1Department of Environmental Health, Health Promotion Research Center, School of Public Health, Zahedan University of Medical Sciences, Zahedan, Iran
2MSc Student of Environmental Health Engineering, Student Research Committee, Qom University of Medical Sciences, Qom, Iran
Corresponding author Email: alijoghatayi69@gmail.com
Article Publishing History
Received: 11/11/2016
Accepted After Revision: 29/03/2017
The objective of this study is to remove the Ciprofloxacin (CPX), from synthetic wastewater by using the acid activated red mud in batch adsorption experiments. The effects of contact time, adsorbent dosage and initial CPX concentration on the adsorption were investigated. It was found that the sufficient time to attain equilibrium was 75 min. The adsorption isotherms were analyzed using the Langmuir, the Freundlich, Temkin and Dubinin Radushkevich isotherms. The Freundlich isotherm was the best-fit adsorption isotherm model for the experimental data obtained from the linear chi-square statistic test. The maximum adsorption capacities were 19.12 at room temperature according to the Langmuir model. The adsorption kinetics analysis indicates that pseudo-second order model is better fitted than other kinetics model for the description of the adsorption rate. The results show that the highest removal efficiency of 96.5% was achieved around adsorbent sosage 5 g/L.
Red Mud, Ciprofloxacin, Adsorption, Kinetics, Isotherms
Balarak D, Mostafapour F. K, Joghataei A. Kinetics and Mechanism of Red Mud in Adsorption of Ciprofloxacin In Aqueous Solution. Biosc.Biotech.Res.Comm. 2017;10(1).
Balarak D, Mostafapour F. K, Joghataei A. Kinetics and Mechanism of Red Mud in Adsorption of Ciprofloxacin In Aqueous Solution. Biosc.Biotech.Res.Comm. 2017;10(1). Available from: https://bit.ly/2YRw8bF
Introduction
For the last years the interest towards the fate of medicines, especially antibiotics, has been arising,(Alexy et al., 2004 and Balarak et al., 2016a). Being refractory substances, antibiotics pass the biological treatment plants intact, either remaining in the liquid phase or, dependent on their hydrophilicity, adsorbing to the active sludge with subsequent desorption to the environment, (Johnson and Mehrvar (2008) and Choi et al., 2008). Pharmaceuticals are considered as an emerging environmental problem due to their continuous input and persistence into the aquatic ecosystem even at low concentrations,( Aksu and Tunc 2005 and Balarak et al 2016 b).
After their use, human pharmaceuticals or their metabolites are excreted into the effluents and reach the sewage treatment plants (STPs) ( Balarak et al 2016c and Su et al., 2016). Unfortunately, conventional STPs are not able to degrade residues of pharmaceutical compounds, and as a result they are introduced into the aquatic environment. Residual amounts of pharmaceuticals can reach surface waters, groundwater or sediments. Many studies have reported a large number of pharmaceuticals at concentrations ranging from ng/L to g/L in STP effluents, in natural waters and even in drinking water. Ciprofloxacin (CPX) is one of the most used antibiotics in aquaculture and veterinary medicine. It has been monitored either in superficial or in potable waters, (Hu et al., 2007, Ji et al 2002, 2009, Jianga et al., 2013, Genec et al., 2013, Zhang et al 2016, Su et al., 2016 and Yu et al., 2016).
Although the amount of drugs introduced in the medium through these routes may be low, its continuous discharge could cause high concentrations in the long term and adverse effects in terrestrial and aquatic organisms. These effects can be slowly accumulated, so that the changes show up suddenly and irreversibly. On the other hand, it could be supposed that pharmaceuticals are susceptible of degradation through microorganisms’ action, but not all drugs are biodegradable. The case of antibiotics is obvious, because they are biologically active, and so they have limited biodegradability. A clear example is the fact that, in the last decades, the increase in antibiotics consumption has resulted in the generation of more harmful bacteria, more resistant to antibiotics. The adsorption process is another attractive alternative treatment process if the adsorbent is inexpensive and readily available, (Dutta et al., 1999,Zhang et al., 2003,Gao and Pedersen (2005) Gulkowsk et al., 2008, Kassinos et al,2011, Peterson et al, 2012 and Balarak et al. 2016 d).
Activate carbon is the most powerful and common adsorbent and has been used successfully. But the high cost in the preparation of activated carbon restricts its use in the industrial wastewater treatment, especially in the developing countries, (Chen and Huang 2010).In recent years, many studies have been done on the non-conventional and economic adsorbents, especially those researches on making use of industrial solid waste. Using an industrial solid waste for the treatment of wastewaters from another industry could be helpful not only to the economy, but also to solve the solid waste disposal problem, (Zhang et al., 2003, Zhu et al., 2013 and Parolo et al., 2008).
Red mud (RM), (bauxite wastes of alumina manufacture) emerges as an unwanted byproducts during alkaline-leaching of bauxite in Bayer process, which is used for the production of alumina from bauxite. Studies using red mud residues from alumina refineries as unconventional adsorbents for water and wastewater treatment purposes are motivated by the fact that red mud is a fine-grained mixture of oxides and hydroxides, capable of removing several contaminants, as well as being widely available, (Claudia et al., 2005, Weiwei et al., 2008, Wang et al., 2008, and Tor and Cengeloglu 2006). However, the studies about utilization of activated red mud for removal of antibiotics from aqueous solution are very rare. Therefore, in the present paper, the possibility of utilisation of the red mud in the acid activated form as an adsorbent for removal of CPX from synthetic wastewater was studied.
Material And Methods
Ciprofloxacin with molecular weight 331.34 g/mol and maximum adsorption 285 nm. The CPX (C17H18FN3O3) was purchased from Sigma–Aldrich (>98% purity) and used without further purification.
Red mud was washed thoroughly with distilled water, dried at 110 oC for 24 hours. 10 grams of washed red mud was soaked in 200 ml of 1N H2SO4 for 24 hours, washed with water several times and dried at 110 oC overnight. The acid treated red mud sample thus prepared was sieved and the sample of average size 120 microns was used for the studies. Scanning Electronic Microscopy (SEM) was carried out for surface morphology to compare the relative performance of raw and acid treated red mud. The surface areas of original red mud and acid treated red mud were determined by BET analysis using ASAP 2020 V3.04 H, Micromeritics, USA surface area analyzer.
Batch adsorption experiments were carried out in 200 mL flasks with 100 mL of working volume. 5 grof RM and determined amounts of stock solution were added into flasks and diluted to designed concentrations. The initial pH of the solution was adjusted to 6.5 ± 0.2 using 0.1M HCl or 0.1M NaOH. The loaded flasks were sealed and shaken at an air rotary shaker with 200 rpm for 75 min. The adsorption was examined at 28±2 oC. A blank adsorption experiment without RM addition was conducted following the same procedure as the control. Each test was carried out in triplicate and the average was reported here. Liquid samples were collected from the flasks at predetermined time intervals. The collected liquid samples were centrifuged at 4000 rpm for 10 min and the supernatant was passed through 0.45 µm filter for the determination of residual tetracycline concentration. CPX concentrations are measured by liquid chromatography (HPLC) coupled to a photodiode array detector (PDA, Surveyor, Thermo Scientific, USA). A Luna C18 column (150 mm×3.0 mm, 3 M, Phenomenex, USA) with a mobile phase containing 87.5% water (0.1% formic acid) and 12.5% acetonitrile is used for the chromatographic analysis of CPX.
The amount of CPX adsorbed was calculated from the following equation:
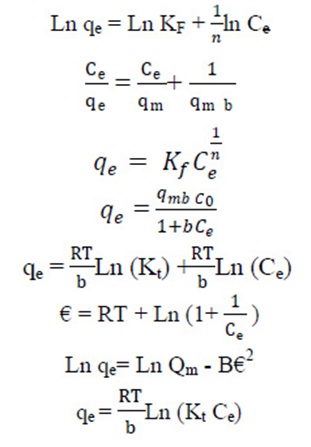
where qe is the amount of CPX adsorbed per unit weight of activated red mud (mg/g); C0 the initial concentration of CPX (mg/L); Ce the concentration of CPX in solution at equilibrium time (mg/L); V the solution volume (L); m is the activated red mud dosage (g).
 |
Figure 1: Chemical structure of CPX |
Results and Discussion
The raw RM and acid treated RM have specific surface area of 21.4 and 28.7 m2/g respectively. The pore size of raw RM and acid treated RM was 18.75 nm and 17.44 nm respectively. The surface area and pore volume of acid treated RM is higher than the raw RM.
SEM of RM after and before CPX adsorbed are shown in Fig. 2a and b. It is clear that, RM has considerable numbers of pores where, there is a good possibility for CPX to be trapped and adsorbed into these pores.
 |
Figure 2: SEM image of Red mud before and after CPXadsorbed |
RM is found to be a complex mixture of phases mainly comprising of Hematite (Fe2O3),Goethite FeO(OH) and Quartz (SiO2). The surface of the RM activated by acid pretreatment was compared with that of raw RM using the results of scanning electron microscopy. The SEM-EDAX micrograph of raw RM and acid treated RM samples are shown in Fig 3a and b. EDAX analysis shows that the intensity for metals like Al, Si, Ti and Fe in raw RM are high. Acid treatment of RM has resulted in drastically decreased intensities for Fe, Ti, Si and Al.
 |
Figure 3: The SEM- EDAX micrograph of a: raw red mud b: red mud treated with H2SO4 |
Effect of contact time and initial CPX concentration
Fig 4 shows the effect of contact time and initial concentration on adsorption of CPX by RM. The removal of CPX Was rapid at initial stage of contact time until saturation. The equilibrium time was 75 min for all the CPX Concentation used (10-100 mg/L). This may be due to the attainment of equilibrium condition at 75 min of contact time, which is fixed as the optimum contact time. At the initial stage, the rate of the removal of CPX was higher, due to the availability of more than required number of active sites on the surface of adsorbent. The rate of the removal became slower at the later stages of contact time, due to the decreased or lesser number of active sites.Low et al 2007, Tor and Cengeloglu 2006 and Balarak et al., 2016e )
The uptake of CPX at equilibrium decreased from 96.5 to 78.4 with the increase of CPX concentration from 10 to 100 mg/L. It can be attributed that the active sites on adsorbent for CPX removal decreases when CPX concentration increases, (Putra et al., 2009).
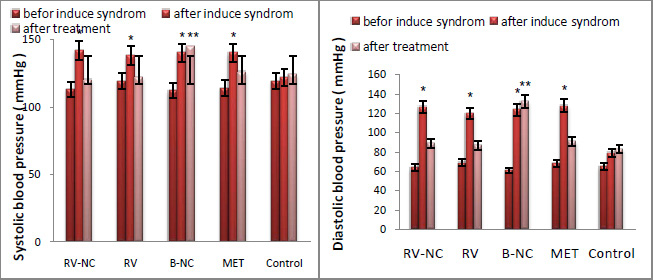 |
Figure 4: Effect of contact time and concentration on CPX removal (pH =7, Adsorbent dosage 5 g/L and temperature= 28±2°C). |
Effect of adsorbent dose
Fig 5 shows that the removal of CPX increased with the increase in adsorbent dosage and reached a maximum of 25.2 mg/g (89.6%) at 5 g/L of adsorbent dose. The increase in the amount of CPX removal with adsorbent dosage is due to the greater availability of adsorbent surface area for adsorption ( Ahmad et al. 2012, Zazouli et al., 2014).
 |
Figure 5: Effect of adsorbent dosage on CPX adsorption (C0 = 50 mg/L, time = 75 min, pH = 7, temp= 28 ± 2°C). |
Adsorption Kinetics
Kinetics is key factor for adsorption investigation because it can predict the rate at which a pollutant is removed from aqueous solution and provides valuable data for understanding the mechanism of adsorption process. Several models are available to investigate the adsorption mechanism and description based on experimental data such as pseudo-first order, pseudo-second order, intramolecular diffusion and Elovich models. The pseudo-first order adsorption rate and pseudo-second order adsorption rate have the following linear forms, (Ersen and Bagd 2013, Ghauch et al., 2009 and Balarak et al., 2015).
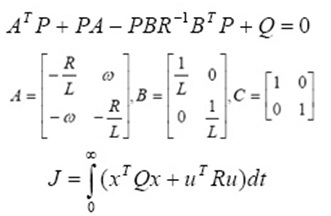
Where k1 (min−1) is pseudo first order rate constant, qe (mg g−1) is the amount of CPX adsorbed on surface at equilibrium, qt (mgg−1) is the amount of CPX adsorbed on surface at time t (min). The adsorption rate constant, k1 and qe were calculated from the plot of log(qe-qt) vs t, and are listed in Table 1.
| Table 1: Adsorption kinetics constants of adsorption of CPX onto RM | ||||||||||||
| First-order model | Second-order model | Weber-Morris model | Elovich model | |||||||||
| qe mg/g exp | qe cal | k1 | R2 | qe cal | k2 | R2 | k | C | R2 | á | â | R2 |
| 19.25 | 17.06 | 0.125 | 0.941 | 20.14 | 0.748 | 0.997 | 0.642 | 0.749 | 0.876 | 4.112 | 0.847 | 0.904 |
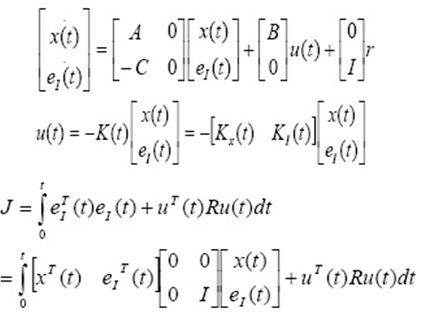
Where, k2 (g mg−1 min−1) is pseudo second order rate constant. The adsorption rate constant, qe and k2 were calculated from the plot of t/qt vs t, and are listed in Table 1. The correlation coefficient of pseudo first order kinetics (0.941) is not lower than that of second order kinetics (0.997). Consequently pseudo second order kinetics is fitted.
Intraparticle diffusion
The limiting step in CPX adsorption may be either the boundary film formation or intraparticle (pore) diffusion of the CPX on the solid surface from bulk of solution. Weber and Morris explain the diffusion mechanism through the following equation:
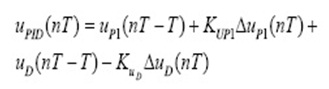
C is the intercept that its value provides information about the thickness of boundary layer. K is intraparticle diffusion rate constant (mg g−1 min−0.5) which are evaluated from the intercept and slope of plot qt and t1/2.
Elovich
It is another rate equation in which the absorbing surface is heterogeneous. It is represented as:
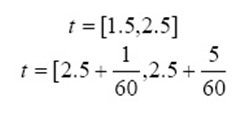
α is the initial adsorption rate (mg g−1 min−1). β is the desorption constant (g mg−1) which are calculated from intercept and slope of plot qt versus lnt.
Adsorption Isotherm Models
The equilibrium data of CPX analyzed by fitting them into Langmuir, Freundlich, Temkin and Dubinin Radushkevich equation to find out the suitable model that may be used for design consideration.
Langmuir Isotherm
The Langmuir isotherm assumes the absence of any interactions between adsorbate molecules and the adsorption process is account for monolayer formation. The linear form of the Langmuir isotherm, assuming monolayer adsorption on a homogeneous adsorbent surface, is expressed as follows:

Where qmax (mg∙g−1) is the maximum adsorption capacity of the adsorbent corresponding to monolayer formation and illustrates the maximum value of qe that can be attained as Ce is increased. The b parameter is a coefficient related to the energy of adsorption and increases with increasing strength of the adsorption bond. Values of qmax and b are determined from the linear regression plot of (Ce/qe) versus Ce. Linear plot in negative direction indicates that Langmuir model fails to explain the process of adsorption and absence of formation of monolayer.
Freundlich Isotherm
It is well established that the Freundlich isotherm is often applied to heterogeneous solid catalyst. The Freundlich equilibrium isotherm equation is an empirical relation involved for the description of multilayer adsorption with interaction between adsorbed molecules. The Freundlich equation is expressed as follows in its linear form, (Rostamian and Behnejad 2016, Balarak et al, 2016 g),
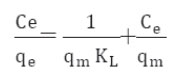
where, KF represents the capacity of the adsorbent for the adsorbate, and 1/n shows adsorption intensity of CPX on solid which is a function of the strength of adsorption. A linear regression plot of log qe versus log Ce, gives the KF and n values. The model is applicable to the adsorption on heterogeneous surfaces by a uniform energy distribution and reversible adsorption. Linear plot with high regression factor indicating the successful model in explaining the adsorption model.
Temkin Isotherm
The Temkin model takes into the account adsorbing species–adsorbent interactions. This isotherm proposed that the heat of adsorption of all the molecules in the layer decreases linearly with coverage due to adsorbent–adsorbate interactions and the adsorption is characterized by a uniform distribution of binding energies, up to some maximum binding energy. The linear Temkin equation is(48, 49):
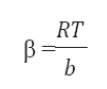
is the equilibrium constant corresponding to the maximum binding energy.
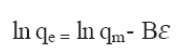
T is the absolute temperature in Kelvin. R is the universal gas constant 8.314 J.mol−1.K−1. b is the Temkin constant related to heat sorption/J∙mg−1.
A and â are calculated from the slope and intercept of qe versus ln Ce. The Temkin equation better holds for the prediction of gas phase equilibria rather than liquid phase. The liquid phase is a more complex phenomenon since the adsorbed molecules do not necessarily organized in a tightly packed structure with identical orientation. Linear plot and high regression value suggest the successful model in explaining the adsorption mechanism.
Dubinin Radushkevich Isotherm
This model is involved to estimate the porosity, free energy and the characteristics of adsorbents. The isotherm assumes the surface heterogeneity and the variation of adsorption potential during sorption process. The model has commonly been applied in the following linear Equation: (Zazouli et al., 2015):
![]()
Polanyi potential, α, can be calculated according the following equation, (Peng et al., 2012):
![]()
Where B is a constant related to the adsorption energy (mol2 J−2), qm the theoretical saturation capacity. Table 2 summarizes Dubinin constants. The mean free energy of adsorption (E) which is energy require to transfer one mole of the CPX from infinity in solution to the surface of the solid can be calculated from the B value using the following relation,( Chang et al., 2012).
Table 2 summarizes isotherm constants. The results of Freundlich, Langmuir, Temkin and Dubinin Radushkevich models suggest that adsorption of CPX is accompanied by multilayer formation. The adsorption energy obtained from Temkin plot 368.08 J.mg−1 which indicates that the adsorption process is endothermic and a strong interaction between RM and CPX. The value of energy is about 875.2 J/mole revealing physisorption of CPX on RM.
| Table 2: Isotherms constants for the removal CPX onto RM | |||||||||||||
| Langmuir model | Freundlich model | Dubinin Radushkevich | Temkin model | ||||||||||
| qm | RL | KL | R2 | n | KF | R2 | B | qm | E | R2 | R2 | ||
| 19.12 | 0.461 | 0.038 | 0.964 | 3.29 | 1.874 | 0.985 | 6.52 | 17.44 | 875.2 | 0.928 | 6.731 | 0.844 | 0.911 |
Conclusions
The adsorption removal of Ciprofloxacin by Red Mud was investigated in this study. The highest removal efficiency of CPX 96.5% was was achieved around adsorbent sosage 5 g/L. The removal efficiency of CPX was affected by the adsorbent dose and the concentration of CPX antibiotics in the solution and contact time. The adsorption isotherms analysis shows that Freundlich model is better fitted than other isotherm models for the adsorption equilibrium. The adsorption behavior of CPX on RM stone was fitted well in the pseudo-second order kinetics model.
References
- Ahmed MJ, Theydan SK.(2012) Adsorption of cephalexin onto activated carbons from Albizia lebbeck seed pods by microwave-induced KOH and K2CO3 activations. Chemical Engineering Journal 211-212; 200–7.
- Aksu Z, Tunc O.(2005) Application of biosorption for Penicillin G removal: Comparison with activated carbon. Process Biochemistry. 40(2):831-47.
- Alexy R, Kumpel T, Kummerer K.(2004) Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere; 57, 505–512.
- Balarak D, Azarpira H, Mostafapour FK. (2016 c)Thermodynamics of removal of cadmium by adsorption on Barley husk biomass. Der Pharma Chemica,8(10):243-247.
- Balarak D, Azarpira H, Mostafapour FK. (2016) Study of the Adsorption Mechanisms of Cephalexin on to Azolla filiculoides. Der Pharma Chemica, 8(10):114-121.
- Balarak D, Jaafari J, Hassani G, Mahdavi Y, Tyagi I, Agarwal S, Gupta VK. (2015) The use of low-cost adsorbent (Canola residues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies. Colloids and Interface Science Communications. Colloids and Interface Science Communications. 7:16–19.
- Balarak D, Mahdavi Y and Mostafapour FK.(2016 b) Application of Alumina-coated Carbon Nanotubes in Removal of Tetracycline from Aqueous Solution. British Journal of Pharmaceutical Research; 12(1): 1-11.
- Balarak D, Mahdavi Y, Maleki A, Daraei H and Sadeghi S. (2016a)Studies on the Removal of Amoxicillin by Single Walled Carbon Nanotubes. British Journal of Pharmaceutical Research. 10(4): 1-9.
- Balarak D, Mahdavi Y, Bazrafshan E , Mahvi AH. (2016e)Kinetic, isotherms and thermodynamic modeling for adsorption of acid blue 92 from aqueous solution by modified Azolla filicoloides. Fresenius Environmental Bulletin.25(5); 1321-30.
- Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH, Esfandyari Y.(2016f) Adsorption of fluoride from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetic and thermodynamic parameters. Flouride. 49(1):35-42.
- Balarak D, Mostafapour FK, Joghataei A.( 2016) Experimental and Kinetic Studies on Penicillin G Adsorption by Lemna minor. British Journal of Pharmaceutical Research. 9(5): 1-10.
- Balarak D.(2016) Kinetics, Isotherm and Thermodynamics Studies on Bisphenol A Adsorption using Barley husk. International Journal of ChemTech Research ;9(5);681-690.
- Chang PH, Li Z, Jean JS, Jiang WT, Wang CJ, Lin KH.(2012) Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 67;158–163.
- Chen WR, Huang CH. (2010)Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere. 79, 779–785
- Choi KJ, Kim SG, Kim SH. (2008) Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater.151;38–43.
- Dutta M, Dutta NN, Bhattachary KG.(1999) Aqueous phase adsorption of certain beta-lactam antibiotics onto polymeric resins and activated carbon. Separation and Purification Technology.16(3);213-24.
- Ers˛an M, Bag˘d E.(2013) Investigation of kinetic and thermodynamic characteristics of removal of tetracycline with sponge like, tannin based cryogels. Colloids and Surfaces B: Biointerfaces. 104;75-82.
- Gao J and Pedersen JA. (2005)Adsorption of Sulfonamide Antimicrobial Agents to Clay Minerals. Environ. Sci. Technol. 39(24). 9509-16.
- Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, Su X.(2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide, J. Colloid. Interface Sci. 368; 540–546.
- Garoma T, Umamaheshwar SH, Mumper A. (2010) Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere. 79;814–20.
- Genç N, Can Dogan E, Yurtsever M.(2013) Bentonite for ciprofloxacin removal from aqueous solution. Water Sci Technol. 68(4):848-55.
- Ghauch A, Tuqan A, Assi HA: Elimination of amoxicillin and ampicillin by micro scale and nano scale iron particles. Environ Pollut 2009,157:1626–1635.
- Gulkowsk A, Leung HW, So MK, Taniyasu S, Yamashita N. (2008) Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water research. 42:395-403.
- Haq, I. Bhatti, HN. Asgher, M. (2011)Removal of solar red BA textile dye from aqueous solution by low cost barley husk: Equilibrium, kinetic and thermodynamic study. Canadian Journal of Chemical Engineering. 89(3);593–600.
- Ji K, Kim S, Han S, Seo J, Lee S, Y.(2012) Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: are the current environmental concentrations safe?, Ecotoxicology 21;2031-2050.
- Ji L, Chen W, Duan L and Zhu D. (2009) Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 43 (7), 2322–27.
- Jianga WT, Changa PH, Wanga YS, Tsaia Y, Jeana JS, Zhaohui Lia Z,(2013) Removal of ciprofloxacin from water by birnessite, Journal of Hazardous Materials. 250–251, 362–36.
- Johnson MB, Mehrvar M.(2008) Aqueous metronidazole degradation by UV/H2O2 process in single-and multi-lamp tubular photoreactors: kinetics and reactor design, Ind. Eng. Chem. Res. 47; 6525–6537.
- Kassinos F D, Meric S, Nikolau A. (2011) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research, Anal. Bioanal. Chem. 2011; 399; 251-275.
- Lanhua Hu L, Flanders PM, Penney L. Miller PL, Timothy J. Strathmann TJ.(2007) Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis Water Research 2007;41(12); 2612-26.
- Low KS, Lee CK, Tan BF.(2000) Quaternized wood as sorbent for reactive dyes. Biochem Biotechnol. ;87:233-45.
- Peng H, Pana B, Wu M, Ran Liu R, Zhanga D, Wu D, Xing B.(2012)Adsorption of ofloxacin on carbon nanotubes: Solubility, pH and cosolvent effects. Journal of Hazardous Materials. 211-212;342-48.
- Peng X, Hu F, Dai H, Xiong Q. (2016) Study of the adsorption mechanism of ciprofloxacin antibiotics onto graphitic ordered mesoporous carbons. Journal of the Taiwan Institute of Chemical Engineers. 8; 1–10.
- Peterson JW. Petrasky LJ, Seymourc MD, Burkharta RS, Schuilinga AB.(2012) Adsorption and breakdown of penicillin antibiotic in the presence of titanium oxide nanoparticles in water. Chemosphere. 87(8); 911–7.
- Putra EK, Pranowoa R, Sunarsob J, Indraswatia N, Ismadjia S. (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanisms, isotherms and kinetics. Water Res. 43, 2419-2430.
- Robinson, T. Chandran, B. Nigam, P. (2002)Removal of dyes from an artificial textile dye effluent by two agricultural waste residues, corncob and barley husk. Environment International. 28(1-2);29–33.
- Rostamian R, Behnejad H. (2016) A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Safety and Environmental Protection.102;20-29.
- Su YF, Wang GB, Kuo DTF, Chang ML.(2016) Photoelectrocatalytic degradation of the antibiotic sulfamethoxazole using TiO2/Tiphotoanode. Applied Catalysis B: Environmental. 186;184-192.
- Su YF, Wang GB, Kuo DTF, Chang ML, Shih YH.(2016) Photo electrocatalytic degradation of the antibiotic sulfamethoxazole using TiO2/Ti photoanode. Applied Catalysis B: Environmental. 186;184–192.
- Tor A, Cengeloglu Y. (2006) Removal of Congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater 138(2): 409-15.
- Wang S, Ang HM, (2008)Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72(11):1621-35.
- Weiwei H, Shaobin W, Zhonghua Z, Li L, Xiangdong Y, Victor R (2008) Phosphate removal from wastewater using red mud. Journal of Hazardous Materials158: 35–42.
- Yu F, Li Y, Han S, Jie Ma J. (2016) Adsorptive removal of antibiotics from aqueous solution using carbon Materials. Chemosphere153;365–385.
- Zazouli MA, Mahvi AH, Dobaradaran S, Barafrashtehpour M, Mahdavi Y, Balarak D.(2014) Adsorption of fluoride from aqueous solution by modified Azolla filiculoides. Fluoride. 47(4):349-58.
- Zazouli MA, Mahvi AH, Mahdavi Y, Balarakd D.(2015) Isothermic and kinetic modeling of fluoride removal from water by means of the natural biosorbents Sorghum and Canola. Fluoride;48(1):15-22.
- Zhang L, Song X, Liu X, Yang L, Pan F, Lv J. (2011) Studies on the removal of tetracycline by multi-walled carbon nanotubes, Chem. Eng. J. 178;26–33.
- Zhang W, He G, Gao P, Chen G: (2003)Development and characterization of composite nanofiltration membranes and their application in concentration of antibiotics. Sep Purif Technol 30:27–35.
- Zhang W, He G, Gao P, Chen G: (2003)Development and characterization of composite nano filtration membranes and their application in concentration of antibiotics. Sep Purif Technol 2003, 30:27–35
- Zhu XD, Wang YJ, Sun RJ, Zhou DM. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere. 92; 925–932.


