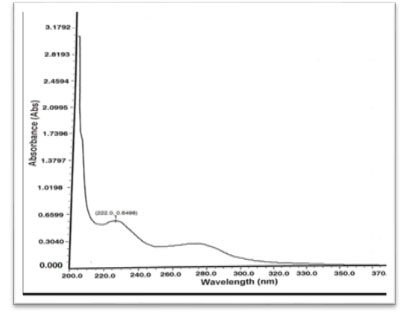
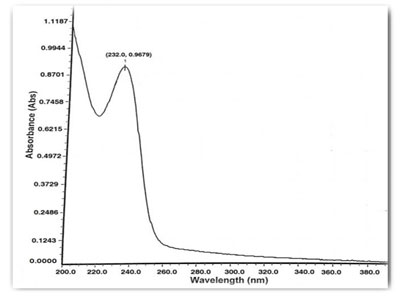
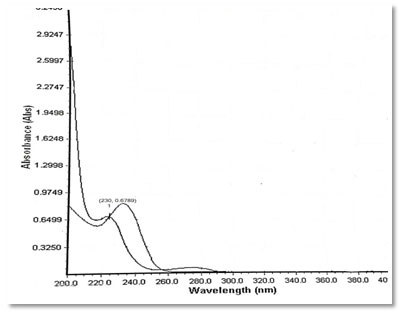
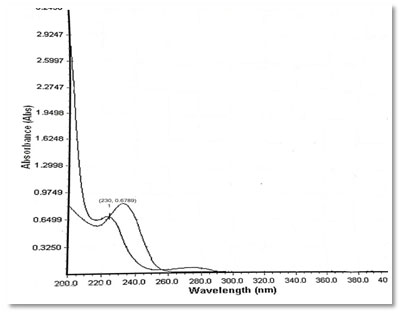
Preparation of standard drug solution was done using 10 mg of Dapagliflozin and Metformin HCL was accurately weighed separately and dissolved in 5 ml diluent (Water), then transferred into a 10 ml volumetric flask, sonicated it for 10 min, finally, volume was made up to the mark with the same solvent to make 1000 µg/ml stock solution. From this 0.1 ml was again diluted to 10 ml to get a concentration of 10 µg/ml solution. It was scanned in UV range [200-400 nm] in 1.0 cm cell against solvent blank. The spectrum of drugs was recorded. After the study of spectrum of drugs, the λmax of Dapagliflozin was found to be 222 nm and the absorbance was found to be 0.6498 and the λmax of Metformin was found to be 232 nm and the absorbance was found to be 0.9679. Isosbestic point was found to be at 230 nm and the absorbance was found to be 0.6789.
Figure 3: Spectrum of Dapagliflozin
Figure 4: Spectrum of Metformin
Figure 5: Overlay Spectrum
RESULTS AND DISCUSSION
Method validation is defined as the process that confirms the analytical procedure employed for a particular test is suitable for its intended use. Validation assures that a measurement process produces valid measurements. Results from method validation are used to judge the quality, reliability of analytical results. It is an integral part of any good analytical practice. The proposed method was validated for the parameters like linearity, accuracy and robustness as per ICH guideline (Parmar, Luhar and Narkhede, 2018).
Accuracy indicates the deviation between the mean and true value. The accuracy is the closeness of agreement between the true value and test result. Accuracy was determined by means of recovery experiments. Solution containing known concentration of Dapagliflozin and Metformin HCL was used for this purpose. The accuracy was assessed from the test results as the percentage of the drug recovered by the assay at 3 levels (Patel, Chaudhary and Bhadani, 2017).
Table 1. Accuracy of Dapagliflozin
| Accuracy Level | Sample Conc (Ppm) | Standard
Conc (Ppm) |
Drugconc (Ppm) | %Recovery | % Mean |
| 50% | 10 | 5 | 15 | 102.16%
99.11% 98.13% |
99.8% |
| 100% | 10 | 10 | 20 | 98.55%
95.44% 96.46% |
96.82% |
| 150% | 10 | 15 | 25 | 98.70%
99.53% 99.71% |
99.31% |
Table 2. Accuracy of Metformin
| Accuracy Level
|
Sample Conc (Ppm) | Standard Conc (Ppm) | Drug Conc (Ppm) | %Recovery | % Mean |
| 50% | 5 | 2.5 | 7.5 | 98.23%
99.20% 100.61% |
99.35% |
| 100% | 5 | 5 | 10 | 98.03%
98.92% 97.49% |
98.15% |
| 150% | 5 | 7.5 | 12.5 | 95.08%
94.39% 95.06% |
98.85% |
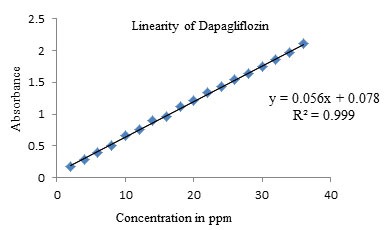
The linearity of an analytical method is its ability to elicit that test results are proportional to the concentration of drug in samples within a given range. Linearity of the method was determined by constructing calibration curves by taking. Standard solutions Dapagliflozin and Metformin HCL of different concentrations level (1mcg – 20mcg\ ml) and (2 – 36 mcg/ml) respectively were used for this purpose. Each absorbance was plotted against the concentrations to obtain the calibration curves and correlation coefficients. Characteristic parameters for regression equation (y = mx+ c) of the method were obtained by least squares treatment of the results and these parameters were used to confirm the good linearity of the method (Jani, Shah and Kapupara 2015).
Figure 5: Linearity of Metformin
Table 3. Data of Linearity of Metformin
| Concentration (ppm) | Absorbance at 232 nm |
| 1 | 0.1132 |
| 2 | 0.2116 |
| 4 | 0.4215 |
| 6 | 0.6251 |
| 8 | 0.8243 |
| 10 | 1.025 |
| 12 | 1.2035 |
| 14 | 1.4098 |
| 16 | 1.6342 |
| 18 | 1.8213 |
| 20 | 2.0198 |
Figure 6: Linearity of Dapagliflozin
Table 4. Data of Linearity of Dapagliflozin
| Concentration (ppm) | Absorbance at 222 nm |
| 2 | 0.1725 |
| 4 | 0.2934 |
| 6 | 0.3942 |
| 8 | 0.5123 |
| 10 | 0.6563 |
| 12 | 0.7624 |
| 14 | 0.8946 |
| 16 | 0.9654 |
| 18 | 1.1145 |
| 20 | 1.2065 |
| 22 | 1.3458 |
| 24 | 1.4268 |
| 26 | 1.5368 |
| 28 | 1.6321 |
| 30 | 1.7524 |
| 32 | 1.8564 |
| 34 | 1.9751 |
| 36 | 2.1024 |
Precision was estimated by studying repeatability by injecting 10 ppm concentration of Dapagliflozin and Metformin. The results were calculated as standard deviation, relative standard deviation (Heerspink, Zeeuw, and Wie, 2013).
Table 5. Data for Precision
| S.NO | Dapagliflozin | Metformin |
| 1 | 0.5264 | 0.9963 |
| 2 | 0.5268 | 0.9953 |
| 3 | 0.5263 | 0.9965 |
| 4 | 0.5269 | 0.9946 |
| 5 | 0.5265 | 0.9998 |
| 6 | 0.5266 | 0.9996 |
| Standard deviation | 0.000787 | 0.00184 |
| %RSD | 0.1845 | 0.2052 |
Limit of Detection (LOD) is defined as the lowest level of concentration of analyte that can be detected, though not necessarily quantitated. It can be calculated from the below formula (Mishra, Soni and Nayak, 2011; Heerspink, Zeeuw, and Wie, 2013).
LOD = 3.3 σ/S
Where,
σ = Standard deviation of the response,
S = Slope of calibration curve.
Limit of Quantization (LOQ) is defined as the lowest concentration of analyse that can be determined with acceptable accuracy and precision when the specified procedure is applied. It can be calculated from the below formula.
LOQ = 10 σ/S
Where,
σ = Standard deviation of the response,
S = Slope of calibration curve.
Table 6. Data for LOD & LOQ
| DRUG NAME | LOD | LOQ |
| DAPAGLIFLOZIN | 0.0241 | 0.0732 |
| METFORMIN | 0.0293 | 0.0890 |
Robustness: It is the capacity of the method to remain unaffected by small but deliberate variations in method parameters. The analysis was performed by slightly changing the wavelength. To determine the robustness at +1 nm and -1nm from the fixed wave length. The results were calculated as % RSD Table 7 & 8 .10 ppm solutions of both the samples are used for the analysis (Nalwade, Reddy and Rao, 2012).
Table 7. Evaluation data for Dapagliflozin Robustness study.
| S.NO | 221 nm | 222 nm | 223 nm |
| 1 | 0.6479 | 0.6580 | 0.5499 |
| 2 | 0.6475 | 0.6582 | 0.5498 |
| 3 | 0.6478 | 0.6792 | 0.5588 |
| Standard deviation | 0.0002 | 0.0005 | 0.00516 |
| %RSD | 0.0008 | 0.0008 | 0.0079 |
Table 8. Evaluation data for Metformin Robustness study
| S.NO | 231 nm | 232 nm | 233 nm |
| 1 | 0.9955 | 0.9952 | 0.9952 |
| 2 | 0.9950 | 0.9959 | 0.9955 |
| 3 | 0.9954 | 0.9958 | 0.9954 |
| Standard deviation | 0.00083 | 0.00038 | 0.00017 |
| %RSD | 0.00083 | 0.0381 | 00.0017 |
Assay of Tablets Formulation: For estimation of drugs in the commercial formulations, twenty tablets were weighed and average weight was calculated. The tablets were crushed and powdered in glass mortar. For the analysis of drugs, quantity of powder equivalent to 10 mg equivalent to Dapagliflozin and Metformin was transferred to 10 ml volumetric flask and dissolved in sufficient quantity of water. It was sonicated for 10 min and volume was made up to obtain a stock solution 1000μg/ml of Sample. Further dilutions were made from this stock solution to get 10μg/ml. The concentration of Dapagliflozin and Metformin was determined by measuring absorbance of sample solutions at 222 nm (λmax of Dapagliflozin) and232 nm (λmax of Metformin) using Q AbsorptionMethod. The results of analysis for the marketed tablet formulation (OXRAMET which contains 10 mg of Dapagliflozin and 500 mg of Metformin) are reported in Table 9. The amount of Dapagliflozin and Metformin was calculated using Q absorption ratio method given below (Sanagapati, Lakshmi and Reddy, 2014).
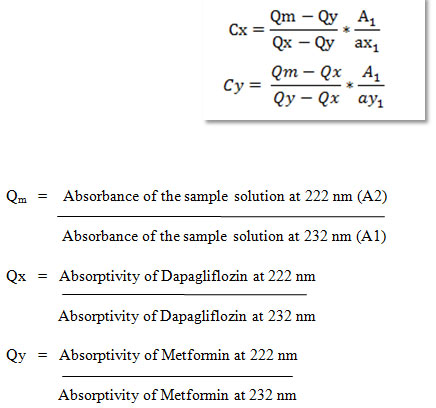
Table 9. Evaluation data for Assay of tablets.
| Drug Name | Label claim (mg) | Amount found |
| Dapagliflozin | 10 mg | 0.1957 μg/ml |
| Metformin | 500 mg | 9.7913 μg/ml |
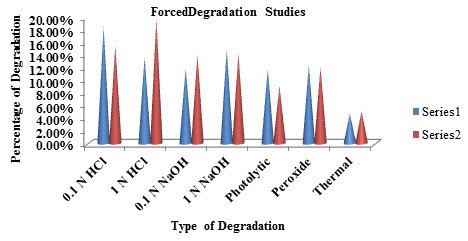
Table 10. Percentage of Degradation studies
| S.No | Type of Degradation | Dapagliflozin
(Series 1) |
Metformin
(Series 2) |
| 1. | Acid Degradation (0.1N HCl ) | 18.35 % | 15.32% |
| 2. | Acid Degradation(1N HCl ) | 13.47% | 19.64% |
| 3. | Alkali Degradation(0.1N NaOH) | 11.82% | 13.98% |
| 4. | Alkali Degradation(1N NaOH) | 14.74% | 14.04% |
| 5. | Photolytic Degradation | 11.68% | 9.02% |
| 6. | Peroxide Degradation | 12.27% | 11.95% |
| 7. | Thermal Degradation | 4.65% | 5.02% |
Forced Degradation: To assess the stability indicating property of the developed UV method stress studies were carried out under ICH recommended conditions.
Acid Degradation: From the 100 ppm of drug solution, take 1 ml of the drug solution into 10 ml volumetric flask and 1 mL of 1 N HCL was added and was kept for 24 hours. After 24 hours neutralize with 1 ml of 1N NaOH room temperature, and further dilute with water to get concentration of 10 µg/mL and determine its absorbance (Satheeshkumar, Pradeepkumar and Shanthikumar 2014; Venkataraman and Manasa 2018).
Alkali Degradation: From the 100 ppm of drug solution, take 1 ml of the drug solution into 10 ml volumetric flask and 1 mL of 1N NaOH was added and was kept for 24 hours. After 24 hours neutralize with 1 ml of 1 N HCL room temperature, and further dilute with water to get concentration of 10 µg/mL and determine its absorbance (Venkataraman and Manasa 2018).
Peroxide Degradation: From the 100 ppm of drug solution, take 1 ml of the drug solution into 10 ml volumetric flask and 1 mL of 30% Hydrogen peroxide solution was added and was kept for 24 hours. After 24 hours dilute with water to get concentration of 10 µg/mL and determine its absorbance.
Photolytic Degradation: The bulk sample was exposed to UV light in UV chamber for 2 hrs by placing 10 mg of drugs in closed Petridish. The samples were appropriately diluted to get a final concentration of 10 µg/mL solution and were scannedover a range of 400 to 200 nm by placing respective solvents as blank (Swartz and Krull 2012; Urooj, Sundar and Vasanthi 2017).
Thermal Degradation: The bulk sample was exposed to dry heat 80⁰C in oven at for 2 hrs by placing 10 mg of drugs in closed petridish. The samples were appropriately diluted to get a final concentration of 10 µg/mL solution and were scanned over a range of 400 to 200 nm by placing the blank solutions and calculate the percentage of degradation (Urooj, Sundar and Vasanthi 2017).
Table 11. Degradation studies Graph
CONCLUSION
From this validation study we can conclude that the developed UV method is accurate, rapid, precise, reproducible and inexpensive with acceptable correlation co-efficient, accuracy and robustness. The method is versatile for simultaneous determination of Dapagliflozin and Metformin with the use of low-cost reagents are the additional benefit of this method. So this method can be used for routine analysis in the quality control for determination of Dapagliflozin and Metformin.
ACKNOWLEDGMENTS
We are very thankful for RBVRR College of Pharmacy, Barkatpura, Narayanaguda and Hyderabad for gratis sample of Dapagliflozin and Metformin and also for providing sophisticated equipment and other facilities to complete this work successfully.
Conflict of Interests: The authors did not have any conflict in interests.
REFERENCES
Jani, B.R., Shah, K.V. and Kapupara, P.P., (2015). Development and validation of UV spectroscopic first derivative method for simultaneous estimation of dapagliflozin and metformin hydrochloride in synthetic mixture. J Bioequiv, 1(1), p.102.
Lambers Heerspink, H.J., De Zeeuw, D., and Wie, L., (2013). Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes, Obesity and Metabolism, 15(9), pp.853-862.
Mishra, K., Soni, H., and Nayak, G. (2011). Method development and validation of metformin hydrochloride in tablet dosage form. E-Journal of Chemistry, 8.
Nalwade, S.U., Reddy, V.R., and Rao, D.D. (2012). A validated stability indicating ultra performance liquid chromatographic method for determination of impurities in Esomeprazole magnesium gastro resistant tablets. Journal of pharmaceutical and biomedical analysis, 57, pp.109-114.
Parmar, S.H., Luhar, S.V. and Narkhede, S.B., (2018). Development and validation of uv-spectroscopic first derivative and high-performance thin layer chromatography analytical methods for simultaneous estimation of dapagliflozin propanediol monohydrate and saxagliptin hydrochloride in synthetic mixture. European j biomed pharm sci, 5(5), pp.1-14.
Patel, K.J., Chaudhary, A.B., and Bhadani, (2017). Stability indicating RP-HPLC method development and validation for estimation of dapagliflozin and metformin HCL. World Journal of Pharmacy & Pharmaceutical Sciences, 6(9), pp.796-809.
Sanagapati, M., Lakshmi, D.K., and Reddy, (2014) Development and Validation of stability-Indicating RP-HPLC method for determination of Dapagliflozin. Journal of Advanced Pharmacy Education & Research, 4(3).
Satheeshkumar, N., Pradeepkumar, M., and Shanthikumar, S. (2014). Development of validated stability indicating assay method for simultaneous estimation of metformin hydrochloride and vildagliptin by RP-HPLC. Drug research, 64(03), pp.124-129.
Swartz, M.E. and Krull, I.S., (2012). Handbook of analytical validation. CRC Press.
Urooj, A., Sundar, P.S., and Vasanthi, R., (2017). Development and Validation of RP-HPLC method for simultaneous estimation of dapagliflozin and metformin in bulk and in synthetic mixture. World journal of pharmacy and pharmaceutical sciences, 6(7), pp. 2139-2150.
Venkataraman, S. and Manasa, M., (2018). Forced degradation studies: Regulatory guidance, characterization of drugs, and their degradation products-a review. Drug Invention Today, 10(2).